Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies
Abstract
:1. Introduction
2. Methods
3. Clinical Evidence Regarding the Impact of Oral Supplementation with Saffron or One of Its Constituents on Vision-Related Parameters in Adults with Ocular Diseases
4. Action Time-Course of Oral Saffron Supplementation
5. Safety Profile of Oral Saffron Supplementation
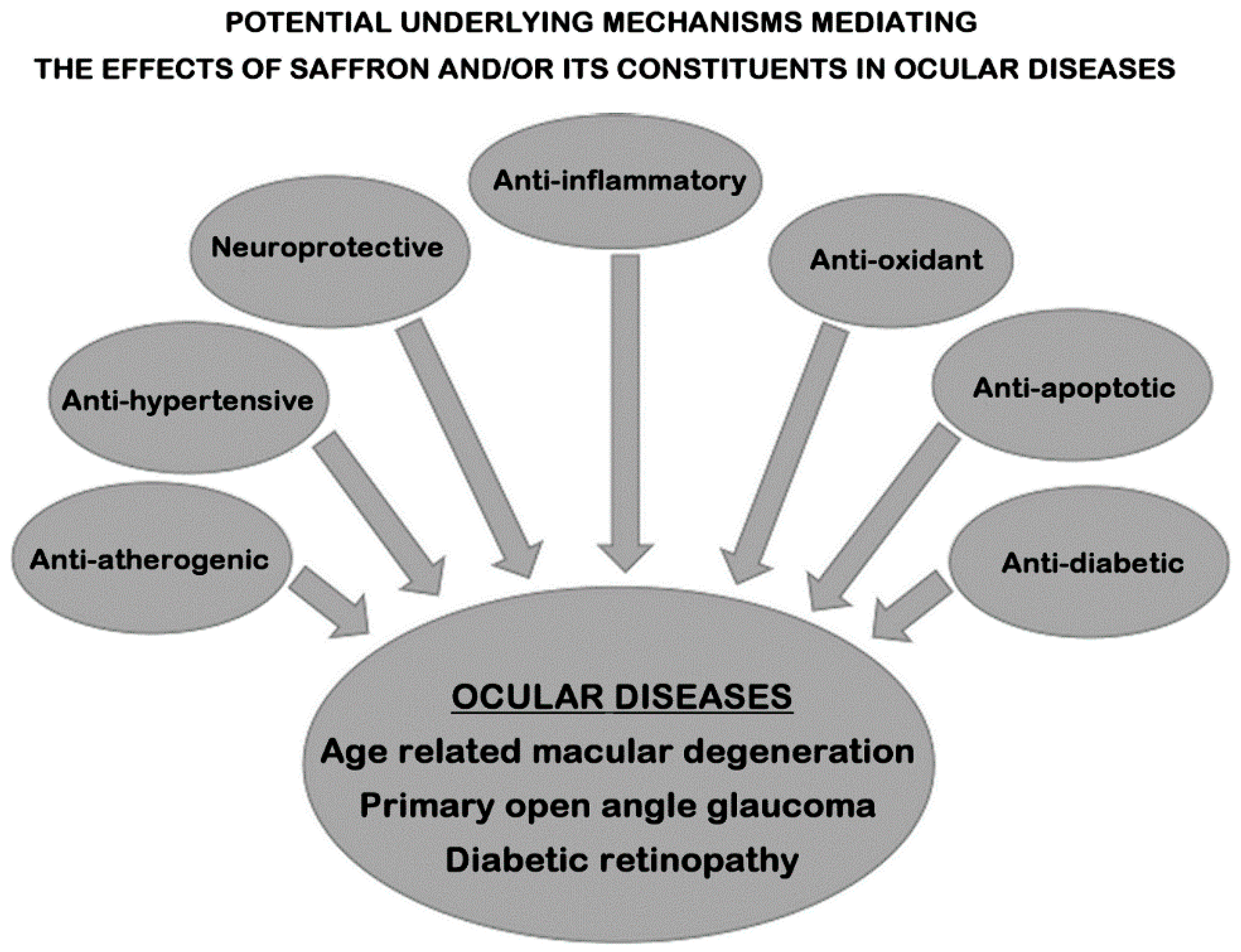
6. Proposed Mechanisms/Pathways Mediating the Effects of Saffron and/or Its Constituents in Ocular Diseases
7. Summary of the Literature
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Vision Loss Expert Group. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Wong, T.Y.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 17, 16012. [Google Scholar] [CrossRef]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxid. Med. Cell. Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278973/ (accessed on 28 February 2019).
