Traceability of Functional Volatile Compounds Generated on Inoculated Cocoa Fermentation and Its Potential Health Benefits
Abstract
:1. Introduction
2. Microbial Composition of Fermented Cocoa Beans
2.1. Yeasts Species Used as Starter during Cocoa Fermentation
2.2. Quality Evaluation of the Chocolate Produce from Inoculated Cocoa Beans
3. Changes in the Nutrient Composition from Fermented to Roasted Cocoa Beans
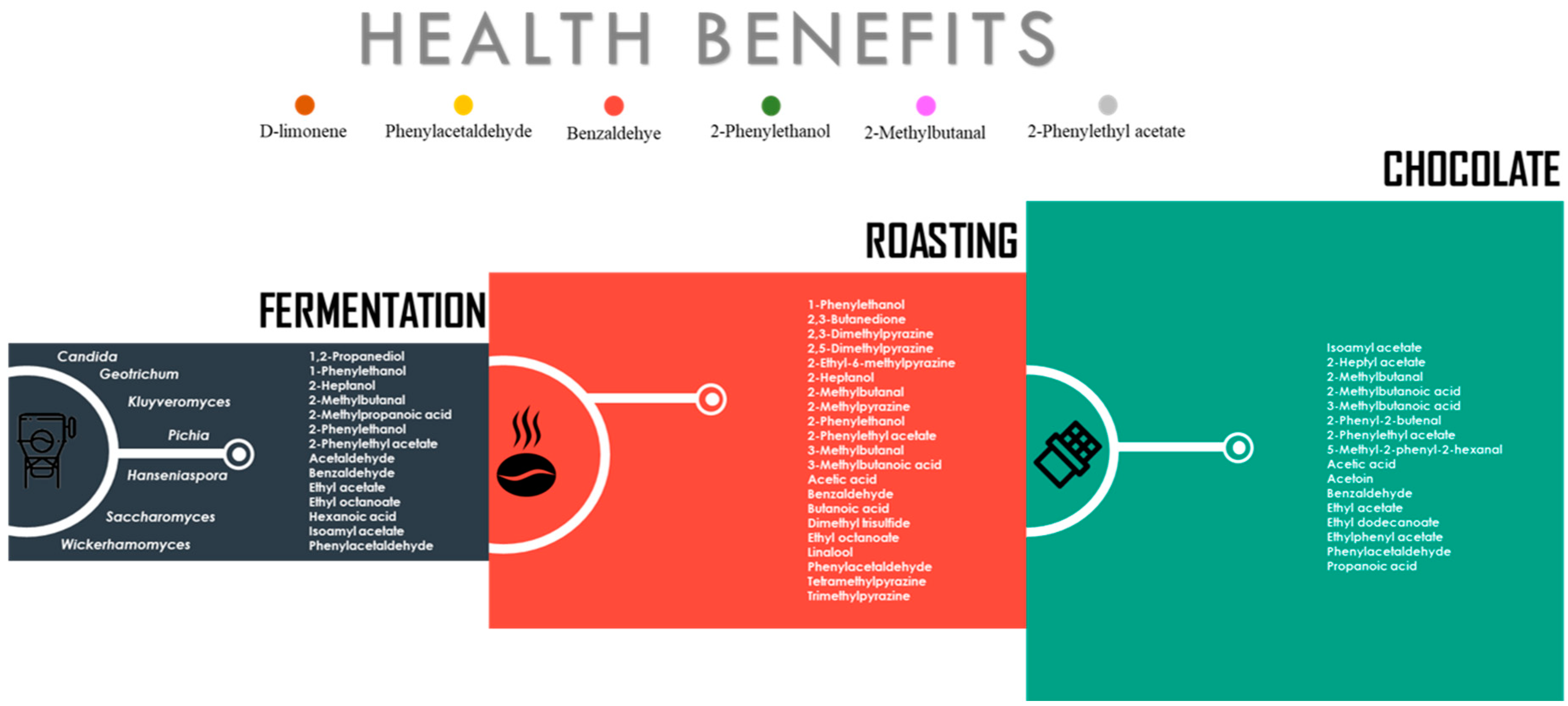
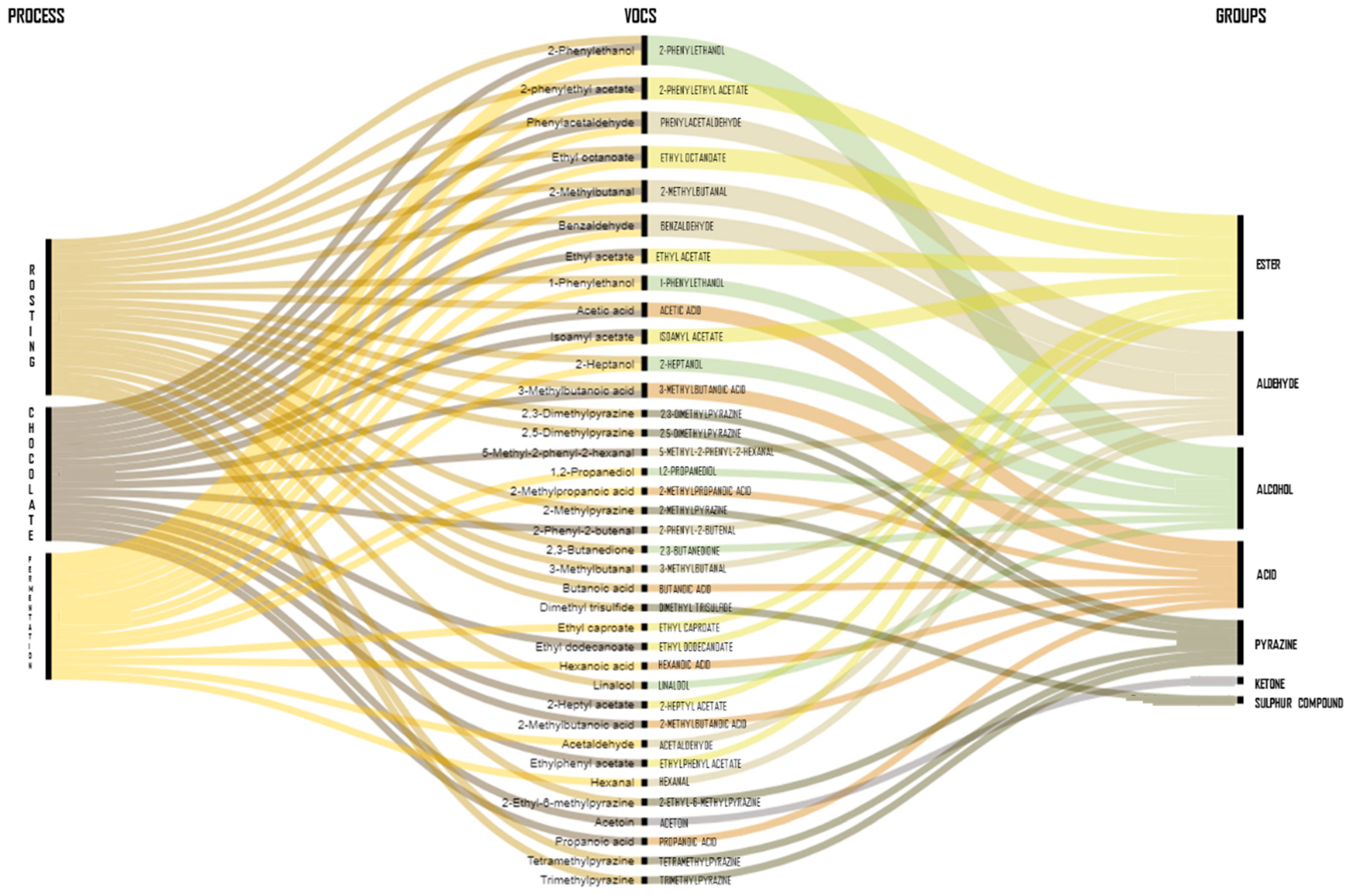
3.1. Composition of Volatile Compounds from Cocoa Beans
3.1.1. VOCs Associated with Inoculated Cocoa Beans
3.1.2. Dynamics of VOCs during Roasting
4. Synthesis of VOCs by Fungal Communities and their Potential Health Benefits
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wadhwa, S.S. Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: A Review. Food Bioprocess Technol. 2013, 6, 607–627. [Google Scholar] [CrossRef]
- Ayseli, M.T.; Ipek Ayseli, Y. Flavors of the future: Health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci. Technol. 2016, 48, 69–77. [Google Scholar] [CrossRef]
- Gobbetti, M.; Di Cagno, R.; de Angelis, M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010, 50, 716–727. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Teniola, O.D.; Ban-Koffi, L.; Owusu, M.; Andersson, T.S.; Holzapfel, W.H. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2007, 114, 168–186. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Cannoni, M.; Cocolin, L. Dynamics and biodiversity of bacterial and yeast communities during the fermentation of cocoa beans. Appl. Environ. Microbiol. 2018, AEM.01164-18. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; De Vuyst, L. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1809–1824. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Van Schoor, A.; De Bruyne, K.; Vandamme, P.; Takrama, J.S.; Addo, S.K.; De Vuyst, L. Influence of turning and environmental contamination on the dynamics of populations of lactic acid and acetic acid bacteria involved in spontaneous cocoa bean heap fermentation in Ghana. Appl. Environ. Microbiol. 2008, 74, 86–98. [Google Scholar] [CrossRef]
- Jespersen, L.; Nielsen, D.S.; Hønholt, S.; Jakobsen, M. Occurrence and diversity of yeasts involved in fermentation of West African cocoa beans. FEMS Yeast Res. 2005, 5, 441–453. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Vrancken, G.; De Bruyne, K.; Vandamme, P.; De Vuyst, L. Spontaneous organic cocoa bean box fermentations in Brazil are characterized by a restricted species diversity of lactic acid bacteria and acetic acid bacteria. Food Microbiol. 2011, 28, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Papalexandratou, Z.; De Vuyst, L. Assessment of the yeast species composition of cocoa bean fermentations in different cocoa-producing regions using denaturing gradient gel electrophoresis. FEMS Yeast Res. 2011, 11, 564–574. [Google Scholar] [CrossRef]
- Kostinek, M.; Ban-Koffi, L.; Ottah-Atikpo, M.; Teniola, D.; Schillinger, U.; Holzapfel, W.H.; Franz, C.M.A.P. Diversity of predominant lactic acid bacteria associated with cocoa fermentation in Nigeria. Curr. Microbiol. 2008, 56, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Miescher Schwenninger, S.; Freimüller Leischtfeld, S.; Gantenbein-Demarchi, C. High-throughput identification of the microbial biodiversity of cocoa bean fermentation by MALDI-TOF MS. Lett. Appl. Microbiol. 2016, 63, 347–355. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. The effect of lactic acid bacteria on cocoa bean fermentation. Int. J. Food Microbiol. 2015, 205, 54–67. [Google Scholar] [CrossRef] [PubMed]
- García-Alamilla, P.; Lagunes-Gálvez, L.M.; Barajas-Fernández, J.; García-Alamilla, R.; Garcia-Armisen, T.; Papalexandratou, Z.; Hendryckx, H.; Camu, N.; Vrancken, G.; De Vuyst, L.; et al. Diversity of the total bacterial community associated with Ghanaian and Brazilian cocoa bean fermentation samples as revealed by a 16 S rRNA gene clone library. Appl. Microbiol. Biotechnol. 2010, 87, 2281–2292. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Falony, G.; Romanens, E.; Jimenez, J.C.; Amores, F.; Daniel, H.; De Vuyst, L. Species diversity, community dynamics, and metabolite kinetics of the microbiota associated with traditional Ecuadorian spontaneous cocoa bean fermentations. Appl. Environ. Microbiol. 2011, 77, 7698–7714. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Lefeber, T.; Bahrim, B.; Seng, O.; Daniel, H.; De Vuyst, L. Acetobacter pasteurianus predominate during well-performed Malaysian cocoa bean box fermentations, underlining the importance of these microbial species for a successful cocoa bean fermentation process. Food Microbiol. 2013, 35, 73–85. [Google Scholar] [CrossRef]
- Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int. J. Food Microbiol. 2016, 236, 98–106. [Google Scholar] [CrossRef]
- Miguel, M.G.D.C.P.; de Castro Reis, L.V.; Efraim, P.; Santos, C.; Lima, N.; Schwan, R.F. Cocoa fermentation: Microbial identification by MALDI-TOF MS, and sensory evaluation of produced chocolate. LWT—Food Sci. Technol. 2017, 77. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Magalhães, K.T.; de Almeida, E.G.; da Silva Coelho, I.; Schwan, R.F. Spontaneous cocoa bean fermentation carried out in a novel-design stainless steel tank: Influence on the dynamics of microbial populations and physical-chemical properties. Int. J. Food Microbiol. 2013, 161, 121–133. [Google Scholar] [CrossRef]
- Illeghems, K.; De Vuyst, L.; Papalexandratou, Z.; Weckx, S. Phylogenetic analysis of a spontaneous cocoa bean fermentation metagenome reveals new insights into its bacterial and fungal community diversity. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- da Veiga Moreira, I.M.; Gabriela, M.; Miguel, P.; Ferreira, W.; Ribeiro, D.; Freitas, R. Microbial succession and the dynamics of metabolites and sugars during the fermentation of three different cocoa (Theobroma cacao L.) hybrids. Food Res. Int. 2013, 54, 9–17. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Guehi, T.; Durand, N.; Kedjebo, K.B.D.; Montet, D.; Meile, J.C. Dynamics of microbial ecology during cocoa fermentation and drying: Towards the identification of molecular markers. Food Control 2015, 48, 117–122. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Alvarez, J.P.; de Neto, D.P.C.; Soccol, V.T.; Tanobe, V.O.A.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT—Food Sci. Technol. 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Segura-García, L.E.; Kirchmayr, M.; Orozco-Ávila, I.; Lugo-Cervantes, E.; Gschaedler-Mathis, A. Identification of predominant yeasts associated with artisan Mexican cocoa fermentations using culture-dependent and culture-independent approaches. World J. Microbiol. Biotechnol. 2015, 31, 359–369. [Google Scholar] [CrossRef]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-kof, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Leal, G.A.; Gomes, L.H.; Efraim, P.; De Almeida Tavares, F.C.; Figueira, A. Fermentation of cacao (Theobroma cacao L.) seeds with a hybrid Kluyveromyces marxianus strain improved product quality attributes. FEMS Yeast Res. 2008, 8, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Batista, N.N.; Ramos, C.L.; Ribeiro, D.D.; Pinheiro, A.C.M.; Schwan, R.F. Dynamic behavior of Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum during spontaneous and inoculated cocoa fermentations and their effect on sensory characteristics of chocolate. LWT—Food Sci. Technol. 2015, 63, 221–227. [Google Scholar] [CrossRef]
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii starter cultures on cocoa beans fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeasts in foods and beverages: Impact on product quality and safety. Curr. Opin. Biotechnol. 2007, 18, 170–175. [Google Scholar] [CrossRef]
- Ramos, C.L.; Dias, D.R.; Miguel, M.G.D.C.P.; Schwan, R.F. Impact of different cocoa hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 inoculation on microbial communities and volatile compounds of cocoa fermentation. Food Res. Int. 2014, 64, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.G.T.; Batista, N.N.; Ramos, C.L.; e Silva, A.R.D.A.; Efraim, P.; Pinheiro, A.C.M.; Schwan, R.F. Investigation of chocolate produced from four different Brazilian varieties of cocoa (Theobroma cacao L.) inoculated with Saccharomyces cerevisiae. Food Res. Int. 2016, 81, 83–90. [Google Scholar] [CrossRef]
- Cempaka, L.; Aliwarga, L.; Purwo, S.; Kresnowati, M.T.A.P. Dynamics of cocoa bean pulp degradation during cocoa bean fermentation: Effects of yeast starter culture addition. J. Math. Fundam. Sci. 2014, 46, 14–25. [Google Scholar] [CrossRef]
- Mahazar, N.H.; Sufian, N.F.; Meor Hussin, A.S.; Norhayati, H.; Mathawan, M.; Rukayadi, Y. Candida sp. as a starter culture for cocoa (Theobroma cacao L.) beans fermentation. Int. Food Res. J. 2015, 22, 1783–1787. [Google Scholar]
