Boosting GSH Using the Co-Drug Approach: I-152, a Conjugate of N-acetyl-cysteine and β-mercaptoethylamine
Abstract
:1. Introduction
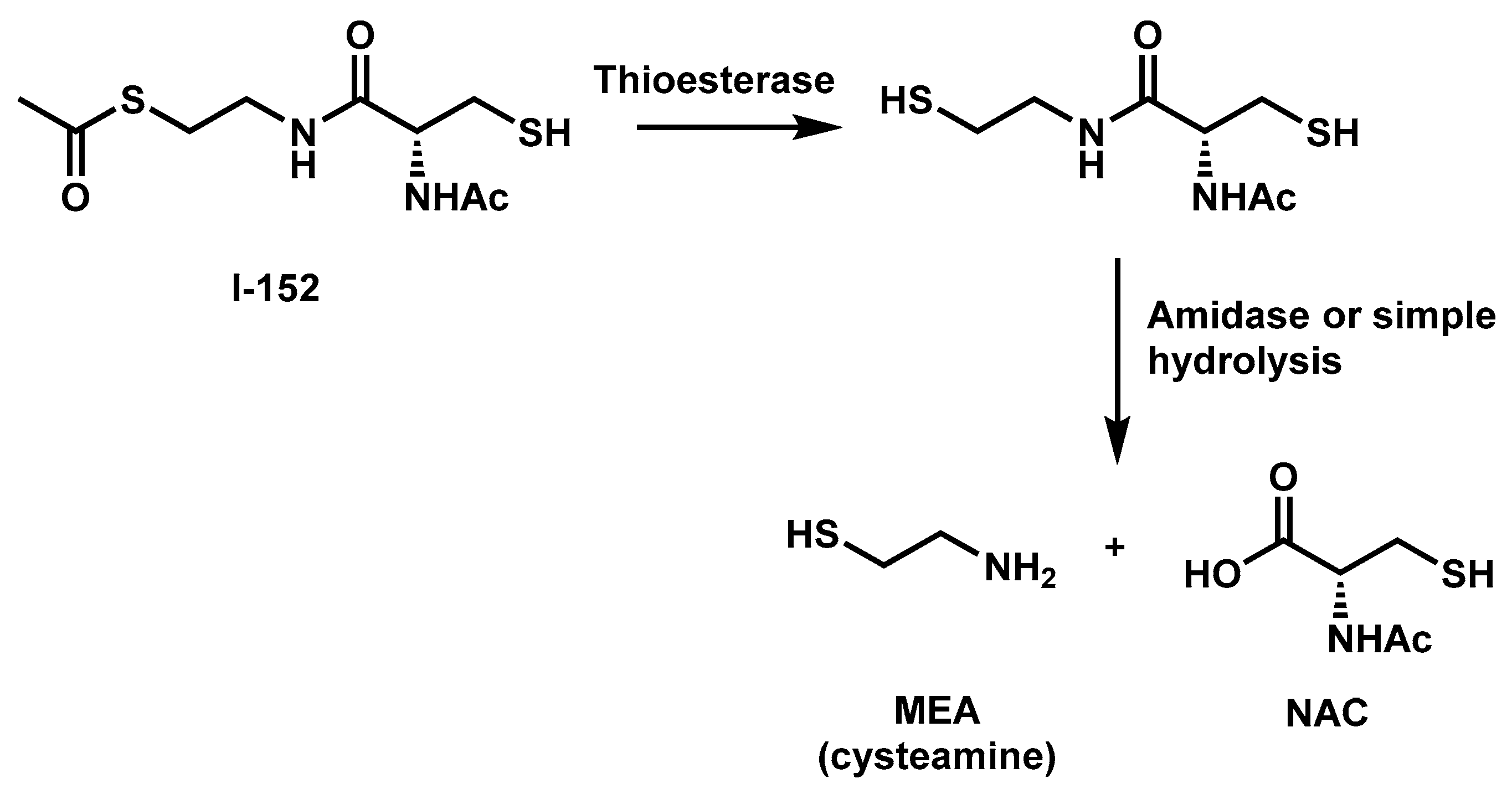
2. I-152 Design and Synthesis
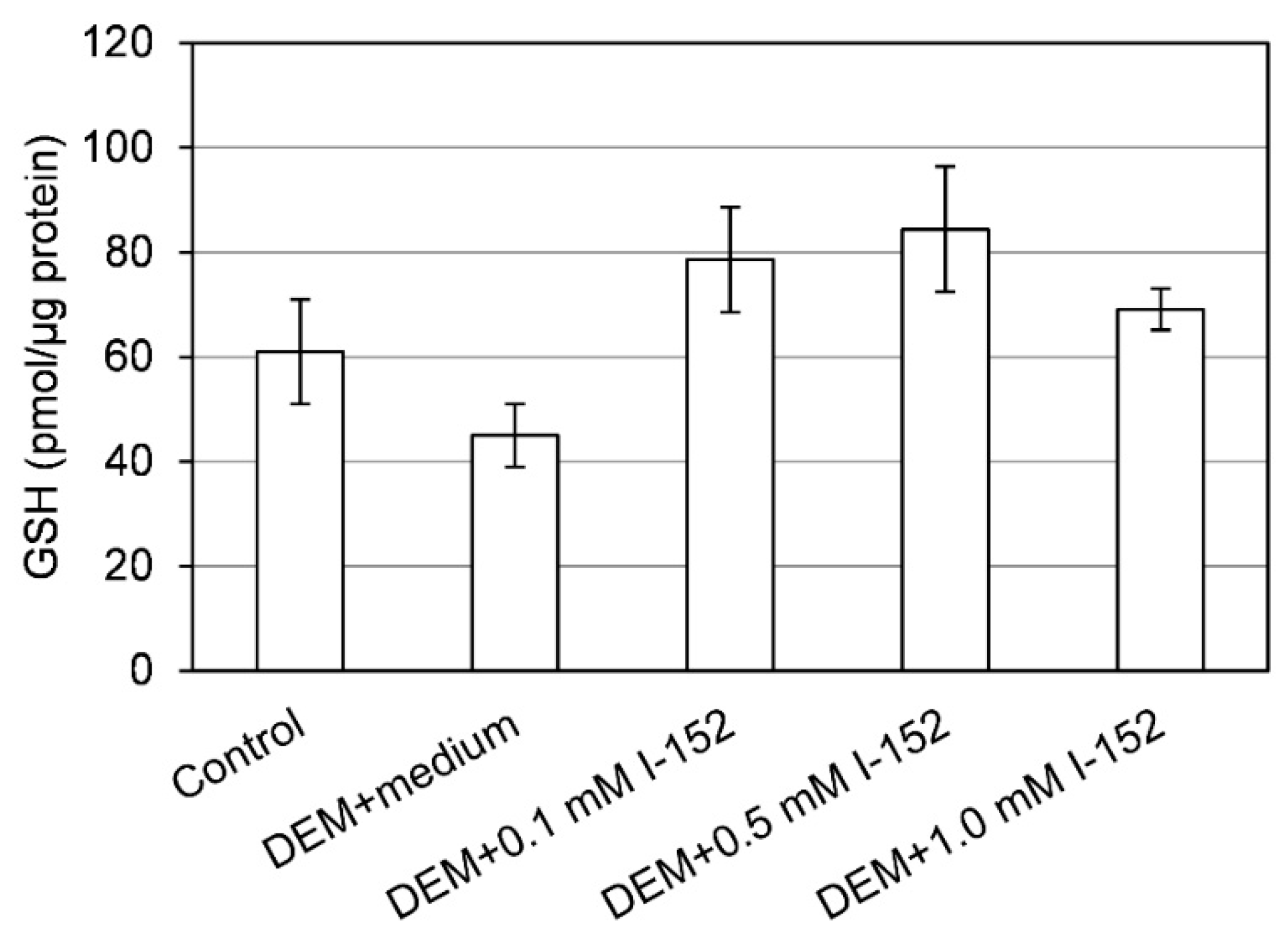
3. I-152: Metabolism and Effects on GSH Levels
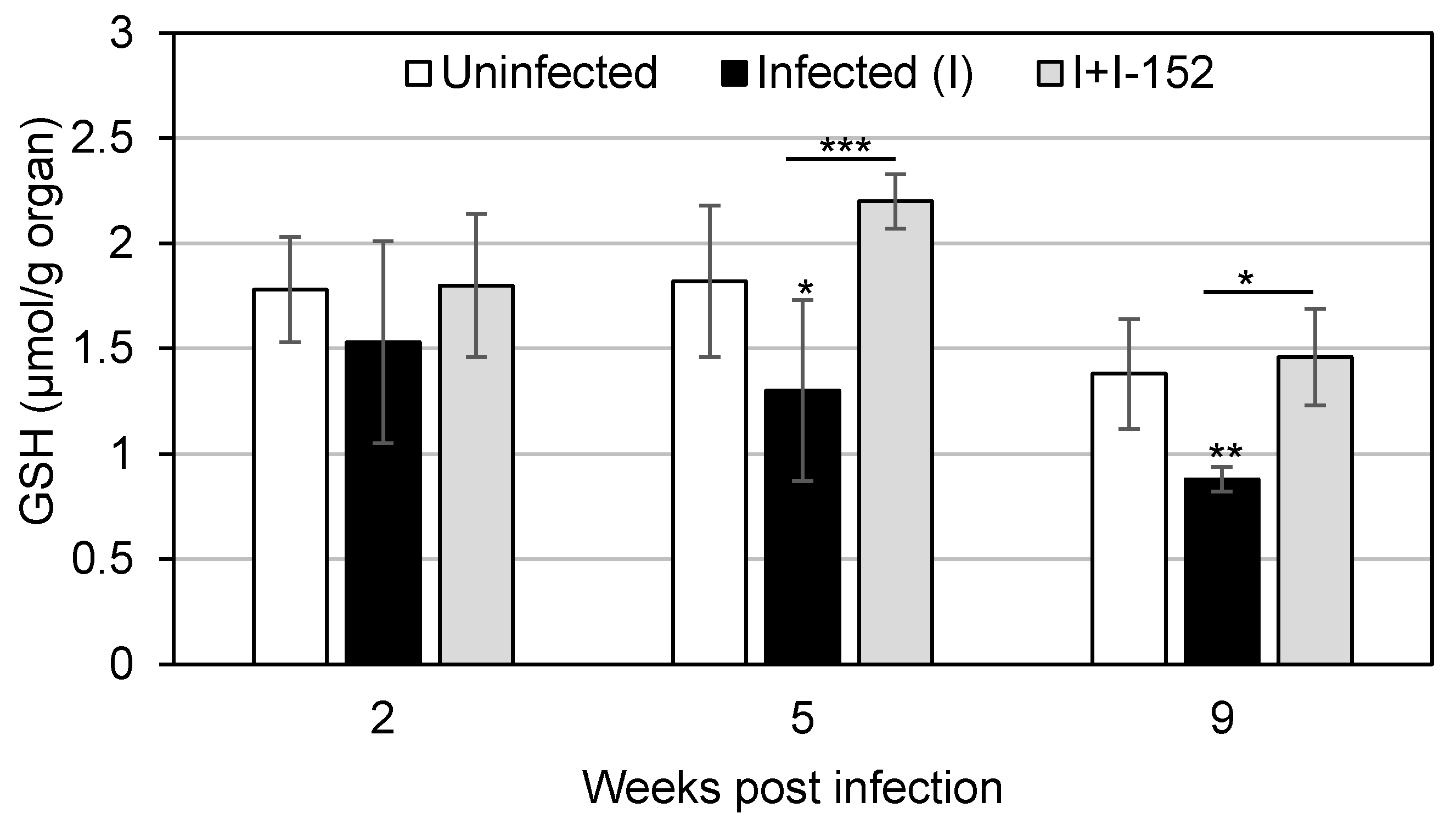
4. Antiviral and Immunomodulatory Properties of I-152
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Yi, J. Redox sensing by proteins: Oxidative modifications on cysteines and the consequent events. Antioxid. Redox Signal. 2012, 16, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ristoff, E.; Larsson, A. Inborn errors in the metabolism of glutathione. Orphanet J. Rare Dis. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [Green Version]
- Morris, D.; Khurasany, M.; Nguyen, T.; Kim, J.; Guilford, F.; Mehta, R.; Gray, D.; Saviola, B.; Venketaraman, V. Glutathione and infection. Biochim. Biophys. Acta 2013, 1830, 3329–3349. [Google Scholar] [CrossRef] [PubMed]
- Palamara, A.T.; Brandi, G.; Rossi, L.; Millo, E.; Benatti, U.; Nencioni, L.; Iuvara, A.; Garaci, E.; Magnani, M. New synthetic glutathione derivatives with increased antiviral activities. Antivir. Chem. Chemother. 2004, 15, 83–91. [Google Scholar] [CrossRef]
- Cacciatore, I.; Cornacchia, C.; Pinnen, F.; Mollica, A.; Di Stefano, A. Prodrug approach for increasing cellular glutathione levels. Molecules 2010, 15, 1242–1264. [Google Scholar] [CrossRef]
- Cacciatore, I.; Baldassarre, L.; Fornasari, E.; Mollica, A.; Pinnen, F. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxid. Med. Cell. Longev. 2012, 2012, 240146. [Google Scholar] [CrossRef]
- Oiry, J.; Mialocq, P.; Puy, J.Y.; Fretier, P.; Clayette, P.; Dormont, D.; Imbach, J.L. NAC/MEA conjugate: A new potent antioxidant which increases the GSH level in various cell lines. Bioorg. Med. Chem. Lett. 2001, 11, 1189–1191. [Google Scholar] [CrossRef]
