Maternal Experience of Domestic Violence, Associations with Children’s Lipid Biomarkers at 10 Years: Findings from MINIMat Study in Rural Bangladesh
Abstract
1. Introduction
2. Materials and Methods
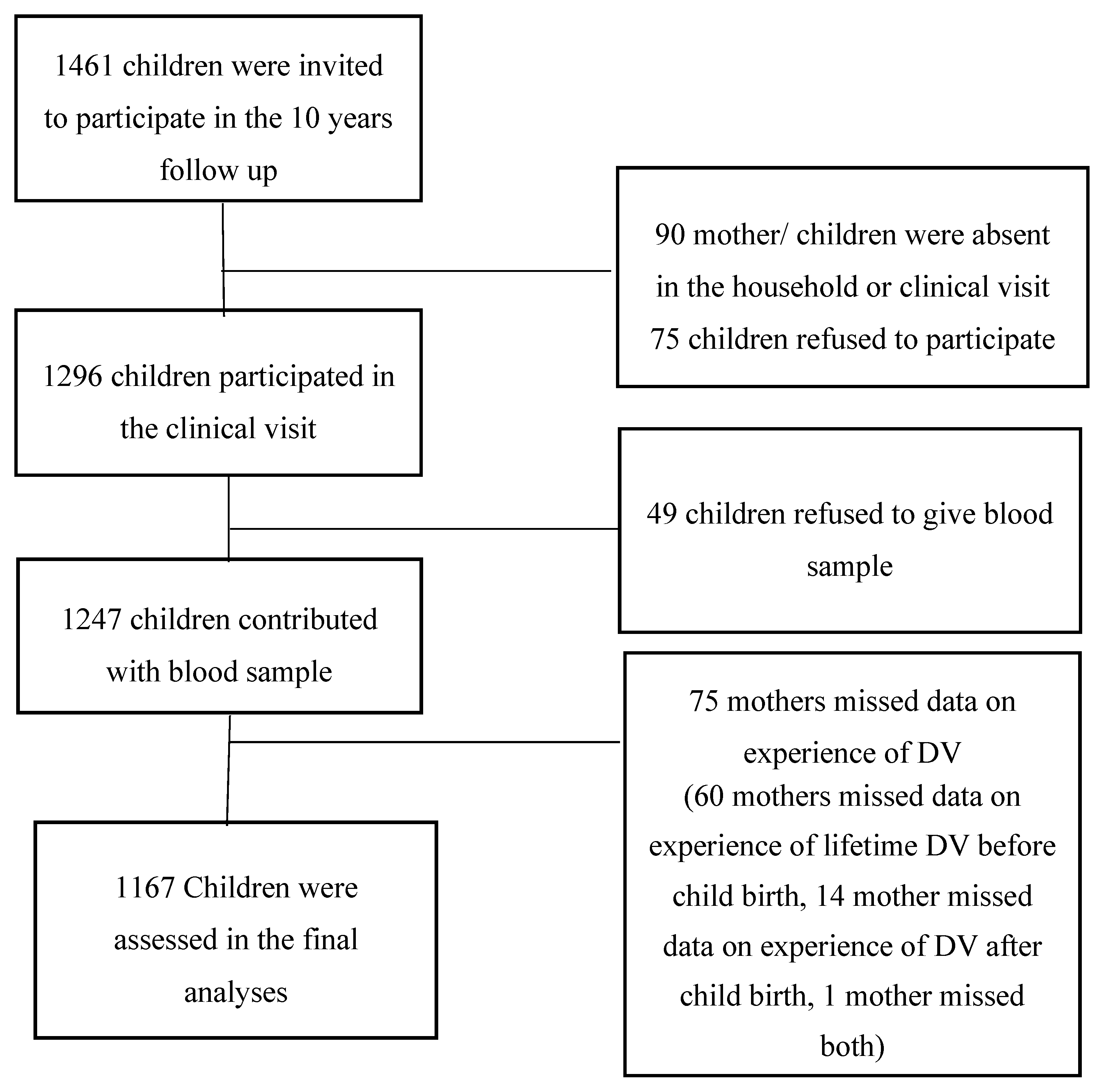
2.1. Study Design and Pupulation
2.2. Data Collection and Measurements
2.2.1. Maternal and Child Characteristics
2.2.2. Explanatory Variables
Women’s Lifetime Experience of DV before Child’s Birth
Women’s Experience of DV after Child’s Birth
2.2.3. Outcome Variables
Children’s Lipid Biomarkers at 10 Years
2.3. Ethical Considerations
2.4. Statistical Analyses
3. Results
Effects of Maternal Experience of DV before and after Childbirth on Their Children’s Lipid Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alwan, A.; MacLean, D.R.; Riley, L.M. Monitoring and surveillance of chronic non-communicable diseases: Progress and capacity in high-burden countries. Lancet 2010, 376, 1861–1868. [Google Scholar] [CrossRef]
- Wagner, K.H.; Brath, H. A global view on the development of non communicable diseases. Prev. Med. 2012, 54, S38–S41. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Noncommunicable Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs355/en/ (accessed on 17 November 2018).
