Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Design
2.3. Induction of Colitis
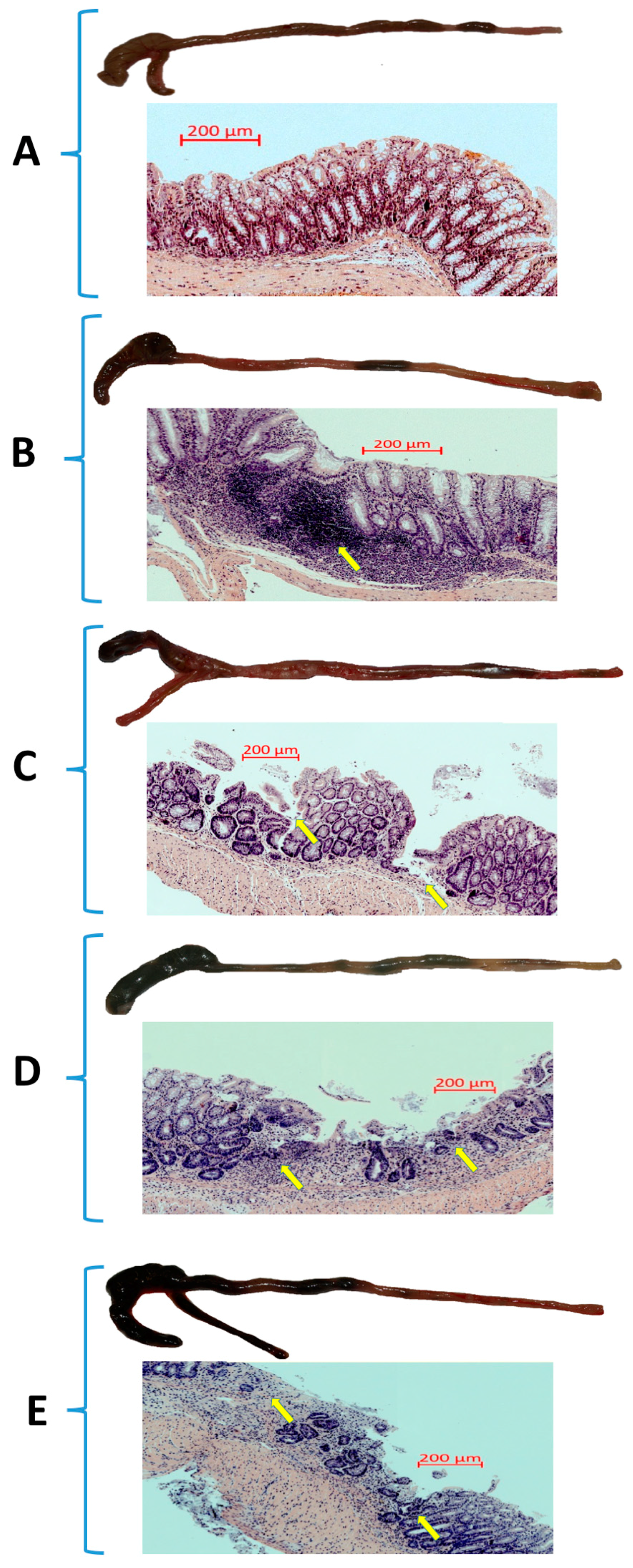
2.4. Assessment of Microscopic Changes in the Colonic Mucosa
2.5. Determination of Lipid Peroxidation
2.6. Measurement of Reduced Glutathione (GSH) Content
2.7. Determination of Superoxide Dismutase (SOD) Activity
2.8. Luminex Microbeads Fluorescent Assays
2.9. Gene Expression in the Mouse Colonic Mucosa Determined by the Real Time Polymerase Chain Reaction (Real-Time PCR)
2.10. Colonic Expression of HO-1 and HIF-1α Proteins Assessed by Western Blot
2.11. Statistical Analysis
3. Results
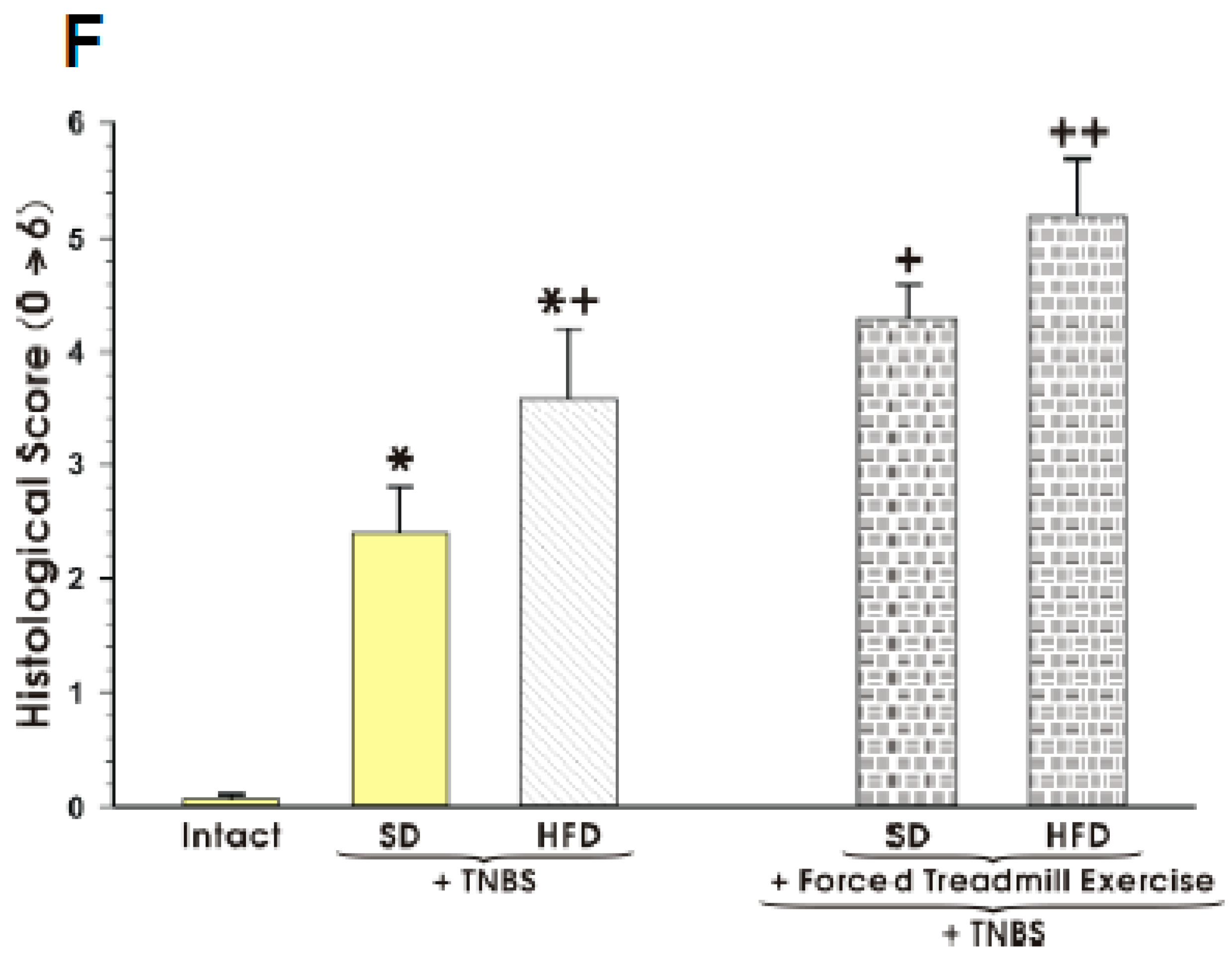
3.1. The Effect of Forced Treadmill Exercise on DAI Activity, CBF, and Macroscopic and Microscopic Appearance of Colonic Mucosa in Mice with Colitis
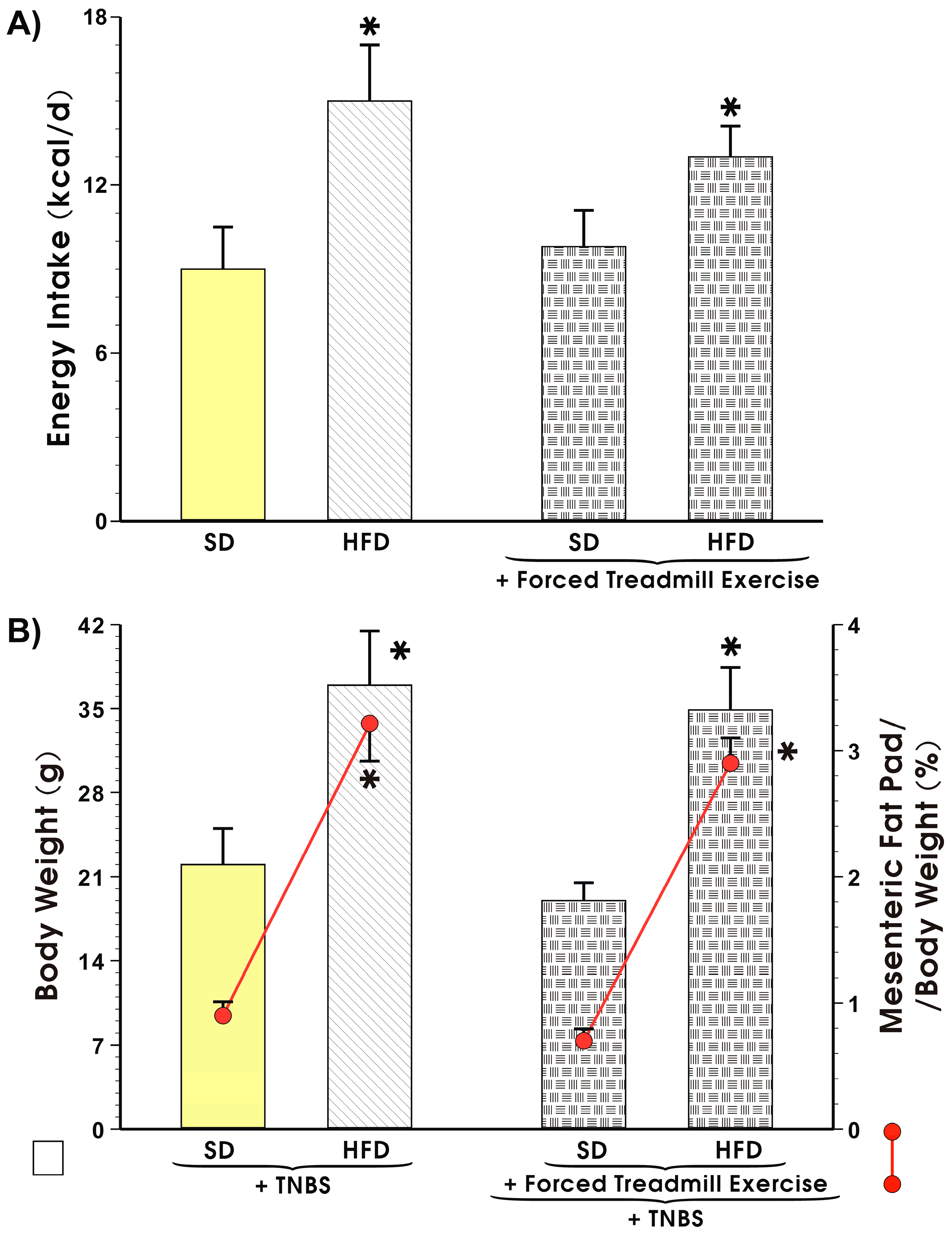
3.2. Energy Intake, Body Weight, and Visceral Adiposity in Mice Fed a SD or HFD with or without Forced Treadmill Exercise.
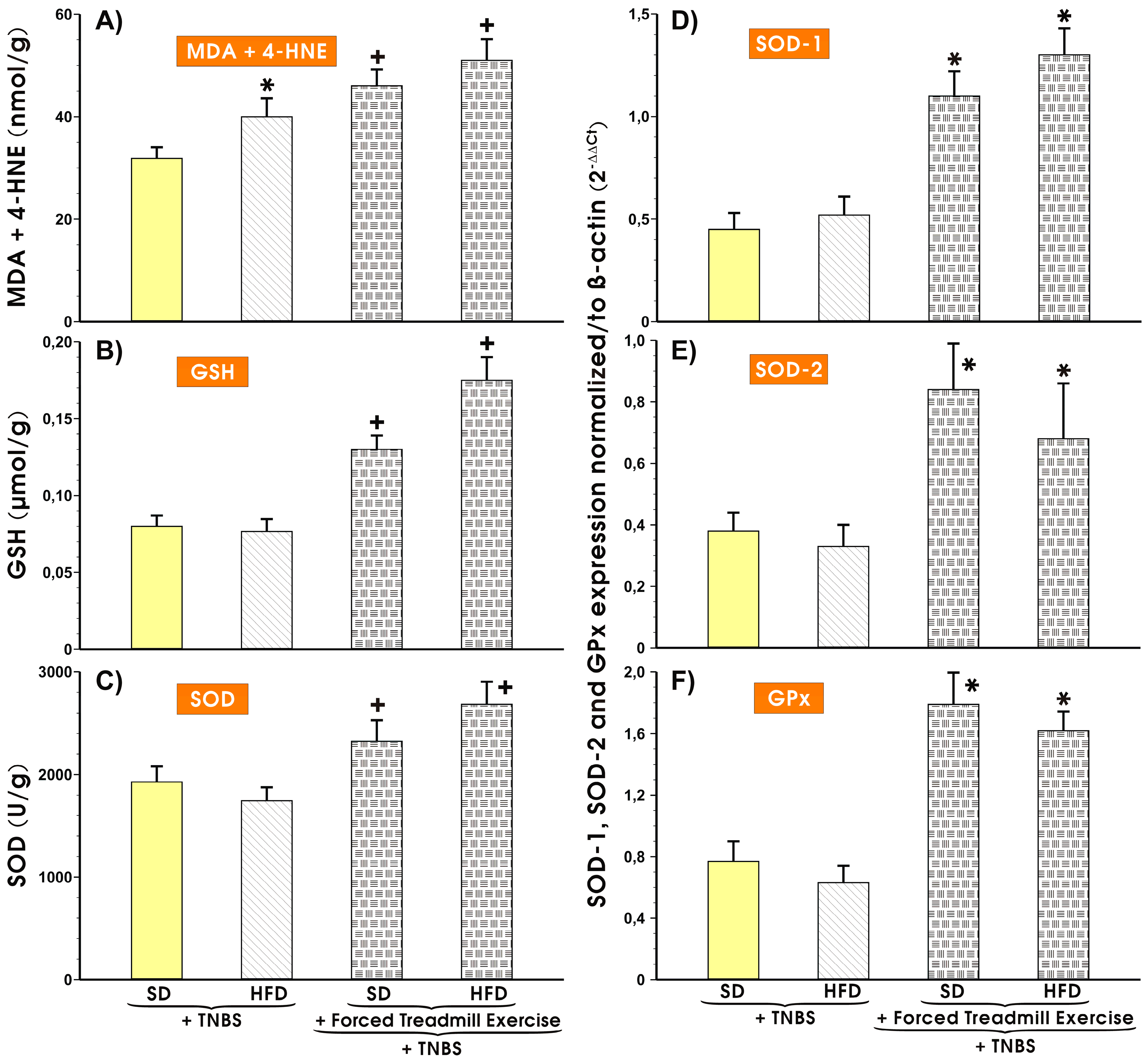
3.3. Effects of Forced Treadmill Exercise on the Mucosal Colonic Content of MDA plus 4-HNE, GSH, and SOD Activity and the mRNA Expression of SOD-1, SOD-2, and GPx-1 in Mice with Experimental Colitis
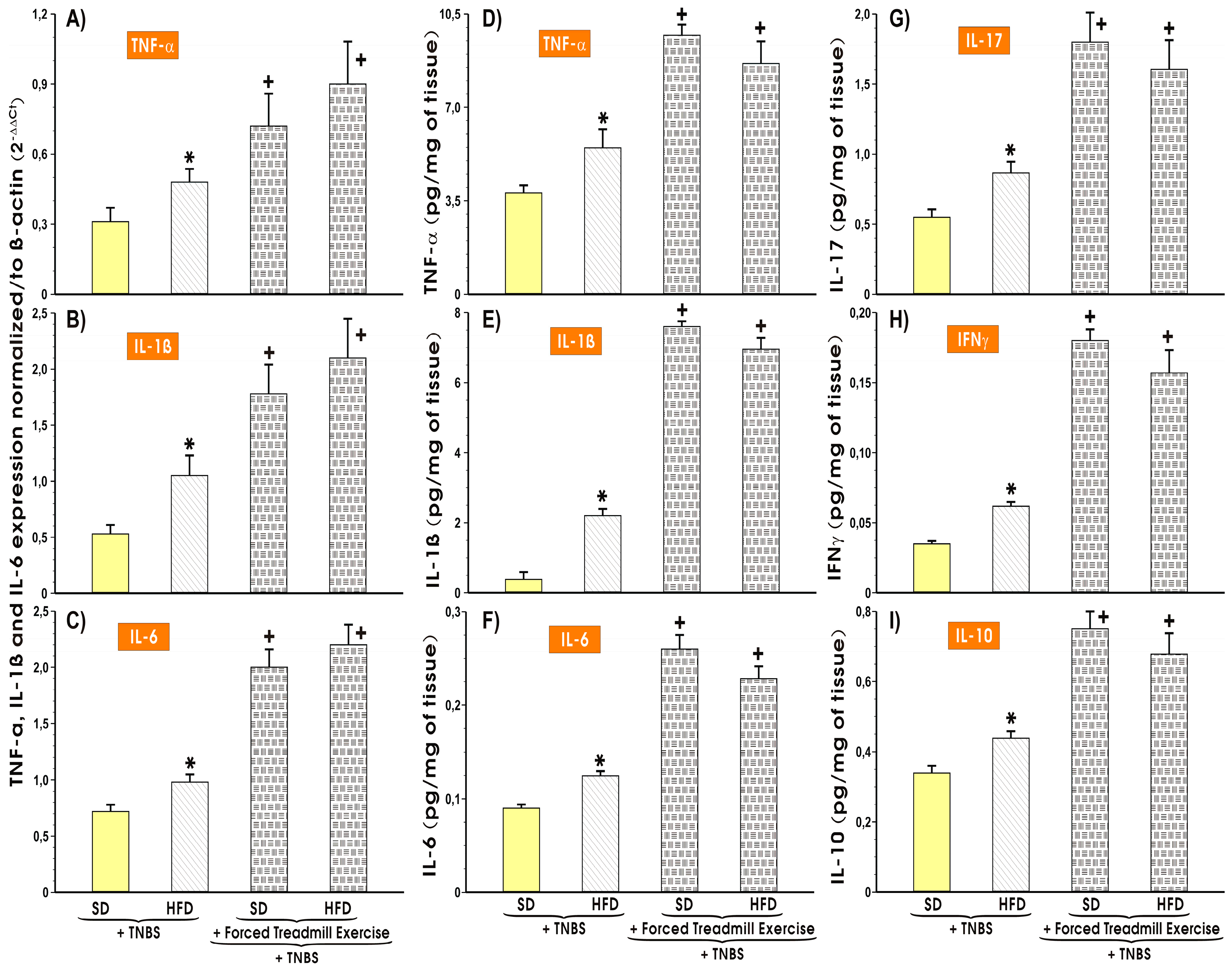
3.4. The Alterations in the Colonic Expression and Changes in Colonic Mucosal Content of Proinflammatory and Oxidative Biomarkers in Mice with TNBS-Induced Colitis fed SD or HFD with or without Forced Treadmill Exercise
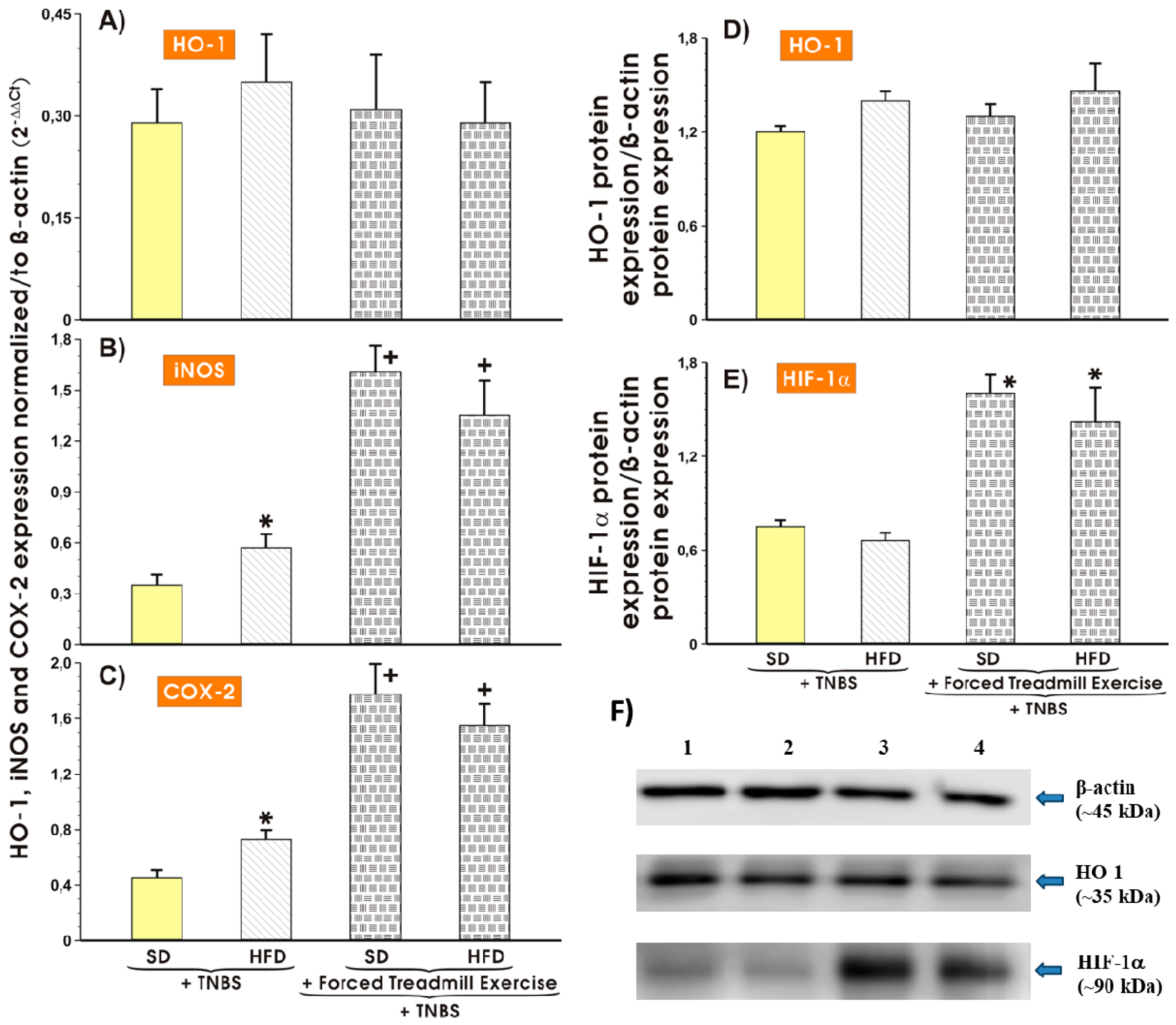
3.5. Effect of Forced Treadmill Exercise on mRNA Expression of COX-2, iNOS and Protein Expression of HO-1 and HIF-1α in Mice with Experimental Colitis Fed SD or HFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Mayberry, J.F.; Lobo, A.; Ford, A.C.; Thomas, A. NICE clinical guideline (CG152): The management of Crohn’s disease in adults, children and young people. Aliment. Pharmacol. Ther. 2013, 37, 195–203. [Google Scholar] [CrossRef]