- Oiry, J.; Mialocq, P.; Puy, J.Y.; Fretier, P.; Dereuddre-Bosquet, N.; Dormont, D.; Imbach, J.L.; Clayette, P. Synthesis and biological evaluation in human monocyte-derived macrophages of N-(N-acetyl-L-cysteinyl)-S-acetylcysteamine analogues with potent antioxidant and anti-HIV activities. J. Med. Chem. 2004, 47, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Whillier, S.; Raftos, J.E.; Chapman, B.; Kuchel, P.W. Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep. 2009, 14, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Huang, Y.H.; Yan, C.Y.; Wei, X.D.; Hou, J.Q.; Pu, J.X.; Lv, J.X. N-acetylcysteine Ameliorates Prostatitis via miR-141 Regulating Keap1/Nrf2 Signaling. Inflammation 2016, 39, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Dominy, J.E., Jr.; Simmons, C.R.; Hirschberger, L.L.; Hwang, J.; Coloso, R.M.; Stipanuk, M.H. Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J. Biol. Chem. 2007, 282, 25189–25198. [Google Scholar] [CrossRef] [PubMed]
- Besouw, M.; Masereeuw, R.; van den Heuvel, L.; Levtchenko, E. Cysteamine: An old drug with new potential. Drug Discov. Today 2013, 18, 785–792. [Google Scholar] [CrossRef] [PubMed]
- O’Brian, C.A.; Chu, F. Post-translational disulfide modifications in cell signaling-role of inter-protein, intra-protein, S-glutathionyl, and S-cysteaminyl disulfide modifications in signal transmission. Free Radic. Res. 2005, 39, 471–480. [Google Scholar] [CrossRef]
- Medic, G.; van der Weijden, M.; Karabis, A.; Hemels, M. A systematic literature review of cysteamine bitartrate in the treatment of nephropathic cystinosis. Curr. Med. Res. Opin. 2017, 33, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Verny, C.; Bachoud-Lévi, A.C.; Durr, A.; Goizet, C.; Azulay, J.P.; Simonin, C.; Tranchant, C.; Calvas, F.; Krystkowiak, P.; Charles, P.; et al. CYST-HD Study Group. A randomized, double-blind, placebo-controlled trial evaluating cysteamine in Huntington’s disease. Mov. Disord. 2017, 32, 932–936. [Google Scholar] [CrossRef]
- Fujisawa, T.; Rubin, B.; Suzuki, A.; Patel, P.S.; Gahl, W.A.; Joshi, B.H.; Puri, R.K. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PLoS ONE 2012, 7, e34437. [Google Scholar] [CrossRef]
- Chu, F.; Koomen, J.M.; Kobayashi, R.; O’Brian, C.A. Identification of an inactivating cysteine switch in protein kinase Cepsilon, a rational target for the design of protein kinase Cepsilon-inhibitory cancer therapeutics. Cancer Res. 2005, 65, 10478–10485. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, M.J.; Kluijtmans, L.A.; van der Velden, T.J.; Willems, P.H.; Scheffer, P.G.; Masereeuw, R.; Monnens, L.A.; van den Heuvel, L.P.; Levtchenko, E.N. Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells. Biochim. Biophys. Acta 2011, 1812, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calkins, M.J.; Townsend, J.A.; Johnson, D.A.; Johnson, J.A. Cystamine protects from 3-nitropropionic acid lesioning via induction of nf-e2 related factor 2 mediated transcription. Exp. Neurol. 2010, 224, 307–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraternale, A.; Crinelli, R.; Casabianca, A.; Paoletti, M.F.; Orlandi, C.; Carloni, E.; Smietana, M.; Palamara, A.T.; Magnani, M. Molecules altering the intracellular thiol content modulate NF-kB and STAT-1/IRF-1 signalling pathways and IL-12 p40 and IL-27 p28 production in murine macrophages. PLoS ONE 2013, 8, e57866. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Larsson, A.; Meister, A. Inhibition of gamma-glutamylcysteine synthetase by cystamine: An approach to a therapy of 5-oxoprolinuria (pyroglutamic aciduria). Biochem. Biophys. Res. Commun. 1977, 79, 919–925. [Google Scholar] [CrossRef]
- Castellani, P.; Angelini, G.; Delfino, L.; Matucci, A.; Rubartelli, A. The thiol redox state of lymphoid organs is modified by immunization: Role of different immune cell populations. Eur. J. Immunol. 2008, 38, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W.; Breitkreutz, R. Glutathione and immune function. Proc. Nutr. Soc. 2000, 59, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, A.; Brundu, S.; Magnani, M. Glutathione and glutathione derivatives in immunotherapy. Biol. Chem. 2017, 398, 261–275. [Google Scholar] [CrossRef]
- Xiaoyuan, R.; Lili, Z.; Xu, Z.; Vasco, B.; Jun, W.; Cristina, C.; Arne, H.; Jun, L. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar]
- Geon, H.K.; Jieun, E.K.; Sandy, J.R.; Sujung, Y. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J.J. Dysregulation of Glutathione Homeostasis in Neurodegenerative Diseases. Nutrients 2012, 4, 1399–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013, 14, 21021–21044. [Google Scholar] [CrossRef] [PubMed]