- Meersman, E.; Steensels, J.; Struyf, N.; Paulus, T.; Saels, V.; Mathawan, M.; Allegaert, L.; Vrancken, G.; Verstrepen, K.J. Tuning chocolate flavor through development of thermotolerant Saccharomyces cerevisiae starter cultures with increased acetate ester. Appl. Environ. Microbiol. 2016, 82, 732–746. [Google Scholar] [CrossRef]
- Meersman, E.; Steensels, J.; Paulus, T.; Struyf, N.; Saels, V.; Mathawan, M.; Koffi, J.; Vrancken, G.; Verstrepen, K.J. Breeding strategy to generate robust yeast starter cultures for cocoa pulp fermentations. Appl. Environ. Microbiol. 2015, 81, 6166–6176. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.M.; Vrancken, G.; Takrama, J.F.; Camu, N.; De Vos, P.; De Vuyst, L. Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res. 2009, 9, 774–783. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; Miguel, M.G.D.C.P.; Ramos, C.L.; Schwan, R.F. Microbiological and physicochemical characterization of small-scale cocoa fermentations and screening of yeast and bacterial strains to develop a defined starter culture. Appl. Environ. Microbiol. 2014, 78, 5395–5405. [Google Scholar] [CrossRef]
- Samagaci, L.; Ouattara, H.; Niamké, S.; Lemaire, M. Pichia kudrazevii and Candida nitrativorans are the most well-adapted and relevant yeast species fermenting cocoa in Agneby-Tiassa, a local Ivorian cocoa producing region. Food Res. Int. 2016, 89, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Francisco, D.; Chambon, C.; Hébraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef]
- Jayani, R.S.; Saxena, S.; Gupta, R. Microbial pectinolytic enzymes: A review. Process Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Batista, N.N.; Ramos, C.L.; Dias, D.R. The impact of yeast starter cultures on the microbial communities and volatile compounds in cocoa fermentation and the resulting sensory attributes of chocolate. J. Food Sci. Technol. 2016, 53, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Francisco, D.; Gori, K.; Arneborg, N.; Gírio, F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010, 86, 965–972. [Google Scholar] [CrossRef]
- Magagna, F.; Guglielmetti, A.; Liberto, E.; Reichenbach, S.E.; Allegrucci, E.; Gobino, G.; Bicchi, C.; Cordero, C. Comprehensive chemical fingerprinting of high-quality cocoa at early stages of processing: Effectiveness of combined untargeted and targeted approaches for classification and discrimination. J. Agric. Food Chem. 2017, 65, 6329–6341. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A.; Ohene, E.; Paterson, A.; Fowler, M.; Ryan, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Efraim, P.; Pezoa-García, N.H.; Calil, D.; Jardim, P.; Nishikawa, A.; Haddad, R.; Eberlin, M.N. Influência da fermentação e secagem de amêndoas de cacau no teor de compostos fenólicos e na aceitação sensorial. Ciência Tecnol. Aliment. 2010, 30, 142–150. [Google Scholar] [CrossRef]
- Caligiani, A.; Cirlini, M.; Palla, G.; Ravaglia, R.; Arlorio, M. GC-MS detection of chiral markers in cocoa beans of different quality and geographic origin. Chirality Pharmacol. Biol. Chem. Consequences Mol. Asymmetry 2007, 334, 329–334. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Trovato, V.; Curti, D. Cocoa bean carbohydrates: Roasting-induced changes and polymer interactions. Food Chem. 2003, 80, 511–516. [Google Scholar] [CrossRef]
- Gu, F.; Tan, L.; Wu, H.; Fang, Y.; Xu, F.; Chu, Z.; Wang, Q. Comparison of cocoa beans from China, Indonesia and Papua New Guinea. Foods 2013, 2, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, R.; Brückner, H.; Oracz, J.; Nebesny, E.; Sukha, D.A.; Butler, D.R.; Umaharan, P.; Boult, E.; Miescher Schwenninger, S.; Freimüller Leischtfeld, S.; et al. Assessment methodology to predict quality of cocoa beans for export. J. Agric. Food Chem. 2008, 56, 773–780. [Google Scholar] [CrossRef]
- Granvogl, M.; Bugan, S.; Schieberle, P. Formation of amines and aldehydes from parent amino acids during thermal processing of cocoa and model systems: New insights into pathways of the strecker reaction. J. Agric. Food Chem. 2006, 54, 1730–1739. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.S. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Kongor, J.E.; Takrama, J.F.; Budu, A.S. Changes in acidification, sugars and mineral composition of cocoa pulp during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int. Food Res. J. 2013, 20, 1215–1222. [Google Scholar]
- Scott-Thomas, C. Raw Food on the Rise. Available online: https://www.foodnavigator.com/Article/2015/06/09/Raw-food-on-the-rise (accessed on 15 January 2019).