- Bilski, J.; Mazur-Bialy, A.; Brzozowski, B.; Magierowski, M.; Zahradnik-Bilska, J.; Wojcik, D.; Magierowska, K.; Kwiecien, S.; Mach, T.; Brzozowski, T. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol. Rep. 2016, 68, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur-Bialy, A.; Bilski, J.; Pochec, E.; Brzozowski, T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J. Physiol. Pharmacol. 2017, 68, 243–251. [Google Scholar] [PubMed]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J. Physiol. Pharmacol. 2018, 69, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bosca-Watts, M.M.; Tosca, J.; Anton, R.; Mora, M.; Minguez, M.; Mora, F. Pathogenesis of Crohn’s disease: Bug or no bug. World J. Gastrointest. Pathophysiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Desreumaux, P.; Ernst, O.; Geboes, K.; Gambiez, L.; Berrebi, D.; Muller-Alouf, H.; Hafraoui, S.; Emilie, D.; Ectors, N.; Peuchmaur, M.; et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 1999, 117, 73–81. [Google Scholar] [CrossRef]
- Drouet, M.; Dubuquoy, L.; Desreumaux, P.; Bertin, B. Visceral fat and gut inflammation. Nutrition 2012, 28, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Karagiannides, I.; Bakirtzi, K.; Pothoulakis, C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2012, 18, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.I.; Wierdak, M.; Brzozowski, T. The impact of physical activity and nutrition on inflammatory bowel disease: The potential role of cross talk between adipose tissue and skeletal muscle. J. Physiol. Pharmacol. 2013, 64, 143–155. [Google Scholar] [PubMed]