- Nowacek, A.; Kosloski, L.M.; Gendelman, H.E. Neurodegenerative disorders and nanoformulated drug development. Nanomedicine 2009, 4, 541–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, P.K.; Sharma, A.K.; Gupta, U. Blood brain barrier: An overview on strategies in drug delivery, realistic in vitro modeling and in vivo live tracking. Tissue Barriers 2015, 4, e1129476. [Google Scholar] [CrossRef] [PubMed]
- Sonam, K.S.; Guleria, S. Synergistic Antioxidant Activity of Natural Products. Ann. Pharmacol. Pharm. 2017, 2, 1086. [Google Scholar]
- Bahat-Stroomza, M.; Gilgun-Sherki, Y.; Offen, D.; Panet, H.; Saada, A.; Krool-Galron, N.; Barzilai, A.; Atlas, D.; Melamed, E. A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson’s disease. Eur. J. Neurosci. 2005, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Githens, S. Glutathione metabolism in the pancreas compared with that in the liver, kidney, and small intestine. Int. J. Pancreatol. 1991, 8, 97–109. [Google Scholar]
- Bhardwaj, P.; Yadav, R.K. Chronic pancreatitis: Role of oxidative stress and antioxidants. Free Radic. Res. 2013, 47, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Brundu, S.; Nencioni, L.; Celestino, I.; Coluccio, P.; Palamara, A.T.; Magnani, M.; Fraternale, A. Validation of a reversed-phase high performance liquid chromatography method for the simultaneous analysis of cysteine and reduced glutathione in mouse organs. Oxid. Med. Cell. Longev. 2016, 2016, 1746985. [Google Scholar] [CrossRef]
- Hara, Y.; McKeehan, N.; Dacks, P.A.; Fillit, H.M. Evaluation of the neuroprotective potential of N-Acetylcysteine for revention and treatment of cognitive aging and dementia. J. Prev. Alzheimers Dis. 2017, 4, 201–206. [Google Scholar]
- Boyland, E.; Chasseaud, L.F. Enzyme-catalysed conjugations of glutathione with unsaturated compounds. Biochem. J. 1967, 104, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundu, S.; Palma, L.; Picceri, G.G.; Ligi, D.; Orlandi, C.; Galluzzi, L.; Chiarantini, L.; Casabianca, A.; Schiavano, G.F.; Santi, M.; et al. Glutathione depletion is linked with Th2 polarization in mice with a retrovirus-induced immunodeficiency syndrome, murine AIDS: Role of proglutathione molecules as immunotherapeutics. J. Virol. 2016, 90, 7118–7130. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Guerra, C.; Donohue, C.; Oh, H.; Khurasany, M.; Venketaraman, V. Unveiling the Mechanisms for Decreased Glutathione in Individuals with HIV Infection. Clin. Dev. Immunol. 