- Ramli, N.; Hassan, O.; Said, M.; Samsudin, W.; Idris, N.A. Influence of roasting conditions on volatile flavor of roasted Malaysian cocoa beans. J. Food Process. Preserv. 2006, 30, 280–298. [Google Scholar] [CrossRef]
- Bonhevi, J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur. Food Res. Tecnhonol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Huang, Y.; Barringer, S.A. Monitoring of cocoa volatiles produced during roasting by selected ion flow tube-mass spectrometry (SIFT-MS). J. Food Sci. 2011, 76, 279–286. [Google Scholar] [CrossRef]
- Van Durme, J.; Ingels, I.; De Winne, A. Online roasting hyphenated with gas chromatography-mass spectrometry as an innovative approach for assessment of cocoa fermentation quality and aroma formation potential. Food Chem. 2016, 205, 66–72. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Changes in key aroma compounds of criollo cocoa. J. Agric. Food Chem. 2008, 56, 10244–10251. [Google Scholar] [CrossRef]
- Tan, J.; Kerr, W.L. Determining degree of roasting in cocoa beans by artificial neural network (ANN)-based electronic nose system and gas chromatography/mass spectrometry (GC/MS). J. Sci. Food Agric. 2018, 98, 3851–3859. [Google Scholar] [CrossRef]
- Magagna, F.; Liberto, E.; Reichenbach, S.E.; Tao, Q.; Carretta, A.; Cobelli, L.; Giardina, M.; Bicchi, C.; Cordero, C. Advanced fingerprinting of high-quality cocoa: Challenges in transferring methods from thermal to differential-flow modulated comprehensive two dimensional gas chromatography. J. Chromatogr. A 2018, 1536, 122–136. [Google Scholar] [CrossRef]
- Janssens, L.; De Pooter, H.L.; Schamp, N.M.; Vandamme, E.J. Production of flavours by microorganisms. Process Biochem. 1992, 27, 195–215. [Google Scholar] [CrossRef]
- Hagedorn, S.; Kaphammer, B. Microbial biocatalysis in the generation of flavor and fragrance chemicals. Annu. Rev. Microbiol. 1994, 46, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin, Germany, 2007; ISBN 9783540493389. [Google Scholar]
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.-M.; Rabenhorst, J. Applied biocatalysis for the synthesis of natural flavour compounds: Curent industrial processes and future prospects. Biotechnol. Lett. 2004, 463–472. [Google Scholar] [CrossRef]
- Kim, B.; Cho, B.R.; Hahn, J.S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 2014, 111, 115–124. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Sell, D.; Schrader, J. Production of 2-phenylethanol and 2-phenylethylacetate from L-phenylalanine by coupling whole-cell biocatalysis with organophilic pervaporation. Biotechnol. Bioeng. 2005, 92, 624–634. [Google Scholar] [CrossRef]
- Wittmann, C.; Hans, M.; Bluemke, W. Metabolic physiology of aroma-producing Kluyveromyces marxianus. Yeast 2002, 19, 1351–1363. [Google Scholar] [CrossRef]
- Moreira, N.; Mendes, F.; Hogg, T.; Vasconcelos, I. Alcohols, esters and heavy sulphur compounds production by pure and mixed cultures of apiculate wine yeasts. Int. J. Food Microbiol. 2005, 103, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Fabre, C.E.; Blanc, P.J.; Goma, G. Screening of yeasts producing 2-phenylethylalcohol. Biotechnol. Tech. 1997, 11, 523–525. [Google Scholar] [CrossRef]
- Cappaert, L.; Larroche, C. Oxidation of a mixture of 2-(R) and 2-(S)-heptanol to 2-heptanone by Saccharomyces cerevisiae in a biphasic system. Biocatal. Biotransform. 2004, 22, 291–296. [Google Scholar] [CrossRef]
- Chen, H.; Fink, G.R. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 2006, 20, 1150–1161. [Google Scholar] [CrossRef]
- Fraud, S.; Rees, E.L.; Mahenthiralingam, E.; Russell, A.D.; Maillard, J.Y. Aromatic alcohols and their effect on Gram-negative bacteria, cocci and mycobacteria (1). J. Antimicrob. Chemother. 2003, 51, 1435–1436. [Google Scholar] [CrossRef]
- Larroy, C.; Parés, X.; Biosca, J.A. Characterization of a Saccharomyces cerevisiae NADP(H)-dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur. J. Biochem. 2002, 269, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Lapadatescu, C.; Feron, G.; Vergoignan, C.; Djian, A.; Durand, A.; Bonnarme, P. Influence of cell immobilization on the production of benzaldehyde and benzyl alcohol by the white-rot fungi Bjerkandera adusta, Ischnoderma benzoinum and Dichomitus squalens. Appl. Microbiol. Biotechnol. 1997, 47, 708–714. [Google Scholar] [CrossRef]
- Kawabe, T.; Morita, H. Production of benzaldehyde and benzyl alcohol by the mushroom Polyporus tuberaster K2606. J. Agric. Food Chem. 1994, 42, 2556–2560. [Google Scholar] [CrossRef]
- Pal, S.; Park, D.H.; Plapp, B.V. Activity of yeast alcohol dehydrogenases on benzyl alcohols and benzaldehydes. Characterization of ADH1 from Saccharomyces carlsbergensis and transition state analysis. Chem. Biol. Interact. 2009, 178, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Van Laere, S.D.M.; Saerens, S.M.G.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Flavour formation in fungi: Characterisation of KlAtf, the Kluyveromyces lactis orthologue of the Saccharomyces cerevisiae alcohol acetyltransferases Atf1 and Atf2. Appl. Microbiol. Biotechnol. 2008, 78, 783–792. [Google Scholar] [CrossRef]
- Duetz, W.A.; Bouwmeester, H.; Beilen, J.B.; Witholt, B. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl. Microbiol. Biotechnol. 2003, 61, 269–277. [Google Scholar] [CrossRef]
- Ariyoshi-Kishino, K.; Hashimoto, K.; Amano, O.; Saitoh, J.; Kochi, M.; Sakagami, H. Tumor-specific cytotoxicity and type of cell death induced by benzaldehyde. Anticancer Res. 2010, 30, 5069–5076. [Google Scholar]
- Stringer, T.; Therrien, B.; Hendricks, D.T.; Guzgay, H.; Smith, G.S. Mono- and dinuclear (η6-arene) ruthenium (II) benzaldehyde thiosemicarbazone complexes: Synthesis, characterization and cytotoxicity. Inorg. Chem. Commun. 2011, 14, 956–960. [Google Scholar] [CrossRef]