- Liu, W.X.; Wang, T.; Zhou, F.; Wang, Y.; Xing, J.W.; Zhang, S.; Gu, S.Z.; Sang, L.X.; Dai, C.; Wang, H.L. Voluntary exercise prevents colonic inflammation in high-fat diet-induced obese mice by up-regulating PPAR-gamma activity. Biochem. Biophys. Res. Commun. 2015, 459, 475–480. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Bilski, J.; Wojcik, D.; Brzozowski, B.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Magierowski, M.; Magierowska, K.; Mach, T.; et al. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 2017, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Brzozowski, B.; Mazur-Bialy, A.; Sliwowski, Z.; Brzozowski, T. The role of physical exercise in inflammatory bowel disease. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Slattery, K.; Bentley, D.; Coutts, A.J. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: Implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 2015, 45, 453–471. [Google Scholar] [CrossRef]
- Vanhees, L.; Geladas, N.; Hansen, D.; Kouidi, E.; Niebauer, J.; Reiner, Z.; Cornelissen, V.; Adamopoulos, S.; Prescott, E.; Borjesson, M.; et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: Recommendations from the EACPR. Part II. Eur. J. Prev. Cardiol. 2012, 19, 1005–1033. [Google Scholar] [CrossRef]
- Hoffman-Goetz, L.; Pedersen, B.K. Exercise and the immune system: A model of the stress response? Immunol. Today 1994, 15, 382–387. [Google Scholar] [CrossRef]
- Cook, M.D.; Martin, S.A.; Williams, C.; Whitlock, K.; Wallig, M.A.; Pence, B.D.; Woods, J.A. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013, 33, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Fletcher, E.; Larsen, B.; Baliga, M.S.; Durstine, J.L.; Fayad, R. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J. Inflamm. 2012, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.I.; Brzozowski, B.; Magierowski, M.; Jasnos, K.; Krzysiek-Maczka, G.; Urbanczyk, K.; Ptak-Belowska, A.; Zwolinska-Wcislo, M.; Mach, T.; et al. Moderate exercise training attenuates the severity of experimental rodent colitis: The importance of crosstalk between adipose tissue and skeletal muscles. Mediators Inflamm. 2015. [Google Scholar] [CrossRef] [PubMed]