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. (Lond.) 2016, 3, 34. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Tie, L.J.; Wu, S.S.; Lv, P.L.; Huang, H.W.; Wang, W.Q.; Wang, H.; Ma, L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1276–1283. [Google Scholar] [CrossRef]
- Adams, M.K.; Simpson, J.A.; Aung, K.Z.; Makeyeva, G.A.; Giles, G.G.; English, D.R.; Hopper, J.; Guymer, R.H.; Baird, P.N.; Robman, L.D. Abdominal obesity and age-related macular degeneration. Am. J. Epidemiol. 2011, 173, 1246–1255. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2018. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martínez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Ehrlich, R.; Harris, A.; Kheradiya, N.S.; Winston, D.M.; Ciulla, T.A.; Wirostko, B. Age-related macular degeneration and the aging eye. Clin. Interv. Aging 2008, 3, 473–482. [Google Scholar] [PubMed]
- Broadhead, G.K.; Grigg, J.R.; Chang, A.A.; McCluskey, P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr. Rev. 2015, 73, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y. Nutrition effects on ocular diseases in the aging eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF42–ORSF47. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Mann, S.N.; Mandal, N.A. Botanical compounds: Effects on major eye diseases. Evid.-Based Complement. Altern. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [PubMed]
- José Bagur, M.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef]
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C.; Giner, R.M.; Máñez, S. An Update Review of Saffron and its Active Constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iran. J. Basic Med. Sci. 2018, 21, 1091–1099. [Google Scholar] [PubMed]
- WHO. Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2007; Volume 3, Available online: http://apps.who.int/medicinedocs/en/m/abstract/Js14213e/ (accessed on 28 February 2019).
- Ghaffari, S.; Roshanravan, N. Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother. 2019, 109, 21–27. [Google Scholar] [CrossRef]
- Poma, A.; Fontecchio, G.; Carlucci, G.; Chichiriccò, G. Anti-inflammatory properties of drugs from saffron crocus. Antiinflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Pourmasoumi, M.; Hadi, A.; Najafgholizadeh, A.; Kafeshani, M.; Sahebkar, A. Clinical evidence on the effects of saffron (crocus sativus L.) on cardiovascular risk factors: A systematic review meta-analysis. Pharmacol. Res. 2019, 139, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Chang, A.; Grigg, J.; McCluskey, P. Efficacy and Safety of Saffron Supplementation: Current Clinical Findings. Crit. Rev. Food Sci. Nutr. 2016, 56, 2767–2776. [Google Scholar] [CrossRef]
- Rahiman, N.; Akaberi, M.; Sahebkar, A.; Emami, S.A.; Tayarani-Najaran, Z. Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc. Res. 2018, 118, 82–89. [Google Scholar] [CrossRef]
- Pashirzad, M.; Shafiee, M.; Avan, A.; Ryzhikov, M.; Fiuji, H.; Bahreyni, A.; Khazaei, M.; Soleimanpour, S.; Hassanian, S.M. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J. Cell. Physiol. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hany, H.O.; Atef, H.; Said, E.; Elkashef, H.A.; Salem, H.A. Crocin mediated amelioration of oxidative burden and inflammatory cascade suppresses diabetic nephropathy progression in diabetic rats. Chem. Biol. Interact. 2018, 284, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Hosseinzadeh, H. Saffron: A promising natural medicine in the treatment of metabolic syndrome. J. Sci. Food Agric. 2017, 97, 1679–1685. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A Longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid.-Based Complement. Altern. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, D.; Falsini, B.; Piccardi, M.; Ambrosio, L.; Minnella, A.M.; Savastano, M.C.; Bisti, S.; Maccarone, R.; Fadda, A.; Mello, E.; et al. Functional effect of saffron supplementation and risk genotypes in early age-related macular degeneration: A preliminary report. J. Transl. Med. 2013, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Lashay, A.; Sadough, G.; Ashrafi, E.; Lashay, M.; Movassat, M.; Akhondzadeh, S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: A double-blind, Placebo-controlled, randomized trial. Med. Hypothesis Discov. Innov. Ophthalmol. 2016, 5, 32. [Google Scholar] [PubMed]
- Riazi, A.; Panahi, Y.; Alishiri, A.A.; Hosseini, M.A.; Zarchi, A.A.K.; Sahebkar, A. The impact of saffron (crocus sativus) supplementation on visual function in patients with dry age-related macular degeneration. Ital. J. Med. 2017, 11, 758. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Grigg, J.R.; McCluskey, P.; Hong, T.; Schlub, T.E.; Chang, A.A. Saffron Therapy for the treatment of mild/ moderate age-related macular degeneration: A randomized clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, M.H.J.; Yazdani, S.; Saadat, S. The ocular hypotensive effect of saffron extractin primary open angle glaucoma: A pilot study. BMC Complement. Altern. Med. 2014, 14, 399. [Google Scholar]
- Sepahi, S.; Mohajeri, S.A.; Hosseini, S.M.; Khodaverdi, E.; Shoeibi, N.; Namdari, M.; Tabassi, S.A.S. Effects of crocin on diabetic maculopathy: A placebo-controlled randomized clinical trial. Am. J. Ophthalmol. 2018, 190, 89–98. [Google Scholar] [CrossRef]
- Di Marco, F.; Romeo, S.; Nandasena, C.; Purushothuan, S.; Adams, C.; Bisti, S.; Stone, J. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am. J. Neurodegener. Dis. 2013, 2, 208–220. [Google Scholar]
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical applications of saffron (crocus sativus) and its constituents: A review. Drug Res. 2015, 65, 287–295. [Google Scholar] [CrossRef]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Vis. Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Betti, G.; Hensel, A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien. Med. Wochenschr. 2007, 157, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, A.H.; Ayati, Z.; Parizadeh, M.R.; Rajbai, O.; Hosseinzadeh, H. Safety Evaluation of Crocin (a constituent of saffron) Tablets in Healthy Volunteers. Iran. J. Basic Med. Sci. 2013, 16, 39–46. [Google Scholar] [PubMed]
- Modaghegh, M.H.; Shahabian, M.; Esmaeili, H.A.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008, 15, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Heekin, K.; Mutchie, H.L.; Anton, S. A systematic review of randomized controlled trials examining the effectiveness of saffron (Crocus sativus L.) on psychological and behavioral outcomes. J. Integr. Med. 2015, 13, 231–240. [Google Scholar] [CrossRef]
- Ernst, E. Herbal medicinal products during pregnancy: Are they safe? BJOG 2002, 109, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, D.; Griffiths, H.R.; Hilton, E.J.; Cunliffe, I.A.; Hosking, S.L. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, H.K. Crocin ameliorates atopic dermatitis symptoms by down regulation of Th2 response via blocking of NF-κB/STAT6 signaling pathways in mice. Nutrients 2018, 10, 1625. [Google Scholar] [CrossRef]