- Berenson, G.S. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease: The Bogalusa Heart Study. Am. J. Cardiol. 2002, 90, L3–L7. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Gluckman, P.D.; Hanson, M.A. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol. Met. 2010, 21, 199–205. [Google Scholar] [CrossRef]
- Charmandari, E.; Kino, T.; Souvatzoglou, E.; Chrousos, G.P. Pediatric stress: Hormonal mediators and human development. Horm. Res. 2003, 59, 161–179. [Google Scholar] [CrossRef]
- Entringer, S.; Buss, C.; Wadhwa, P.D. Prenatal stress and developmental programming of human health and disease risk: Concepts and integration of empirical findings. Curr. Opin. Endocrinol. 2010, 17, 507–516. [Google Scholar] [CrossRef]
- Pervanidou, P.; Chrousos, G.P. Metabolic consequences of stress during childhood and adolescence. Metabolism 2012, 61, 611–619. [Google Scholar] [CrossRef]
- Pervanidou, P.; Chrousos, G.P. Stress and obesity/metabolic syndrome in childhood and adolescence. Int. J. Pediatr. Obes. 2011, 6 (Suppl. 1), 21–28. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Wust, S.; Kumsta, R.; Layes, I.M.; Nelson, E.L.; Hellhammer, D.H.; Wadhwa, P.D. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am. J. Obstet. Gynecol. 2008, 199, 498.e1–498.e7. [Google Scholar] [CrossRef]
- Flory, J.D.; Bierer, L.M.; Yehuda, R. Maternal exposure to the holocaust and health complaints in offspring. Dis. Markers 2011, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.O.; Olsen, J.; Vestergaard, M.; Obel, C.; Baker, J.L.; Sorensen, T.I.A. Prenatal Stress Exposure Related to Maternal Bereavement and Risk of Childhood Overweight. PLoS ONE 2010, 5, e11896. [Google Scholar] [CrossRef]
- Hochberg, Z.; Feil, R.; Constancia, M.; Fraga, M.; Junien, C.; Carel, J.C.; Boileau, P.; Le Bouc, Y.; Deal, C.L.; Lillycrop, K.; et al. Child Health, Developmental Plasticity, and Epigenetic Programming. Endocr. Rev. 2011, 32, 159–224. [Google Scholar] [CrossRef]
- Vaiserman, A.M. Epigenetic Programming by Early-Life Stress: Evidence from Human Populations. Dev. Dynam. 2015, 244, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, A.F.; Kopf, D.; Westphal, S.; Lederbogen, F.; Banaschewski, T.; Esser, G.; Schmidt, M.H.; Zimmermann, U.S.; Laucht, M.; Deuschle, M. Impact of Early Parental Child-Rearing Behavior on Young Adults’ Cardiometabolic Risk Profile: A Prospective Study. Psychosom. Med. 2010, 72, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ravaja, N.; Katainen, S.; Keltikangas-Jarvinen, L. Perceived difficult temperament, hostile maternal child-rearing attitudes and insulin resistance syndrome precursors among children: A 3-year follow-up study. Psychother. Psychosom. 2001, 70, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Mason, S.; Rexrode, K.; Spiegelman, D.; Hibert, E.; Kawachi, I.; Jun, H.J.; Wright, R.J. Physical and Sexual Abuse in Childhood as Predictors of Early-Onset Cardiovascular Events in Women. Circulation 2012, 126, 920–927. [Google Scholar] [CrossRef]