- Karczewska, E.; Wojtas, I.; Sito, E.; Trojanowska, D.; Budak, A.; Zwolinska-Wcislo, M.; Wilk, A. Assessment of co-existence of Helicobacter pylori and Candida fungi in diseases of the upper gastrointestinal tract. J. Physiol. Pharmacol. 2009, 60, 33–39. [Google Scholar] [PubMed]
- Cheng, L.; Jin, H.; Qiang, Y.; Wu, S.; Yan, C.; Han, M.; Xiao, T.; Yan, N.; An, H.; Zhou, X. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int. Immunopharmacol. 2016, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoydal, M.A.; Wisloff, U.; Kemi, O.J.; Ellingsen, Ø. Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. Eur. J. Cardiovascular. Prev. Rehabil. 2007, 14, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Schefer, V.; Talan, M.I. Oxygen consumption in adult and Aged C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996, 31, 387–392. [Google Scholar] [CrossRef]
- Gambero, A.; Marostica, M.; Abdalla Saaad, M.J.; Pedrazzoli, J. Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm. Bowel Dis. 2007, 13, 1357–1364. [Google Scholar] [CrossRef]
- Te Velde, A.A.; Verstege, M.I.; Hommes, D.W. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm. Bowel Dis. 2006, 12, 995–999. [Google Scholar] [CrossRef] [Green Version]
- Magierowski, M.; Magierowska, K.; Surmiak, M.; Hubalewska-Mazgaj, M.; Kwiecien, S.; Wallace, J.L.; Brzozowski, T. The effect of hydrogen sulfide-releasing naproxen (ATB-346) versus naproxen on formation of stress-induced gastric lesions, the regulation of systemic inflammation, hypoxia and alterations in gastric microcirculation. J. Physiol. Pharmacol. 2017, 68, 749–756. [Google Scholar] [PubMed]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, K.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Adamski, J.; Bakalarz, D.