- Su, W.; Zhou, Q.; Huang, Y.; Huang, Q.; Huo, L.; Xiao, Q.; Huang, S.; Huang, C.; Chen, R.; Qian, Q.; et al. Synthesis, crystal and electronic structure, anticancer activity of ruthenium (II) arene complexes with thiosemicarbazones. Appl. Organomet. Chem. 2013, 27, 307–312. [Google Scholar] [CrossRef]
- Liu, Y.; Sakagami, H.; Hashimoto, K.; Kikuchi, H.; Amano, O.; Ishibara, M.; Yu, G. Tumor-specific cytotoxicity and type of cell death induced by beta-cyclodextrin benzaldehyde inclusion compound. Anticancer Res. 2008, 28, 229–236. [Google Scholar] [PubMed]
- Alamri, A.; El-Newehy, M.H.; Al-Deyab, S.S. Biocidal polymers: Synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chem. Cent. J. 2012, 6, 1–13. [Google Scholar] [CrossRef]
- Rabi, T.; Bishayee, A. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009, 8, 9. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother. Pharmacol. 1998, 42, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Igimi, H.; Watanabe, D.; Yamamoto, F.; Asakawa, S.; Toraishi, K.; Shimura, H. A useful cholesterol solvent for medical dissolution of gallstones. Gastroenterol. Jpn. 1992, 27, 536–545. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.A.C.; Costa, J.P.; De Carvalho, R.B.F.; De Sousa, D.P.; De Freitas, R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012, 1448, 56–62. [Google Scholar] [CrossRef]
- do Amaral, J.F.; Silva, M.I.G.; de Aquino Neto, M.R.A.; Neto, P.F.T.; Moura, B.A.; de Melo, C.T.V.; de Sousa, F.C.F. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol. Pharm. Bull. 2007, 30, 1217–1220. [Google Scholar] [CrossRef]
- Giri, R.K.; Parija, T.; Das, B.R. D-limonene chemoprevention of hepatocarcinogenesis in AKR mice: Inhibition of c-jun and c-myc. Oncol. Rep. 1999, 6, 1123–1127. [Google Scholar] [CrossRef]
- Kaji, I.; Tatsuta, M.; Lishi, H.; Baba, M.; Inoue, A.; Kasugai, H. Inhibition by d-limonene of experimental hepatocarcinogenesis in sprague-dawley rats does not involve P21ras plasma membrane association. Int. J. Cancer 2001, 93, 441–444. [Google Scholar] [CrossRef]
- Vuuren, S.V.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combinatio. Flavours Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Purity, antimicrobial activities and olfactory evaluations of 2-phenylethanol and some derivatives. J. Essent. Oil Res. 2008, 20, 82–85. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.J.; Shin, J.H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Zhao, J.; Gao, H.; Zhou, L.; Liu, Z.; Chen, Y.; Sui, P. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component 2-hydroxy-4-methoxybenzaldehyde. Molecules 2010, 15, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, B.T.; Prasad, M.; Lingappa, Y.; Hunt, D.F.; Biemann, K. Phenethyl alcohol and tryptophol: Autoantibiotics produced by the fungus Candida albicans. Science 1969, 163, 192–194. [Google Scholar] [CrossRef]
- Wang, Q.; Ke, L.; Xue, C.; Luo, W.; Chen, Q. Inhibitory kinetics of p-substituted benzaldehydes on polyphenol oxidase from the fifth instar of Pieris rapae L. Tsinghua Sci. Technol. 2007, 12, 400–404. [Google Scholar] [CrossRef]
- Wilkinson, S.M.; Love, S.B.; Westcombe, A.M.; Gambles, M.A.; Burgess, C.C.; Cargill, A.; Young, T.; Maher, E.J.; Ramirez, A.J. Effectiveness of aromatherapy massage in the management of anxiety and depression in patients with cancer: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 532–539. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Rawal, S.; Li, C.; Duffy, V.B. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): First-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2017, 17, 221–240. [Google Scholar] [CrossRef]
- Naka, A.; Riedl, M.; Luger, A.; Hummel, T.; Mueller, C.A. Clinical significance of smell and taste disorders in patients with diabetes mellitus. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 547–550. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Scholze, N.; Joraschky, P.; Hummel, T. Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J. Psychiatr. Res. 2008, 43, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Skrandies, W.; Zschieschang, R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef]
- Clepce, M.; Reich, K.; Gossler, A.; Kornhuber, J.; Thuerauf, N. Olfactory abnormalities in anxiety disorders. Neurosci. Lett. 2012, 511, 43–46. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Mattes, R.D. Nutrition and taste and smell dysfunction. World J. Otorhinolaryngol.-Head Neck Surg. 2018, 4, 3–10. [Google Scholar] [CrossRef]
- Pekala, K.; Chandra, R.K.; Turner, J.H. Efficacy of olfactory training in patients with olfactory loss: A systematic review and meta-analysis. Int. Forum Allergy Rhinol. 2016, 6, 299–307. [Google Scholar] [CrossRef]
- Jiang, R.S.; Twu, C.W.; Liang, K.L. The effect of olfactory training on the odor threshold in patients with traumatic anosmia. Am. J. Rhinol. Allergy 2017, 31, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Reden, K.R.J.; Hähner, A.; Weidenbecher, M.; Hüttenbrink, K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Tosch, C.; Wolz, M.; Klingelhoefer, L.; Fauser, M.; Storch, A.; Reichmann, H.; Hummel, T. Olfactory training in patients with Parkinson’s disease. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Geißler, K.; Reimann, H.; Gudziol, H.; Bitter, T.; Guntinas-Lichius, O. Olfactory training for patients with olfactory loss after upper respiratory tract infections. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Pikart, L.K.; Reimann, H.; Burkert, S.; Göktas, Ö.; Haxel, B.; Frey, S.; Charalampakis, I.; Beule, A.; Renner, B.; Hummel, T.; et al. Olfactory training is helpful in postinfectious olfactory loss: A randomized, controlled, multicenter study. Laryngoscope 2014, 124, 826–831. [Google Scholar] [CrossRef]