- Slopen, N.; Goodman, E.; Koenen, K.C.; Kubzansky, L.D. Socioeconomic and Other Social Stressors and Biomarkers of Cardiometabolic Risk in Youth: A Systematic Review of Less Studied Risk Factors. PLoS ONE 2013, 8, e64418. [Google Scholar] [CrossRef]
- Devries, K.M.; Mak, J.Y.; Bacchus, L.J.; Child, J.C.; Falder, G.; Petzold, M.; Astbury, J.; Watts, C.H. Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies. PLoS Med. 2013, 10, e1001439. [Google Scholar] [CrossRef] [PubMed]
- Devries, K.M.; Seguin, M. Violence against women and suicidality: Does violence cause suicidal behaviour? In Violence against Women and Mental Health; García-Moreno, C., Riecher-Rössler, A., Eds.; Karger: Basel, Switzerland, 2013. [Google Scholar]
- Ellsberg, M.; Jansen, H.A.; Heise, L.; Watts, C.H.; Garcia-Moreno, C. Intimate partner violence and women’s physical and mental health in the WHO multi-country study on women’s health and domestic violence: An observational study. Lancet 2008, 371, 1165–1172. [Google Scholar] [CrossRef]
- Holt, S.; Buckley, H.; Whelan, S. The impact of exposure to domestic violence on children and young people: A review of the literature. Child Abuse Negl. 2008, 32, 797–810. [Google Scholar] [CrossRef]
- Shay-Zapien, G.; Bullock, L. Impact of Intimate Partner Violence on Maternal Child Health. MCN Am. J. Matern./Child Nurs. 2010, 35, 206–212. [Google Scholar] [CrossRef]
- Bangladesh Bureau of Statistics (BBS). Report on National Violence against Women Survey 2015; Bangladesh Bureau of Statistics: Dhaka, Bangladesh, 2016. [Google Scholar]
- Eapen, D.; Kalra, G.L.; Merchant, N.; Arora, A.; Khan, B.V. Metabolic syndrome and cardiovascular disease in South Asians. Vasc. Health Risk Manag. 2009, 5, 731–743. [Google Scholar]
- Bangladesh Health Watch Report 2016. Non-Communicable Diseasesin BangladeshCurrent Scenario and Future Directions; School of Public Health, BRAC University: Dhaka, Bangladesh, 2017. [Google Scholar]
- Arifeen, S.E.; Ekstrom, E.C.; Frongillo, E.A.; Hamadani, J.; Khan, A.I.; Naved, R.T.; Rahman, A.; Raqib, R.; Rasmussen, K.M.; Selling, K.E.; et al. Cohort Profile: The Maternal and Infant Nutrition Interventions in the Matlab (MINIMat) Cohort in Bangladesh. Int. J. Epidemiol. 2018, 47, 1737e–1738e. [Google Scholar] [CrossRef]
- Persson, L.A.; Arifeen, S.; Ekstrom, E.C.; Rasmussen, K.M.; Frongillo, E.A.; Yunus, M.; MINIMat Study Team. Effects of Prenatal Micronutrient and Early Food Supplementation on Maternal Hemoglobin, Birth Weight, and Infant Mortality Among Children in Bangladesh The MINIMat Randomized Trial. JAMA J. Am. Med. Assoc. 2012, 307, 2050–2059. [Google Scholar] [CrossRef]
- Gwatkin, D.R.; Rutstein, S.; Johnson, K.; Pande, R.; Wagstaff, A. Socio-Economic Differences in Health, Nutrition, and Population; HNP/Poverty Thematic Group, World Bank: Washington, DC, USA, 2000. [Google Scholar]