- Lv, B.; Huo, F.; Zhu, Z.; Xu, Z.; Dang, X.; Chen, T.; Zhang, T.; Yang, X. Crocin Upregulates CX3CR1 expression by suppressing NF-κB/YY1 signaling and inhibiting lipopolysaccharide-induced microglial activation. Neurochem. Res. 2016, 41, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kakkar, R.; Wang, J. In vivo and in vitro approach to anti-arthritic and anti-inflammatory effect of crocetin by alteration of nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 and NF-κB expression. Front. Pharmacol. 2018, 9, 1341. [Google Scholar] [CrossRef]
- Giaccio, M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef]
- Xuan, B.; Zhou, Y.H.; Li, N.; Min, Z.D.; Chiou, G.C. Effects of crocin analogs on ocular blood flow and retinal function. J. Ocul. Pharmacol. Ther. 1999, 15, 143–152. [Google Scholar] [CrossRef]
- Izzotti, A.; Saccà, S.C.; Longobardi, M.; Cartiglia, C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5251–5258. [Google Scholar] [CrossRef]
- Saccà, S.C.; Gandolfi, S.; Bagnis, A.; Manni, G.; Damonte, G.; Traverso, C.E.; Izzotti, A. The Outflow Pathway: A Tissue with Morphological and Functional Unity. J. Cell. Physiol. 2016, 231, 1876–1893. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Zare, V.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. Antidiabetic potential of saffron and its active constituents. J. Cell. Physiol. 2019, 234, 8610–8617. [Google Scholar] [CrossRef]
- Corso, L.; Cavallero, A.; Baroni, D.; Garbati, P.; Prestipino, G.; Bisti, S.; Nobile, M.; Picco, C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016, 12, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef]
- Laabich, A.; Vissvesvaran, G.P.; Lieu, K.L.; Murata, K.; McGinn, T.E.; Manmoto, C.C.; Sinclair, J.R.; Karliga, I.; Leung, D.W.; Fawzi, A.; et al. Protective effect of crocin against lue light-and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3156–3163. [Google Scholar] [CrossRef]
- Waugh, N.; Loveman, E.; Colquitt, J.; Royle, P.; Yeong, J.L.; Hoad, G.; Lois, N. Treatments for dry age-related macular degeneration and Stargardt disease: A systematic review. Health Technol. Assess. 2018, 22, 1–167. [Google Scholar] [CrossRef]
- Potnuri, A.G.; Allakonda, L.; Lahkar, M. Crocin attenuates cyclophosphamide induced testicular toxicity by preserving glutathione redox system. Biomed. Pharmacother. 2018, 101, 1740180. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Xu, G.; Zheng, S.; Sun, S.; Wen, N.; Sheng, L.; Shi, Y.; Zhang, Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007, 18, 64–72. [Google Scholar] [CrossRef]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J. Agric. Food Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef]
- Umigai, N.; Murakami, K.; Ulit, M.V.; Antonio, L.S.; Shirotori, M.; Morikawa, H.; Nakano, T. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine 2011, 18, 575–578. [Google Scholar] [CrossRef]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D.; Inarejos-García, A.M.; Prodanov, M. affron®, a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2018, 232, 349–357. [Google Scholar] [CrossRef]
- Tóth, B.; Hegyi, P.; Lantos, T.; Szakács, Z.; Kerémi, B.; Varga, G.; Tenk, J.; Pétervári, E.; Balaskó, M.; Rumbus, Z.; et al. The Efficacy of Saffron in the Treatment of Mild to Moderate Depression: A Meta-analysis. Planta Med. 2019, 85, 24–31. [Google Scholar] [CrossRef]
- Chen, X.; Lu, L. Depression in Diabetic Retinopathy: A Review and Recommendation for Psychiatric Management. Psychosomatics 2016, 57, 465–471. [Google Scholar] [CrossRef]
- Musch, D.C.; Niziol, L.M.; Janz, N.K.; Gillespie, B.W. Trends in and Predictors of Depression Among Participants in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am. J. Ophthalmol. 2019, 197, 128–135. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Food Supplements. 2019. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 28 February 2019).