2012, 2012, 734125. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Nencioni, L.; Garaci, E.; Palamara, A.T.; Magnani, M. GSH and analogs in antiviral therapy. Mol. Asp. Med. 2009, 30, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Oiry, J.; Clayette, P.; Vogel, J.U.; Cinatl, J., Jr.; Palamara, A.T.; Sgarbanti, R.; Garaci, E.; et al. Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Curr. Med. Chem. 2006, 13, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Orlandi, C.; Schiavano, G.F.; Chiarantini, L.; Clayette, P.; Oiry, J.; Vogel, J.U.; Cinatl, J., Jr.; et al. Inhibition of murine AIDS by pro-glutathione (GSH) molecules. Antivir. Res. 2008, 77, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Sgarbanti, R.; Nencioni, L.; Amatore, D.; Coluccio, P.; Fraternale, A.; Sale, P.; Mammola, C.L.; Carpino, G.; Gaudio, E.; Magnani, M.; et al. Redox regulation of the influenza hemagglutinin maturation process: A new cell-mediated strategy for anti-influenza therapy. Antioxid. Redox Signal. 2011, 15, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Papadopoulos, N.G.; Stanciu, L.A.; Bellettato, C.M.; Pinamonti, S.; Degitz, K.; Holgate, S.T.; Johnston, S.L. Reducing agents inhibit rhinovirus-induced up-regulation of the rhinovirus receptor intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells. FASEB J. 2002, 16, 1934–1936. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Ly, J.; Lagman, M.; Saing, T.; Singh, M.K.; Tudela, E.V.; Morris, D.; Anderson, J.; Daliva, J.; Ochoa, C.; Patel, N.; et al. Liposomal Glutathione Supplementation Restores TH1 Cytokine Response to Mycobacterium tuberculosis Infection in HIV-Infected Individuals. J. Interferon Cytokine Res. 2015, 35, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribbs, S.K.; Guidot, D.M.; Martin, G.S.; Lennox, J.; Brown, L.A. Anti-retroviral therapy is associated with decreased alveolar glutathione levels even in healthy HIV-infected individuals. PLoS ONE 2014, 9, e88630. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Percival, S.S. Immunomodulatory Effects of Glutathione, Garlic Derivatives, and Hydrogen Sulfide. Nutrients 2019, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Ghousunnissa, S.; Nair, S.; Valluri, V.L.; Mukhopadhyay, S. Glutathione-redox balance regulates c-rel-driven IL-12 production in macrophages: Possible implications in antituberculosis immunotherapy. J. Immunol. 