- Straus, M.A.; Douglas, E.M. A short form of the Revised Conflict Tactics Scales, and typologies for severity and mutuality. Violence Vict. 2004, 19, 507–520. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Putting Women First: Ethical and Safety Recommendations for Research on Domestic Violence Against Women; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Naved, R.T.; Rimi, N.A.; Jahan, S.; Lindmark, G. Paramedic-conducted mental health counselling for abused women in rural Bangladesh: An evaluation from the perspective of participants. J. Health Popul. Nutr. 2009, 27, 477–491. [Google Scholar] [CrossRef]
- Weidner, G.; Hutt, J.; Connor, S.L.; Mendell, N.R. Family Stress and Coronary Risk in Children. Psychosom. Med. 1992, 54, 471–479. [Google Scholar] [CrossRef]
- Harris, A.; Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef]
- Sturge-Apple, M.L.; Davies, P.T.; Cicchetti, D.; Manning, L.G. Mother’s parenting practices as explanatory mechanisms in associations between interparental violence and child adjustment. Partner Abuse 2010, 1, 45–60. [Google Scholar] [CrossRef]
- Øverlien, C. Children exposed to domestic violence: Conclusions from the literature and challenges ahead. J. Soc. Work. 2010, 10, 80–97. [Google Scholar] [CrossRef]
- Herrenkohl, T.I.; Sousa, C.; Tajima, E.A.; Herrenkohl, R.C.; Moylan, C.A. Intersection of child abuse and children’s exposure to domestic violence. Trauma Violence Abuse 2008, 9, 84–99. [Google Scholar] [CrossRef]
- Lee, C.; Tsenkova, V.; Carr, D. Childhood trauma and metabolic syndrome in men and women. Soc. Sci. Med. 2014, 105, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Naved, R.T. Sexual violence towards married women in Bangladesh. Arch. Sex. Behav. 2013, 42, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Cavalin, C. WHO Multi-country Study on Women’s Health and Domestic Violence Against Women. Initial Results on Prevalence, Health Outtcomes and Women’s Responses. Population 2010, 65, 837–839. [Google Scholar]
- Jina, R.; Thomas, L.S. Health consequences of sexual violence against women. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Alim, A.; Noguchi, T. Spousal abuse against women and its consequences on reproductive health: A study in the urban slums in Bangladesh. Matern. Child Health J. 2006, 10, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Hamby, S.L.; Finkelhor, D.; Turner, H.; Ormrod, R. Children’s Exposure to Intimate Partner Violence and Other Forms of Family Violence: Nationally Representative Rates among US Youth; Department of Justice Juvenile Justice Clearinghouse: Washington, DC, USA, 2011.
- Grebstein, L.C.; Van Wyk, J.A. Turning the Tide of Male Juvenile Delinquency: The Ocean Tides Approach; Springer: New York, NY, USA, 2016. [Google Scholar]
- Adams, C.M. The consequences of witnessing family violence on children and implications for family counselors. Fam. J. 2006, 14, 334–341. [Google Scholar] [CrossRef]