- Kollndorfer, K.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Pollak, M.; Trattnig, S.; Schöpf, V. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.; Tsakiropoulou, E.; Bekiaridou, P.; Kazantzidou, C.; Constantinidis, J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope 2013, 123. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Certain Food Additives: Fifty-Ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/iris/bitstream/handle/10665/42601/WHO_TRS_913.pdf?sequence=1&isAllowed=y (accessed on 10 January 2019).

| Genera/Species | Year | Country | Type of Cocoa Bean | Type of Fermentation | Amount | VOCs | Sensorial Analysis | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| F | C | ||||||||
| Kluyveromyces marxianus MMIII-41 | 2008 | Brazil | NM | Plastic basket | 45 kg | - | - | + | Leal et al., 2008 [28] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | PH16 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | PS1030 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | FA13 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | PS1319 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum | 2015 | Brazil | PS1319 | Wooden box | 100 kg | - | - | + | Batista et al., 2015 [30] |
| Candida sp. | 2015 | Malaysia | NM | Basket | 5 kg | - | - | - | Mahazar et al., 2015 [36] |
| Saccharomyces cerevisiae H19 | 2015 | Malaysia | NM | Basket | 50 kg | - | + | + | Meersman et al., 2016 [37] |
| Saccharomyces cerevisiae H28 | 2015 | Malaysia | NM | Basket | 50 kg | - | + | + | Mersman et al., 2016 [37] |
| Saccharomyces cerevisiae H37 | 2015 | Malaysia | NM | Basket | 50 kg | - | + | + | Mersman et al., 2016 [37] |
| Saccharomyces cerevisae var. chevalieri | 2015 | Indonesia | Forastero | Plastic bags | NM | - | - | - | Cempaka et al., 2014 [35] |
| Saccharomyces cerevisiae | 2016 | Brazil | CCN51 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Saccharomyces cerevisiae | 2016 | Brazil | CEPEC2004 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Saccharomyces cerevisiae | 2016 | Brazil | FA13 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Saccharomyces cerevisiae | 2016 | Brazil | PS1030 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Torulaspora delbrueckii | 2017 | Brazil | PS1319 | Wooden box | 300 kg | - | + | + | Visintin et al., 2017 [31] |
| T. delbrueckii | 2017 | Brazil | SJ02 | Wooden box | 300 kg | - | + | + | Visintin et al., 2017 [31] |
| S. cerevisiae and T. delbrueckii | 2017 | Brazil | PS1319 | Wooden box | 300 kg | - | + | + | Visintin et al., 2017 [31] |
| Pichia kudriavzevii LPB06 | 2017 | Brazil | NM | Lab scale | 400 g | + | - | - | Pereira et al., 2017 [25] |
| Pichia kudriavzevii LPB07 | 2017 | Brazil | NM | Lab scale | 400 g | + | - | - | Pereira et al., 2017 [25] |
| Saccharomyces cerevisiae | 2018 | Cameroon | Forastero | Wooden box | 200 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
| Saccharomyces cerevisiae | 2018 | Cameroon | Forastero | Heap | 100 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
| Saccharomyces cerevisiae and T. delbrueckii | 2018 | Cameroon | Forastero | Wooden box | 200 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
| Saccharomyces cerevisiae and T. delbrueckii | 2018 | Cameroon | Forastero | Heap | 100 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
| Source | Origin | Variety | Genetic Material | Carbohydrates | Lipids | Proteins | |||
|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Fructose | Glucose | Total Carbohydrates | ||||||
| Afoakwa et al., 2013 [56] | Ghana | NM | Unfermented | 155.00 | 552.00 | 216.00 | |||
| Efraim et al., 2010 [50] | Brazil | Forastero | Unfermented | 548.20 | 238.80 | ||||
| Afoakwa et al., 2013 [56] | Ghana | NM | Fermented | 210.00 | 534.00 | 188.00 | |||
| Efraim et al., 2010 [50] | Brazil | Forastero | Fermented | 556.00 | 169.90 | ||||
| Redgwell et al., 2003 [52] | Ghana | NM | Dry cocoa beans | 1.58 | 4.18 | 0.62 | |||
| Redgwell et al., 2003 [52] | Ivory Coast | NM | Dry cocoa beans | 1.55 | 2.80 | 0.80 | |||
| Redgwell et al., 2003 [52] | Ecuador | NM | Dry cocoa beans | 4.83 | 1.72 | 0.84 | |||
| Gu et al., 2013 [53] | Papua New Guinea | Trinitario | Roasted | 458.60 | |||||
| Gu et al., 2013 [53] | Indonesia | Trinitario | Roasted | 498.50 | |||||
| Gu et al., 2013 [53] | China | Trinitario | Roasted | 392.40 | |||||
| Gu et al., 2013 [53] | China | Trinitario | Roasted | 434.40 | |||||
| Redgwell et al., 2003 [52] | Ghana | NM | Roasted | 1.41 | 0.60 | 0.05 | |||
| Redgwell et al., 2003 [52] | Ivory Coast | NM | Roasted | 2.03 | 0.44 | 0.05 | 134.40 | ||
| Redgwell et al., 2003 [52] | Ecuador | NM | Roasted | 6.24 | 0.61 | 0.11 | 181.70 | ||
| Volatile Aroma Compounds | Raw Beans [6,33] | End of Fermentation [6,33] | Roasting [59,60,61,62,63,64] | Chocolate [31,34,37] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | ||||||||||||
| 2-Methylbutanal | 0.70 | - | 1.24 | 0.49 | - | 1.46 | 111.00 | - | 4500.00 | 0.21 | - | 38.30 |
| Acetaldehyde | 0.02 | - | 0.85 | 0.00 | - | 0.18 | 285.00 | - | 285.00 | 0.60 | - | 41.70 |
| Benzaldehyde | 0.21 | - | 0.55 | 0.59 | - | 0.75 | 28.00 | - | 895.00 | 2.77 | - | 53.50 |
| Decanal | 0.03 | - | 0.06 | 0.02 | - | 0.04 | 1.00 | - | 1.00 | |||
| Dodecanal | 0.00 | - | 0.02 | 0.00 | - | 0.01 | 0.10 | - | 0.50 | |||
| Furfural | 0.00 | - | 0.24 | 0.00 | - | 0.25 | 26.00 | - | 87.00 | |||
| Hexanal | 0.02 | - | 3.65 | 0.01 | - | 6.55 | ||||||
| Nonanal | 0.14 | - | 0.19 | 0.09 | - | 0.14 | 46.00 | - | 46.00 | 0.05 | - | 1.52 |
| Phenylacetaldehyde | 4.06 | - | 6.09 | 3.49 | - | 12.37 | 60.00 | - | 5500.00 | 0.06 | - | 0.15 |
| (E)-2-Undecenal | 0.00 | - | 0.01 | 0.00 | - | 0.05 | ||||||
| 2-Phenyl-2-butenal | 0.00 | - | 0.00 | 0.00 | - | 0.05 | ||||||
| Alcohols | ||||||||||||
| (Z)-3-Hexen-1-ol | 0.00 | - | 37.65 | 0.01 | - | 0.02 | ||||||
| 1,2-Propanediol | 0.00 | - | 0.00 | 0.07 | - | 0.35 | 1.10 | - | 1.70 | |||
| 1-Butanol | 3.20 | - | 33.26 | 0.91 | - | 10.50 | ||||||
| 1-Decanol | 0.01 | - | 0.01 | 0.01 | - | 0.01 | ||||||