2010, 184, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.D.; Herzenberg, L.A.; Vasquez, K.; Waltenbaugh, C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 3071–3076. [Google Scholar] [CrossRef] [Green Version]
- Fraternale, A.; Paoletti, M.F.; Dominici, S.; Caputo, A.; Castaldello, A.; Millo, E.; Brocca-Cofano, E.; Smietana, M.; Clayette, P.; Oiry, J.; et al. The increase in intra-macrophage thiols induced by new pro-GSH molecules directs the Th1 skewing in ovalbumin immunized mice. Vaccine 2010, 28, 7676–7682. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Dominici, S.; Buondelmonte, C.; Caputo, A.; Castaldello, A.; Tripiciano, A.; Cafaro, A.; Palamara, A.T.; Sgarbanti, R.; et al. Modulation of Th1/Th2 immune responses to HIV-1 Tat by new pro-GSH molecules. Vaccine 2011, 29, 6823–6829. [Google Scholar] [CrossRef]
- Liu, X.; Wetzler, L.; Massari, P. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 2008, 26, 786–796. [Google Scholar] [CrossRef] [Green Version]
- Lefeber, D.J.; Benaissa-Trouw, B.; Vliegenthart, J.F.; Kamerling, J.P.; Jansen, W.T.; Kraaijeveld, K.; Harme, S. Th1-directing adjuvants increase the immunogenicity of oligosaccharide-protein conjugate vaccines protein related to Streptococcus pneumoniae type 3. Infect. Immun. 2003, 71, 6915–6920. [Google Scholar] [CrossRef]
- Green, K.A.; Cook, W.J.; Green, W.R. Myeloid-derived suppressor cells in murine retrovirus-induced AIDS inhibit T- and B-cell responses in vitro that are used to define the immunodeficiency. J. Virol. 2013, 87, 2058–2071. [Google Scholar] [CrossRef]
- O’Connor, M.A.; Fu, W.W.; Green, K.A.; Green, W.R. Subpopulations of M-MDSCs from mice infected by an immunodeficiency-causing retrovirus and their differential suppression of T- vs. B-cell responses. Virology 2015, 485, 263–273. [Google Scholar] [CrossRef]
- Ohnishi, T.; Bandow, K.; Kakimoto, K.; Kusuyama, J.; Matsuguchi, T. Long-Time Treatment by Low-Dose N-Acetyl-L-Cysteine Enhances Proinflammatory Cytokine Expressions in LPS-Stimulated Macrophages. PLoS ONE 2014, 9, e87229. [Google Scholar] [CrossRef]