- Stark, E. Coercive Control; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Ziaei, S.; Frith, A.L.; Ekstrom, E.C.; Naved, R.T. Experiencing Lifetime Domestic Violence: Associations with Mental Health and Stress among Pregnant Women in Rural Bangladesh: The MINIMat Randomized Trial. PLoS ONE 2016, 11, e0168103. [Google Scholar] [CrossRef]
- Hurley, K.M.; Black, M.M.; Papas, M.A.; Caulfield, L.E. Maternal symptoms of stress, depression, and anxiety are related to nonresponsive feeding styles in a statewide sample of WIC participants. J. Nutr. 2008, 138, 799–805. [Google Scholar] [CrossRef]
- Park, H.; Walton-Moss, B. Parenting style, parenting stress, and children’s health-related behaviors. J. Dev. Behav. Pediatr. 2012, 33, 495–503. [Google Scholar] [CrossRef]
- Michels, N.; Sioen, I.; Braet, C.; Eiben, G.; Hebestreit, A.; Huybrechts, I.; Vanaelst, B.; Vyncke, K.; De Henauw, S. Stress, emotional eating behaviour and dietary patterns in children. Appetite 2012, 59, 762–769. [Google Scholar] [CrossRef]
- Ellsberg, M.; Heise, L.; Pena, R.; Agurto, S.; Winkvist, A. Researching domestic violence against women: Methodological and ethical considerations. Stud. Fam. Plan. 2001, 32, 1–16. [Google Scholar] [CrossRef]
- Flury, M.; Nyberg, E.; Riecher-Rossler, A. Domestic violence against women: Definitions, epidemiology, risk factors and consequences. Swiss. Med. Wkly. 2010, 140, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.M.; Katre, P.A.; Kumaran, K.; Joglekar, C.; Osmond, C.; Bhat, D.S.; Lubree, H.; Pandit, A.; Yajnik, C.S.; Fall, C.H. Tracking of cardiovascular risk factors from childhood to young adulthood—The Pune Children’s Study. Int. J. Cardiol. 2014, 175, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Perusse, L.; Malina, R.M.; Bergeron, J.; Despres, J.P.; Bouchard, C. Stability of indicators of the metabolic syndrome from childhood and adolescence to young adulthood: The Quebec Family Study. J. Clin. Epidemiol. 2001, 54, 190–195. [Google Scholar] [CrossRef]
- Sleight, P. Cardiovascular Risk-Factors and the Effects of Intervention. Am. Heart J. 1991, 121, 990–995. [Google Scholar] [CrossRef]
- Gotto, A.M., Jr. Jeremiah Metzger Lecture: Cholesterol, inflammation and atherosclerotic cardiovascular disease: Is it all LDL? Trans. Am. Clin. Climatol. Assoc. 2011, 122, 256–289. [Google Scholar] [PubMed]
| Variable | n (%) or Mean ± SD (N = 1167) | Variable | n (%) or Mean ± SD (N = 1167) |
|---|---|---|---|
| Maternal characteristics at pregnancy week 8 | Child characteristics | ||
| Age in years | 26.50 ± 5.9 | Sex (female) | 567 (48.8) |
| BMI | 20.04 ± 2.6 | Age in years | 10.08 ± 0.1 |
| Height | 149.84 ± 5.2 | Height (cm) | 129.53 ± 6.3 |
| Education | Weight (Kg) | 23.97 ± 4.2 | |
| None | 430 (36.8) | BMI (Kg/m2) | 14.20 ± 1.6 |
| 1–5 years | 264 (22.6) | Stunted | 306 (28.2) |
| ≥6 years | 473 (40.5) | Underweight | 414 (48.5) |
| Lifetime experience of DV before child birth | |||
| Any DV | 661 (56.6) | Level of lipid biomarkers | |