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Brzozowski, T. Interaction between endogenous carbon monoxide and hydrogen sulfide in the mechanism of gastroprotection against acute aspirin-induced gastric damage. Pharmacol. Res. 2016, 114, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Reber, S.O.; Obermeier, F.; Straub, R.H.; Falk, W.; Neumann, I.D. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology 2006, 147, 4968–4976. [Google Scholar] [CrossRef] [PubMed]
- Gue, M.; Bonbonne, C.; Fioramonti, J.; More, J.; Del Rio-Lacheze, C.; Comera, C.; Bueno, L. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 272, G84–G91. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, B.; Mazur-Bialy, A.; Pajdo, R.; Kwiecien, S.; Bilski, J.; Zwolinska-Wcislo, M.; Mach, T.; Brzozowski, T. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD). Role of brain-gut axis. Curr. Neuropharmacol. 2016, 14, 892–900. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhou, F.; Wang, Y.; Wang, T.; Xing, J.W.; Zhang, S.; Sang, L.X.; Gu, S.Z.; Wang, H.L. Voluntary exercise protects against ulcerative colitis by up-regulating glucocorticoid-mediated PPAR-gamma activity in the colon in mice. Acta Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.J.; Valentine, R.J.; Wilund, K.R.; Antao, N.; Baynard, T.; Woods, J.A. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1164–E1171. [Google Scholar] [CrossRef] [Green Version]
- Pincu, Y.; Linden, M.A.; Zou, K.; Baynard, T.; Boppart, M.D. The effects of high fat diet and moderate exercise on TGFβ1 and collagen deposition in mouse skeletal muscle. Cytokine 2015, 73, 23–29. [Google Scholar] [CrossRef]
- Konstandi, M.; Johnson, E.; Lang, M.A.; Malamas, M.; Marselos, M. Noradrenaline, dopamine, serotonin: Different effects of psychological stress on brain biogenic amines in mice and rats. Pharmacol. Res. 2000, 41, 341–346. [Google Scholar] [CrossRef]