- Office of Dietary Supplements (ODS). Dietary Supplements. Background Information. 2011. Available online: https://ods.od.nih.gov/factsheets/dietarysupplements-healthprofessional/#disc (accessed on 28 February 2019).
| Parameters | Descriptions |
|---|---|
| Population/Participants/Problem | Adults with ocular disease |
| Intervention | Any intervention with oral administration of saffron or one of its constituents |
| Comparison | Studies with any comparator/control that incorporated a non-intervention group or studies with a pre- vs. post-intervention comparison without a comparator/control group |
| Outcomes | Vision-related outcome measures, such as visual acuity, visual field parameters, contrast sensitivity, electrophysiology parameters (ERG, fERG, mfERG), macular thickness measures, and IOP |
| Setting | Clinical studies/trials |
| Ocular Disease | Number of Subjects | Constituent Dosage | Study Design | Primary Outcome Measures—Findings | Proposed Mechanisms | Reference |
|---|---|---|---|---|---|---|
| AMD [bilateral early AMD] | N = 25 | Saffron 20 mg daily | Double-blind, placebo controlled, cross over, RCT three-month period with cross over for another three months | fERG: increased amplitude in saffron, but not in placebo group BCVA: increased (one line) in saffron, but not in placebo group | Anti-oxidant Neuroprotective | Falsini et al. (2010) [32] |
| AMD [bilateral early AMD] | N = 29 | Saffron 20 mg daily | Longitudinal interventional open-label study three monthly follow-ups over a 15-month period | fERG: increased amplitude that stabilized after three months BCVA: increased (two lines) that stabilized after three months | Anti-oxidant Neuroprotective | Piccardi et al. (2012) [33] |
| AMD [bilateral early AMD] | N = 33 | Saffron 20 mg daily | Longitudinal, 3 monthly follow-ups over a 12-month period | fERG: increased amplitude and sensitivity amplitude that stabilized after three months independent of genotype | Anti-oxidant Anti-inflammatory Neuroprotective | Marangoni et al. (2013) [34] |
| AMD [dry and wet AMD] | N = 40 | Saffron 15 mg twice daily | Placebo controlled, RCT six-month period with follow-ups at three and six months | CMT: decreased in saffron and placebo groups in wet AMD, but not in dry AMD ERG: amplitude increased in the saffron group (dry and wet AMD) compared to placebo after three months, but not six months | Neuroprotective Anti-depressant | Lashay et al. (2016) [35] |
| AMD [mild/moderate dry AMD] | N = 54 | Saffron 50 mg daily | Placebo controlled, RCT three months | CMT: unchanged BCVA: increased (one line) in saffron, but not in placebo group CS: increased in saffron, but not in placebo group | Anti-oxidant Hemorheological activity | Riazi et al. (2017) [36] |
| AMD [mild/moderate AMD] | N = 96 | Saffron 20 mg daily | Double-blind, placebo controlled, cross over, RCT three months followed by cross over into the other arm for three months | BCVA: increased in saffron group [and AREDS * + saffron], but not in placebo mfERG response density: increased in AREDS+saffron, but not in the saffron or placebo group mfERG latency: decreased in saffron group, but not in placebo group | Anti-oxidant Neuroprotective | Broadhead et al. (2019) [37] |
| POAG [clinically stable POAG] | N = 34 | Saffron 30 mg daily | Double-blind, placebo controlled RCT 1 month duration 1 month wash-out | IOP: reduction after three and four weeks compared to placebo IOP returned to pre-intervention levels after a 4-week wash out period | Antioxidant Neuroprotective | Bonyadi et al. (2014) [38] |
| DME | N = 60 (101 DME eyes) | Crocin 5 mg or 15 mg daily | Double-masked, placebo controlled, phase 2 RCT 3 months | CMT: significantly decreased after three months compared to placebo only in the 15 mg group BCVA: significantly improved after three months compared to placebo only in the 15 mg group HbA1c and FBG: significantly decreased after three months compared to placebo only in the 15 mg group | Anti-oxidant Hemorheological activity Anti-inflammatory | Sepahi et al. (2018) [39] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. https://doi.org/10.3390/nu11030649
Heitmar R, Brown J, Kyrou I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients. 2019; 11(3):649. https://doi.org/10.3390/nu11030649
Chicago/Turabian StyleHeitmar, Rebekka, James Brown, and Ioannis Kyrou. 2019. "Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies" Nutrients 11, no. 3: 649. https://doi.org/10.3390/nu11030649