| Any physical DV | 246 (21.1) | Apo A (g/l) | 1.25 ± 0.18 |
| Any sexual DV | 312 (26.7) | Apo B (g/l) | 0.74 ± 0.16 |
| Any emotional DV | 276 (23.7) | Apo B/Apo A | 0.61 ± 0.16 |
| Any controlling behavior | 419 (35.9) | HDL (mmol/l) | 1.11 ± 0.23 |
| LDL (mmol/l) | 1.90 ± 0.67 | ||
| Experience of DV after child birth | LDL/HDL | 1.79 ± 0.73 | |
| Any DV | 770 (66.0) | Cholesterol (mmol/l) | 3.77 ± 0.70 |
| Any physical DV | 454 (38.9) | Triglycerides 1 (mmol/l) | 0.95 (0.32–4.10) |
| Any sexual DV | 439 (37.6) | ||
| Any emotional DV | 458 (39.2) | ||
| Any controlling behavior | 388 (33.2) |
| Maternal Experience of Any Lifetime DV | |||||
|---|---|---|---|---|---|
| Biomarkers | Model | No Experience | Before Childbirth | After Childbirth | Both before and after Child Birth |
| ß (95% CI) | ß (95% CI) | ß (95% CI) | |||
| Apo A (g/l) | Unadjusted | Ref | −0.04 (−0.08, −0.01) * | −0.02 (−0.06, 0.01) | −0.03 (−0.06, −0.00) * |
| Adjusted 1 | Ref | −0.04 (−0.08, −0.01) * | −0.02 (−0.05, 0.01) | −0.03 (−0.06, 0.00) | |
| Apo B (g/l) | Unadjusted | Ref | 0.00 (−0.03, 0.03) | −0.02 (−0.05, 0.01) | −0.00 (−0.03, 0.02) |
| Adjusted 1 | Ref | 0.00 (−0.03, 0.03) | −0.02 (−0.05, 0.01) | −0.00 (−0.03, 0.02) | |
| Apo B/Apo A | Unadjusted | Ref | 0.02 (−0.02, 0.05) | −0.01 (−0.04, 0.02) | 0.01 (−0.01, 0.04) |
| Adjusted 1 | Ref | 0.02 (−0.02, 0.05) | −0.01 (−0.04, 0.02) | 0.01 (−0.02, 0.03) | |
| HDL (mmol/l) | Unadjusted | Ref | −0.04 (−0.09, 0.00) | −0.03 (−0.07, 0.01) | −0.03 (−0.07, 0.01) |
| Adjusted 1 | Ref | −0.04 (−0.09, 0.00) | −0.03 (−0.07, 0.01) | −0.03 (−0.06, 0.01) | |
| LDL (mmol/l) | Unadjusted | Ref | −0.00 (−0.14, 0.13) | −0.00 (−0.12, 0.12) | 0.03 (−0.08, 0.13) |
| Adjusted 1 | Ref | 0.01 (−0.13, 0.14) | 0.01 (−0.11, 0.13) | 0.04 (−0.07, 0.15) | |
| LDL/HDL | Unadjusted | Ref | 0.08 (−0.07, 0.22) | 0.03 (−0.10, 0.16) | 0.09 (−0.03, 0.20) |
| Adjusted 1 | Ref | 0.09 (−0.06, 0.23) | 0.04 (−0.09, 0.17) | 0.10 (−0.02, 0.21) | |
| Cholesterol (mmol/l) | Unadjusted | Ref | −0.07 (−0.20, 0.07) | −0.10 (−0.22, 0.03) | −0.04 (−0.15, 0.07) |
| Adjusted 1 | Ref | −0.05 (−0.20, 0.09) | −0.08 (−0.20, 0.04) | −0.02 (−0.14, 0.09) | |
| Triglycerides 2 (mmol/l) | Unadjusted | Ref | 0.03 (−0.04, 0.10) | −0.02 (−0.08, 0.04) | 0.01 (−0.04, 0.07) |
| Adjusted 1 | Ref | 0.03 (−0.04, 0.10) | −0.02 (−0.08, 0.04) | 0.01 (−0.05, 0.07) | |
| Maternal Experience of Any Physical DV | |||||
|---|---|---|---|---|---|
| Biomarkers | Model | No Experience | Before Childbirth | After Childbirth | Both before and after Child Birth |
| ß (95% CI) | ß (95% CI) | ß (95% CI) | |||
| Apo A (g/l) | Unadjusted | Ref | −0.03 (−0.07, 0.02) | −0.02 (−0.05, 0.00) | −0.03 (−0.06, 0.00) |
| Adjusted 1 | Ref | −0.02 (−0.07, 0.02) | −0.02 (−0.04, 0.01) | −0.02 (−0.05, 0.01) | |
| Apo B (g/l) | Unadjusted | Ref | 0.02 (−0.02, 0.06) | −0.01 (−0.03, 0.01) | 0.01 (−0.02, 0.03) |
| Adjusted 1 | Ref | 0.01 (−0.03, 0.05) | −0.01 (−0.04, 0.01) | 0.00 (−0.03, 0.03) | |