| 1-Dodecanol | 0.02 | - | 0.17 | 0.05 | - | 0.38 | ||||||
| 1-Heptadecanol | 0.03 | - | 0.10 | 0.06 | - | 0.21 | ||||||
| 1-Heptanol | 0.04 | - | 0.05 | 0.00 | - | 0.00 | 0.03 | - | 0.05 | |||
| 1-Hexanol | 0.21 | - | 0.43 | 0.15 | - | 0.22 | ||||||
| 1-Octanol | 0.06 | - | 0.09 | 0.09 | - | 0.17 | ||||||
| 1-Octen-3-ol | 0.03 | - | 0.05 | 0.00 | - | 0.18 | ||||||
| 1-Pentanol | 0.13 | - | 0.83 | 0.07 | - | 0.14 | ||||||
| 1-Phenylethanol | 0.29 | - | 0.55 | 0.22 | - | 0.34 | ||||||
| 1-Propanol | 0.00 | - | 1.01 | 0.02 | - | 1.02 | ||||||
| 2,3-Butanediol | 0.00 | - | 9.60 | 0.00 | - | 2.07 | 62.00 | - | 356.00 | 35.40 | - | 65.35 |
| 2-Ethyl-1-hexanol | 0.31 | - | 0.49 | 0.14 | - | 0.34 | 0.37 | - | 0.71 | |||
| Furfuryl alcohol | 0.00 | - | 0.00 | 0.00 | - | 10.71 | 0.49 | - | 0.90 | |||
| 2-Heptanol | 0.35 | - | 0.54 | 0.00 | - | 8.97 | 32.00 | - | 1070.00 | 0.00 | - | 0.00 |
| 2-Hexanol | 0.42 | - | 1.13 | 0.07 | - | 0.18 | ||||||
| 2-Methyl-1-butanol | 0.00 | - | 3.36 | 0.00 | - | 2.75 | 0.10 | - | 3.70 | |||
| 2-Methyl-1-propanol | 0.00 | - | 0.22 | 0.00 | - | 10.33 | ||||||
| 2-Nonanol | 0.04 | - | 0.06 | 0.16 | - | 0.78 | 1.00 | - | 1.00 | |||
| 2-Pentanol | 25.70 | - | 47.70 | 1.52 | - | 4.32 | 0.47 | - | 0.47 | |||
| 2-Phenylethanol | 0.31 | - | 0.55 | 0.00 | - | 6.87 | 63.00 | - | 7500.00 | 3.60 | - | 142.00 |
| 3-Methyl-1-butanol | 1.09 | - | 1.30 | 0.88 | - | 1.86 | 27.00 | - | 238.00 | 0.10 | - | 27.10 |
| 3-Methyl-1-pentanol | 0.63 | - | 7.64 | 0.00 | - | 3.08 | ||||||
| Benzyl alcohol | 0.04 | - | 0.05 | 0.03 | - | 0.07 | 104.00 | - | 104.00 | 0.20 | - | 0.23 |
| Ethanol | 2.17 | - | 3.89 | 1.25 | - | 3.81 | 124.00 | - | 124.00 | 4.06 | - | 6.71 |
| Isobutanol | 0.10 | - | 1.54 | 0.06 | - | 0.14 | ||||||
| Methanol | 0.00 | - | 15.74 | 0.00 | - | 24.41 | 9068.00 | - | 9068.00 | |||
| (E)-3-Hexen-1-ol | 0.00 | - | 43.75 | |||||||||
| Acids | ||||||||||||
| 2-Methylpropanoic acid | 0.00 | - | 0.00 | 0.00 | - | 0.60 | 79.00 | - | 79.00 | 7.70 | - | 48.80 |
| 3-Methylbutanoic acid | 0.05 | - | 0.10 | 3.51 | - | 9.20 | 86.00 | - | 9700.00 | 0.10 | - | 48.10 |
| Acetic acid | 0.68 | - | 1.30 | 4.33 | - | 28.40 | 5.60 | - | 330000.00 | 734.00 | - | 2555.70 |
| Butanoic acid | 0.00 | - | 7.36 | 0.00 | - | 13.10 | 21.00 | - | 570.00 | 1.30 | - | 2555.70 |
| Decanoic acid | 0.00 | - | 1.32 | 0.00 | - | 0.00 | ||||||
| Heptanoic acid | 0.00 | - | 9.79 | 0.00 | - | 0.09 | 31.00 | - | 31.00 | |||
| Hexanoic acid | 0.16 | - | 2.71 | 0.00 | - | 0.50 | 116.00 | - | 116.00 | 0.40 | - | 1.47 |
| Nonanoic acid | 0.00 | - | 10.28 | 0.00 | - | 0.00 | 0.10 | - | 0.10 | |||
| Octanoic acid | 0.03 | - | 0.06 | 0.11 | - | 0.27 | ||||||
| Ketones | ||||||||||||
| 2-Heptanone | 0.66 | - | 1.28 | 0.88 | - | 3.61 | 85.00 | - | 140.00 | 1.10 | - | 5.20 |
| 2-Pentanone | 1.55 | - | 9.73 | 1.01 | - | 2.23 | ||||||
| 2-Undecanone | 0.04 | - | 0.05 | 0.00 | - | 0.03 | 1.00 | - | 1.00 | |||
| Acetoin | 0.38 | - | 0.47 | 1.23 | - | 5.98 | 14.00 | - | 1143.00 | 1.99 | - | 505.20 |
| Acetophenone | 1.17 | - | 3.06 | 0.81 | - | 2.31 | 14.00 | - | 225.00 | |||
| Esters | ||||||||||||
| 1,2-Propanediol diacetate | 6.50 | - | 8.11 | 1.21 | - | 2.53 | ||||||
| Isoamyl acetate | 0.00 | - | 56.50 | 0.00 | - | 17.65 | ||||||
| 2,3-Butanediol diacetate | 0.15 | - | 0.30 | 0.03 | - | 1.20 | ||||||
| 2-Pentanol acetate | 1.42 | - | 2.55 | 1.78 | - | 3.93 | ||||||
| Diethyl malate | 0.00 | - | 0.00 | 0.18 | - | 0.44 | ||||||
| Diethyl succinate | 0.06 | - | 11.65 | 0.00 | - | 0.93 | ||||||
| Ethyl acetate | 0.00 | - | 18.45 | 0.00 | - | 22.82 | 66.00 | - | 66.00 | 1.40 | - | 28.90 |
| Ethyl benzoate | 0.00 | - | 0.02 | 0.13 | - | 0.24 | 2.10 | - | 2.10 | |||
| Ethyl butanoate | 0.26 | - | 3.99 | 0.06 | - | 4.18 | ||||||
| Ethyl caproate | 0.17 | - | 0.22 | 0.43 | - | 0.94 | ||||||
| Ethyl dodecanoate | 0.00 | - | 1.64 | 0.38 | - | 1.77 | 24.00 | - | 24.00 | |||
| Ethyl octanoate | 0.00 | - | 0.03 | 0.00 | - | 74.29 | 3.30 | - | 143.00 | 0.09 | - | 19.10 |
| Ethyl pyruvate | 0.00 | - | 0.88 | 1.78 | - | 20.88 | ||||||
| Ethyl-o-toluate | 0.00 | - | 0.00 | 0.33 | - | 0.62 | ||||||
| Furfuryl acetate | 1.59 | - | 27.04 | 0.13 | - | 3.57 | ||||||
| Hexyl acetate | 0.00 | - | 0.01 | 0.00 | - | 0.04 | ||||||
| Isoamyl benzoate | 0.10 | - | 0.19 | 0.02 | - | 0.56 | ||||||
| Isobutyl acetate | 0.14 | - | 1.97 | 0.06 | - | 1.98 | ||||||
| Methyl octanoate | 0.10 | - | 0.15 | 0.00 | - | 0.00 | ||||||
| Mono-ethyl succinate | 0.00 | - | 1.98 | 0.00 | - | 0.52 | ||||||
| Hexyl butanoate | 0.00 | - | 0.05 | 0.00 | - | 0.00 | ||||||
| Phenyl acetate | 0.00 | - | 0.54 | 0.00 | - | 0.14 | ||||||
| α-Phenylethyl acetate | 0.00 | - | 0.45 | 0.17 | - | 0.89 | 34.00 | - | 930.00 | 2.60 | - | 37.10 |
| Propyl acetate | 0.00 | - | 0.09 | 0.00 | - | 1.34 | ||||||
| β-Phenylethyl acetate | 0.03 | - | 0.12 | 0.73 | - | 1.69 | ||||||
| Terpenes | ||||||||||||
| Carveol | 0.01 | - | 0.05 | 0.00 | - | 0.00 | ||||||
| (Z)-Linalool oxide pyranoid | 0.10 | - | 0.15 | 0.06 | - | 0.17 | ||||||
| (Z)-Linalool oxide furanoid | 0.03 | - | 0.10 | 0.00 | - | 0.10 | 21.00 | - | 21.00 | |||
| Nerylacetone | 0.02 | - | 0.04 | 0.00 | - | 0.00 | ||||||
| Limonene | 6.65 | - | 12.37 | 6.43 | - | 30.60 | ||||||
| Geraniol | 0.00 | - | 0.31 | 0.00 | - | 0.00 | ||||||
| Limonene epoxide | 0.29 | - | 0.90 | 0.00 | - | 0.02 | ||||||
| Sabinene | 0.05 | - | 0.17 | 0.05 | - | 0.30 | ||||||
| α-Caryophyllene | 0.08 | - | 0.09 | 0.07 | - | 0.18 | ||||||
| α-Citral | 0.03 | - | 0.10 | 0.02 | - | 0.15 | ||||||
| α-Limonene diepoxide | 0.00 | - | 0.02 | 0.00 | - | 0.02 | ||||||
| β-Caryophyllene | 0.01 | - | 0.02 | 0.01 | - | 0.03 | ||||||
| β-Citronellol | 0.00 | - | 0.00 | 0.00 | - | 0.41 | ||||||
| β-Myrcene | 1.98 | - | 2.32 | 0.96 | - | 3.14 | 66.00 | - | 66.00 | |||
| (E)-β-ocimene | 0.08 | - | 0.34 | 0.06 | - | 0.51 | ||||||
| Lactones | ||||||||||||