- Archer, J. Tests for emotionality in rats and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Magierowski, M.; Kwiecien, S.; Wojcik, D.; Ptak-Belowska, A.; Surmiak, M.; Targosz, A.; Magierowska, K.; Brzozowski, T. Exploiting significance of physical exercise in prevention of gastrointestinal disorders. Curr. Pharm. Des. 2018. [Google Scholar] [CrossRef] [PubMed]
- ter Steege, R.W.; Van der Palen, J.; Kolkman, J.J. Prevalence of gastrointestinal complaints in runners competing in a long-distance run: An internet-based observational study in 1281 subjects. Scand. J. Gastroenterol. 2008, 43, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, E.B.; Lowe, D.K.; Goss, J.R.; Wayne, R. Gastrointestinal symptoms of marathon runners. West. J. Med. 1984, 141, 481–484. [Google Scholar] [PubMed]
- Riddoch, C.; Trinick, T. Gastrointestinal disturbances in marathon runners. Br. J. Sports Med. 1988, 22, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Øktedalen, O.; Lunde, O.; Opstad, P.; Aabakken, L.; Kvernebo, K. Changes in the gastrointestinal mucosa after long-distance running. Scand. J. Gastroenterol. 1992, 27, 270–274. [Google Scholar] [CrossRef]
- Clausen, J.P. Effect of physical training on cardiovascular adjustments to exercise in man. Physiol. Rev. 1977, 57, 779–815. [Google Scholar] [CrossRef] [PubMed]
- van Deventer, S.J.; Gouma, D. Bacterial translocation and endotoxin transmigration in intestinal ischaemia and reperfusion. Curr. Opin. Anesthesiol. 1994, 7, 126–130. [Google Scholar] [CrossRef]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.; Vet-Joop, K.; Sturk, A.; Stegen, J.; Senden, J.; Saris, W.; Wagenmakers, A. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.K.; Hankey, J.; Wright, A.; Marczak, S.; Hemming, K.; Allerton, D.M.; Ansley-Robson, P.; Costa, R.J. The impact of a 24-h ultra-marathon on circulatory endotoxin and cytokine profile. Int. J. Sports Med. 2015, 36, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Teixeira, A.; Rama, L.; Prestes, J.; Rosado, F.; Hankey, J.; Scheer, V.; Hemmings, K.; Ansley-Robson, P.; Costa, R.J. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc. Immunol. Rev. 2015, 21, 114–128. [Google Scholar] [PubMed]
- Grootjans, J.; Lenaerts, K.; Buurman, W.A.; Dejong, C.H.; Derikx, J.P. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J. Gastroenterol. 2016, 22, 2760. [Google Scholar] [CrossRef] [PubMed]
- van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.; Poeze, M.; van Loon, L.J.; Dejong, C.H.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef]
- Sasaki, H.; Hattori, Y.; Ikeda, Y.; Kamagata, M.; Iwami, S.; Yasuda, S.; Tahara, Y.; Shibata, S. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2: LUC mice. Sci. Rep. 2016, 6, 27607. [Google Scholar] [CrossRef] [PubMed]
- Borges Lda, S.; Dermargos, A.; da Silva Junior, E.P.; Weimann, E.; Lambertucci, R.H.; Hatanaka, E. Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J. Pineal. Res. 2015, 58, 166–172. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Vergauwen, H.; Tambuyzer, B.; Jennes, K.; Degroote, J.; Wang, W.; De Smet, S.; Michiels, J.; Van Ginneken, C. Trolox and ascorbic acid reduce direct and indirect oxidative stress in the IPEC-J2 cells, an in vitro model for the porcine gastrointestinal tract. PLoS ONE 2015, 10, e0120485. [Google Scholar] [CrossRef]
- Puelo, F.; Meirelles, K.; Navaratnarajah, M.; Fitzpatrick, L.; Shumate, M.L.; Cooney, R.N.; Lang, C.H. Skeletal muscle catabolism in trinitrobenzene sulfonic acid-induced murine colitis. Metabolism 2010, 59, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.M.; Milbury, P.E.; Cannon, J.G.; Roubenoff, R.; Blumberg, J.B. Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic. Biol. Med 2003, 34, 1575–1588. [Google Scholar] [CrossRef]