| Apo B/Apo A | Unadjusted | Ref | 0.03 (−0.01, 0.06) | −0.00 (−0.02, 0.02) | 0.02 (−0.01, 0.04) |
| Adjusted 1 | Ref | 0.02 (−0.02, 0.06) | −0.00 (−0.03, 0.02) | 0.01 (−0.02, 0.03) | |
| HDL (mmol/l) | Unadjusted | Ref | −0.03 (−0.09, 0.02) | −0.03 (−0.06, 0.00) | −0.04 (−0.08, 0.00) |
| Adjusted 1 | Ref | −0.03 (−0.08, 0.03) | −0.02 (−0.05, 0.01) | −0.02 (−0.06, 0.02) | |
| LDL (mmol/l) | Unadjusted | Ref | 0.04 (−0.13, 0.20) | 0.04 (−0.06, 0.13) | 0.06 (−0.06, 0.17) |
| Adjusted 1 | Ref | 0.05 (−0.12, 0.21) | 0.05 (−0.05, 0.15) | 0.07 (−0.05, 0.19) | |
| LDL/HDL | Unadjusted | Ref | 0.10 (−0.08, 0.28) | 0.09 (−0.01, 0.19) | 0.13 (−0.01, 0.25) |
| Adjusted 1 | Ref | 0.10 (−0.08, 0.28) | 0.09 (−0.02, 0.19) | 0.12 (−0.01, 0.25) | |
| Cholesterol (mmol/l) | Unadjusted | Ref | −0.01(−0.18, 0.16) | −0.06 (−0.15, 0.04) | 0.01 (−0.11, 0.13) |
| Adjusted 1 | Ref | 0.00 (−0.17, 0.18) | −0.04 (−0.14, 0.06) | 0.03 (−0.10, 0.15) | |
| Triglycerides 2 (mmol/l) | Unadjusted | Ref | −0.01 (−0.10, 0.07) | 0.01 (−0.04, 0.06) | 0.08 (0.02, 0.14) ** |
| Adjusted 1 | Ref | −0.02 (−0.11, 0.07) | 0.01 (−0.04, 0.06) | 0.07 (0.01, 0.14) * | |
| Maternal Experience of Any Sexual DV | |||||
|---|---|---|---|---|---|
| Biomarkers | Model | No Experience | Before Childbirth | After Childbirth | Both before and after Child Birth |
| ß (95% CI) | ß (95% CI) | ß (95% CI) | |||
| Apo A (g/l) | Unadjusted | Ref | −0.05 (−0.08, −0.01) ** | −0.01 (−0.03, 0.02) | −0.02 (−0.05, 0.02) |
| Adjusted 1 | Ref | −0.05 (−0.08, −0.01) ** | −0.00 (−0.03, 0.02) | −0.02 (−0.05, 0.02) | |
| Apo B (g/l) | Unadjusted | Ref | 0.03 (−0.00, 0.06) | −0.01 (−0.03, 0.02) | 0.02 (−0.01, 0.04) |
| Adjusted 1 | Ref | 0.03 (0.00, 0.06) * | −0.01 (−0.03, 0.02) | 0.02 (−0.01, 0.04) | |
| Apo B/Apo A | Unadjusted | Ref | 0.04 (0.01, 0.07) | −0.00 (−0.02, 0.02) | 0.02 (−0.01, 0.05) |
| Adjusted 1 | Ref | 0.04 (0.02, 0.07) | −0.00 (−0.02, 0.02) | 0.02 (−0.01, 0.05) | |
| HDL (mmol/l) | Unadjusted | Ref | −0.05 (−0.10, −0.01) * | −0.01 (−0.04, 0.03) | −0.00 (−0.04, 0.04) |
| Adjusted 1 | Ref | −0.05 (−0.10, −0.01) ** | −0.00 (−0.03, 0.03) | −0.00 (−0.04, 0.04) | |
| LDL (mmol/l) | Unadjusted | Ref | 0.16 (0.04, 0.29) ** | −0.03 (−0.12, 0.07) | 0.04 (−0.08, 0.15) |
| Adjusted 1 | Ref | 0.17 (0.05, 0.29) ** | −0.02 (−0.12, 0.07) | 0.04 (−0.08, 0.16) | |
| LDL/HDL | Unadjusted | Ref | 0.24 (0.11, 0.37) ** | −0.01 (−0.11, 0.10) | 0.04 (−0.09, 0.17) |
| Adjusted 1 | Ref | 0.24 (0.11, 0.38) ** | −0.01 (−0.11, 0.10) | 0.04 (−0.09, 0.17) | |
| Cholesterol (mmol/l) | Unadjusted | Ref | 0.04 (−0.08, 0.17) | −0.04 (−0.14, 0.06) | 0.08 (−0.04, 0.20) |
| Adjusted 1 | Ref | 0.05 (−0.08, 0.17) | −0.03 (−0.14, 0.07) | 0.08 (−0.04, 0.20) | |
| Triglycerides 2 (mmol/l) | Unadjusted | Ref | 0.06 (−0.01, 0.12) | −0.01 (−0.06, 0.04) | −0.01 (−0.07, 0.06) |
| Adjusted 1 | Ref | 0.06 (−0.01, 0.12) | −0.02 (−0.07, 0.03) | −0.01 (−0.07, 0.05) | |
| Maternal Experience of Any Controlling Behavior | |||||