- Dobashi, K.; Aihara, M.; Araki, T.; Shimizu, Y.; Utsugi, M.; Iizuka, K.; Murata, Y.; Hamuro, J.; Nakazawa, T.; Mori, M. Regulation of LPS induced IL-12 production by IFN-γ and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin. Exp. Immunol. 2001, 124, 290–296. [Google Scholar] [CrossRef]
- Liu, M.; Pelling, J.C.; Ju, J.; Chu, E.; Brash, D.E. Antioxidant action via p53-mediated apoptosis. Cancer Res. 1998, 58, 1723–1729. [Google Scholar]
- Okamura, D.M.; Bahrami, N.M.; Ren, S.; Pasichnyk, K.; Williams, J.M.; Gangoiti, J.A.; Lopez-Guisa, J.M.; Yamaguchi, I.; Barshop, B.A.; Duffield, J.S.; et al. Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD. J. Am. Soc. Nephrol. 2014, 25, 43–54. [Google Scholar] [CrossRef]
| Time from Injection (min) | 30 | 240 | ||||||
|---|---|---|---|---|---|---|---|---|
| Thiol Species | NAC | MEA | GSH | Cysteine | NAC | MEA | GSH | Cysteine |
| BRAIN | + | + | = | = | / | / | ↑ | ↑ |
| LYMPH NODES | + | + | ↓ | ↑ | + | / | = | = |
| PANCREAS | + | + | = | = | / | / | = | = |
| DISEASE PARAMETERS | INFECTED | INFECTED+I-152 | |
|---|---|---|---|
| Splenomegaly | +++ | + | [42] |
| Lymphadenopathy | +++ | + | [42] |
| Hypergammaglobulinemia | +++ | ++ | [42] |
| Proviral DNA in lymphoid organs | +++ | + | [42] |
| B and T cell proliferative index decrease | +++ | ++ | [42] |
| Th1/Th2 unbalance in favour of Th2 | +++ | + | [46] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crinelli, R.; Zara, C.; Smietana, M.; Retini, M.; Magnani, M.; Fraternale, A. Boosting GSH Using the Co-Drug Approach: I-152, a Conjugate of N-acetyl-cysteine and β-mercaptoethylamine. Nutrients 2019, 11, 1291. https://doi.org/10.3390/nu11061291
Crinelli R, Zara C, Smietana M, Retini M, Magnani M, Fraternale A. Boosting GSH Using the Co-Drug Approach: I-152, a Conjugate of N-acetyl-cysteine and β-mercaptoethylamine. Nutrients. 2019; 11(6):1291. https://doi.org/10.3390/nu11061291
Chicago/Turabian StyleCrinelli, Rita, Carolina Zara, Michaël Smietana, Michele Retini, Mauro Magnani, and Alessandra Fraternale. 2019. "Boosting GSH Using the Co-Drug Approach: I-152, a Conjugate of N-acetyl-cysteine and β-mercaptoethylamine" Nutrients 11, no. 6: 1291. https://doi.org/10.3390/nu11061291