| Δ-Decalactone | 0.00 | - | 0.20 | |||||||||
| Other compounds | ||||||||||||
| 1,1-Diethoxyethane | 0.06 | - | 21.65 | 0.12 | - | 5.83 | ||||||
| o-Guaiacol | 0.00 | - | 0.01 | 0.02 | - | 0.62 | 230.00 | - | 230.00 | |||
| Phenol | 0.02 | - | 0.03 | 0.02 | - | 0.37 | 7.00 | - | 7.00 | |||
| trans-Methyl dihydrojasmonate | 0.02 | - | 0.04 | 0.02 | - | 0.04 | ||||||
| Source | Country | Variety | Equipment | Roasting conditions | |
|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | ||||
| Bonhevi et al., 2005 [60] | Ghana, Cameroon, Ivory Coast, Brazil and Ecuador | NM | GC-MS | 130 | 48 |
| Ramli et al., 2006 [59] | Malaysia | NM | GC-MSD | 150 | 30 |
| Frauendorfer and Shieberle, 2008 [63] | Grenada | Criollo | HRGC-MS | 95 | 14 |
| Huang and Barringer, 2011 [61] | Ecuador | NM | SIFT-MS | 150 | 30 |
| Van Durme et al., 2016 [62] | Ghana and Tanzania | NM | HS-SPME-GC-MS | 150 | 30 |
| Magagna et al., 2018 [65] | Mexico | NM | HS-SPME-GCxGC-MS | 100–130 | 20–40 |
| Tan and Kerr, 2018 [64] | United States of America | Forastero | GC-MS and ANN-based-e-nose | 135 | 0–40 |
| Magagna et al., 2017 [48] | Ecuador and Mexico | Trinitario hybrids | GCxGC-MS, GCx2GC-MS/FID | nm | nm |
| Group | VOCs | Microorganism | Reference |
|---|---|---|---|
| Alcohol | 2-heptanol | Saccharomyces cerevisiae | Cappaert and Laroche, 2004 [75] |
| 2-phenylethanol | Candida tropicalis | Koné et al., 2016 [27] | |
| Galactomyces geotrichum | Koné et al., 2016 [27] | ||
| Geotrichum candidum | Janssens et al., 1992 [66] | ||
| Hanseniaspora guilliermondii | Moreira et al., 2005 [73] | ||
| Hanseniaspora uvarum | Moreira et al., 2005 [73] | ||
| Kluyveromyces lactis | Janssens et al., 1992 [66], Fabre et al., 1997 [74] | ||
| Kluyveromyces marxianus | Janssens et al., 1992 [66], Whittmann et al., 2002 [72], Etschman et al., 2005 [71], Fabre et al., 1997 [74] | ||
| Pichia anomala | Janssens et al., 1992 [66] | ||
| Pichia farinosa | Janssens et al., 1992 [66] | ||
| Pichia galeiformis | Koné et al., 2016 [27] | ||
| Pichia kudriavzevii | Koné et al., 2016 [27] | ||
| Saccharomyces cerevisiae | Kim et al., 2014 [70], Koné et al., 2016 [27], Schwan and Wheals, 2004 [7], Moreira et al., 2005 [73], Fabre et al., 1997 [74] | ||
| Wickerhamomyces anomalus | Koné et al., 2016 [27] | ||
| Aldehydes | 2-methylbutanal | Saccharomyces cerevisiae | Janssens et al., 1992 [66], Larroy et al., 2002 [78] |
| Benzaldehyde | Agaricus bisporus | Janssens et al., 1992 [66] | |
| Bjerkandera adusta | Lapadatescu et al., 1997 [79] | ||
| Dichomitus squales | Lapadatescu et al., 1997 [79] | ||
| Galactomyces geotrichum | Koné et al., 2016 [27] | ||
| Ischnoderma benzoinum | Lapadatescu et al., 1997 [79] | ||
| Pichia pastoris | Berger, 2007 [68] | ||
| Polyporus tuberaster | Kawabe and Morita, 1994 [80] | ||
| Saccharomyces carlsbergensis | Pal et al., 2009 [81] | ||
| Phenylacetaldehyde | Kluyveromyces marxianus | Etschman et al., 2005 [71] | |
| Acetobacter | Berger, 2007 [68] | ||
| Ester | Ethyl acetate | Candida tropicalis | Koné et al., 2016 [27] |
| Candida utilis | Janssens et al., 1992 [66] | ||
| Geotrichum candidum | Janssens et al., 1992 [66] | ||
| Hanseniaspora guilliermondii | Rojas et al., 2001 [82] | ||
| Hanseniaspora uvarum | Rojas et al., 2001 [82] | ||
| Kloeckera apiculate | Schwan and Wheals, 2004 [7] | ||
| Pichia anomala | Janssens et al., 1992 [66], Rojas et al., 2001 [82] | ||
| Pichia farinosa | Janssens et al., 1992 [66] | ||
| Pichia kudriavzevii | Koné et al., 2016 [27], Pereira et al., 2017 [25] | ||
| Saccharomyces cerevisiae | Janssens et al., 1992 [66], Koné et al., 2016 [27], Rojas et al., 2001 [82], Schwan and Wheals, 2004 [7] | ||
| Wickerhamomyces anomalus | Koné et al., 2016 [27] | ||
| Kluyveromyces lactis | Van Laere et al., 2008 [83] | ||
| 2-Phenylethyl acetate | Cladosporium cladosporoides | Janssens et al., 1992 [66] | |
| Geotrichum candidum | Janssens et al., 1992 [66] | ||
| Hanseniaspora guilliermondii | Rojas et al., 2001 [82], Moreira et al., 2005 [73] | ||
| Hanseniaspora uvarum | Rojas et al., 2001 [82] | ||
| Kluyveromyces marxianus | Janssens et al., 1992 [66], Whittmann et al., 2002 [72], Etschman et al., 2005 [71] | ||
| Pichia anomala | Janssens et al., 1992 [66], Rojas et al., 2001 [82] | ||
| Pichia farinosa | Janssens et al., 1992 [66] | ||
| Saccharomyces cerevisiae | Kone et al., 2016 [27], Rojas et al., 2001 [82] | ||
| Terpenoid | Limonene | Ascoidea hylecoeti | Janssens et al., 1992 [66] |
| Limonene metabolites (terpineol, verbenol) | Armillareira, Aspergillus Cladosporium | Duetz et al., 2003 [84], Janssens et al., 1992 [66] | |
| Limonene metabolites (limonene-1,2-epoxide) | Corynespora Diplodia | Duetz et al., 2003 [84] | |
| Limonene metabolites (verbenone) | Hormonema | Berger, 2007 [68] | |
| Limonene metabolites (carvone, carveol) | Penicillium Pleutotus | Janssens et al., 1992 [66], Duetz et al., 2003 [84] | |
| Limonene metabolites | Pichia angula Ambrosiozyma Fusarium | Janssens et al., 1992 [66] Berger, 2007 [68] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Gutierrez, J.; Barbosa-Pereira, L.; Ferrocino, I.; Cocolin, L. Traceability of Functional Volatile Compounds Generated on Inoculated Cocoa Fermentation and Its Potential Health Benefits. Nutrients 2019, 11, 884. https://doi.org/10.3390/nu11040884
Mota-Gutierrez J, Barbosa-Pereira L, Ferrocino I, Cocolin L. Traceability of Functional Volatile Compounds Generated on Inoculated Cocoa Fermentation and Its Potential Health Benefits. Nutrients. 2019; 11(4):884. https://doi.org/10.3390/nu11040884
Chicago/Turabian StyleMota-Gutierrez, Jatziri, Letricia Barbosa-Pereira, Ilario Ferrocino, and Luca Cocolin. 2019. "Traceability of Functional Volatile Compounds Generated on Inoculated Cocoa Fermentation and Its Potential Health Benefits" Nutrients 11, no. 4: 884. https://doi.org/10.3390/nu11040884