|---|---|---|---|---|---|
| Biomarkers | Model | No Experience | Before Childbirth | After Childbirth | Both before and after Child Birth |
| ß (95% CI) | ß (95% CI) | ß (95% CI) | |||
| Apo A (g/l) | Unadjusted | Ref | −0.02 (−0.04, 0.01) | −0.01 (−0.04, 0.02) | −0.04 (−0.06, −0.00) * |
| Adjusted 1 | Ref | −0.02 (−0.04, 0.01) | −0.01 (−0.04, 0.02) | −0.03 (−0.06, −0.00) * | |
| Apo B (g/l) | Unadjusted | Ref | −0.01 (−0.03, 0.02) | −0.02 (−0.05, 0.00) | 0.00 (−0.02, 0.03) |
| Adjusted 1 | Ref | −0.01 (−0.03, 0.02) | −0.02 (−0.05, 0.00) | 0.00 (−0.02, 0.03) | |
| Apo B/Apo A | Unadjusted | Ref | 0.01 (−0.02, 0.03) | −0.01 (−0.04, 0.01) | 0.02 (−0.01, 0.04) |
| Adjusted 1 | Ref | 0.01 (−0.02, 0.03) | −0.02 (−0.04, 0.01) | 0.01 (−0.01, 0.04) | |
| HDL (mmol/l) | Unadjusted | Ref | −0.01 (−0.04, 0.03) | −0.02 (−0.06, 0.01) | −0.04 (−0.08, −0.00) * |
| Adjusted 1 | Ref | −0.01 (−0.04, 0.03) | −0.02 (−0.06, 0.02) | −0.03 (−0.07, 0.00) | |
| LDL (mmol/l) | Unadjusted | Ref | −0.05 (−0.16, 0.05) | −0.18 (−0.29, −0.07) ** | 0.00 (−0.11, 0.11) |
| Adjusted 1 | Ref | −0.05 (−0.15, 0.06) | −0.17 (−0.28, −0.06) ** | 0.01 (−0.10, 0.12) | |
| LDL/HDL | Unadjusted | Ref | −0.03 (−0.14, 0.08) | −0.13 (−0.25, −0.01) * | 0.08 (−0.04, 0.20) |
| Adjusted 1 | Ref | −0.02 (−0.14, 0.09) | −0.12 (−0.25, −0.00) * | 0.08 (−0.04, 0.20) | |
| Cholesterol (mmol/l) | Unadjusted | Ref | −0.06 (−0.17, 0.05) | −0.14 (−0.25, −0.02) * | −0.05 (−0.16, 0.07) |
| Adjusted 1 | Ref | −0.05 (−0.16, 0.06) | −0.13 (−0.25, −0.02) * | −0.03 (−0.15, 0.08) | |
| Triglycerides 2 (mmol/l) | Unadjusted | Ref | −0.00 (−0.06, 0.05) | −0.00 (−0.06, 0.06) | 0.02 (−0.04, 0.08) |
| Adjusted 1 | Ref | −0.00 (−0.06, 0.05) | −0.01 (−0.07, 0.05) | 0.01 (−0.04, 0.07) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziaei, S.; Naved, R.T.; Rahman, A.; Raqib, R.; Ekström, E.-C. Maternal Experience of Domestic Violence, Associations with Children’s Lipid Biomarkers at 10 Years: Findings from MINIMat Study in Rural Bangladesh. Nutrients 2019, 11, 910. https://doi.org/10.3390/nu11040910
Ziaei S, Naved RT, Rahman A, Raqib R, Ekström E-C. Maternal Experience of Domestic Violence, Associations with Children’s Lipid Biomarkers at 10 Years: Findings from MINIMat Study in Rural Bangladesh. Nutrients. 2019; 11(4):910. https://doi.org/10.3390/nu11040910
Chicago/Turabian StyleZiaei, Shirin, Ruchira Tabassum Naved, Anisur Rahman, Rubhana Raqib, and Eva-Charlotte Ekström. 2019. "Maternal Experience of Domestic Violence, Associations with Children’s Lipid Biomarkers at 10 Years: Findings from MINIMat Study in Rural Bangladesh" Nutrients 11, no. 4: 910. https://doi.org/10.3390/nu11040910
APA StyleZiaei, S., Naved, R. T., Rahman, A., Raqib, R., & Ekström, E.-C. (2019). Maternal Experience of Domestic Violence, Associations with Children’s Lipid Biomarkers at 10 Years: Findings from MINIMat Study in Rural Bangladesh. Nutrients, 11(4), 910. https://doi.org/10.3390/nu11040910