- Hollander, J.; Fiebig, R.; Gore, M.; Ookawara, T.; Ohno, H.; Ji, L. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflügers Arch. 2001, 442, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.D.; Kappelman, M.D.; Martin, C.F.; Chen, W.; Sandler, R.S.; Long, M.D. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflamm. Bowel Dis. 2015, 21, 1063–1071. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| COX-2 | GCCAGCAAAGCCTAGAGCAACAAA | TACTGAGTACCAGGCCAGCACAAA |
| GPx-1 | ACAGTCCACCGTGTATGCCTTC | CTCTTCATTCTTGCCATTCTCCTG |
| HO-1 | CACGCATATACCCGCTACCT | CCAGAGTGTTCATTCGAGCA |
| IL-1β | TGGAGAGTGTGGATCCCAAGCAAT | TGCTTGTGAGGTGCTGATGTACCA |
| IL-6 | TTGTACAGTCCCAGTCAGGCAACA | TCAAGCTACTGCAGGCCAGTTACA |
| iNOS | CAAACACGAGTGCAGCTGGTTGAA | AGGCAGGACTGAGTTCAGTGTGTT |
| SOD-1 | CCACGTCCATCAGTATGGGG | CGTCCTTTCCAGCAGTCACA |
| SOD-2 | GTGTCTGTGGGAGTCCAAGG | CCCCAGTCATAGTGCTGCAA |
| TNF-α | TGAGTTCTGCAAAGGGAGAGTGGT | TGCACCTCAGGGAAGAATCTGGAA |
| β-actin | CCCATCTATGAGGGTTACGC | TTTAATGTCACGCACGATTTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Magierowski, M.; Surmiak, M.; Kwiecien, S.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Brzozowski, T. Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers. Nutrients 2019, 11, 1127. https://doi.org/10.3390/nu11051127
Bilski J, Mazur-Bialy A, Wojcik D, Magierowski M, Surmiak M, Kwiecien S, Magierowska K, Hubalewska-Mazgaj M, Sliwowski Z, Brzozowski T. Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers. Nutrients. 2019; 11(5):1127. https://doi.org/10.3390/nu11051127
Chicago/Turabian StyleBilski, Jan, Agnieszka Mazur-Bialy, Dagmara Wojcik, Marcin Magierowski, Marcin Surmiak, Slawomir Kwiecien, Katarzyna Magierowska, Magdalena Hubalewska-Mazgaj, Zbigniew Sliwowski, and Tomasz Brzozowski. 2019. "Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers" Nutrients 11, no. 5: 1127. https://doi.org/10.3390/nu11051127






