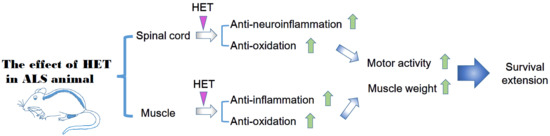
Hochu-Ekki-To Improves Motor Function in an Amyotrophic Lateral Sclerosis Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hochu-Ekki-To (HET) Treatment
2.3. Rota-Rod Test
2.4. Foot Print Test
2.5. Survival Test
2.6. Tissue Preparation
2.7. Western Blotting
2.8. H&E Staining and Immunohistohcemistry
2.9. Statistical Analysis
3. Results
3.1. Hochu-Ekki-(HET) Extended Survival and Improved Motor Function
3.2. Hochu-Ekki-To (HET) Reduces Neuroinflammation and Oxidative Stress in the Spinal Cord of hSOD1G93A Mice
3.3. Hochu-Ekki-To (HET) Attenuates Muscle Dysfunction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orsini, M.; Oliveira, A.B.; Nascimento, O.J.; Reis, C.H.; Leite, M.A.; de Souza, J.A.; Pupe, C.; de Souza, O.G.; Bastos, V.H.; de Freitas, M.R.; et al. Amyotrophic Lateral Sclerosis: New Perpectives and Update. Neurol. Int. 2015, 7, 5885. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, W.; Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Mesika, R.; Reichmann, D. When safeguarding goes wrong: Impact of oxidative stress on protein homeostasis in health and neurodegenerative disorders. Adv. Protein Chem. Struct. Biol. 2019, 114, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, M.; Gerardi, F.; Santus, W.; Lizio, A.; Sansone, V.A.; Lunetta, C.; Zanoni, I.; Granucci, F. Inflammatory role of dendritic cells in Amyotrophic Lateral Sclerosis revealed by an analysis of patients’ peripheral blood. Sci. Rep. 2017, 7, 7853. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.E.; Baloh, R.H. Inflammation in ALS/FTD pathogenesis. Acta. Neuropathol. 2019, 137, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.S.; Wosiski-Kuhn, M.; Gillespie, R.; Caress, J.; Milligan, C. Inflammation, Immunity, and amyotrophic lateral sclerosis: I. Etiology and pathology. Muscle Nerve 2019, 59, 10–22. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Boillee, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 2006, 312, 1389–1392. [Google Scholar] [CrossRef]
- Zhao, W.; Beers, D.R.; Hooten, K.G.; Sieglaff, D.H.; Zhang, A.; Kalyana-Sundaram, S.; Traini, C.M.; Halsey, W.S.; Hughes, A.M.; Sathe, G.M.; et al. Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes. JAMA Neurol. 2017, 74, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Patin, F.; Baranek, T.; Vourc’h, P.; Nadal-Desbarats, L.; Goossens, J.F.; Marouillat, S.; Dessein, A.F.; Descat, A.; Hounoum, B.M.; Bruno, C.; et al. Combined Metabolomics and Transcriptomics Approaches to Assess the IL-6 Blockade as a Therapeutic of ALS: Deleterious Alteration of Lipid Metabolism. Neurotherapeutics 2016, 13, 905–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbour, D.; Vande Velde, C.; Robitaille, R. New perspectives on amyotrophic lateral sclerosis: The role of glial cells at the neuromuscular junction. J. Physiol. 2017, 595, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Nardo, G.; Trolese, M.C.; de Vito, G.; Cecchi, R.; Riva, N.; Dina, G.; Heath, P.R.; Quattrini, A.; Shaw, P.J.; Piazza, V.; et al. Immune response in peripheral axons delays disease progression in SOD1(G93A) mice. J. Neuroinflammation 2016, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Heurich, B.; El Idrissi, N.B.; Donev, R.M.; Petri, S.; Claus, P.; Neal, J.; Morgan, B.P.; Ramaglia, V. Complement upregulation and activation on motor neurons and neuromuscular junction in the SOD1 G93A mouse model of familial amyotrophic lateral sclerosis. J. Neuroimmunol. 2011, 235, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.N.; Navarro, D.S.; Barbosa-Filho, J.M. Plants with central analgesic activity. Phytomedicine 2001, 8, 310–322. [Google Scholar] [CrossRef]
- Bahmani, M.; Shirzad, H.; Majlesi, M.; Shahinfard, N.; Rafieian-Kopaei, M. A review study on analgesic applications of Iranian medicinal plants. Asian Pac. J. Trop. Med. 2014, 7S1, S43–S53. [Google Scholar] [CrossRef]
- Zeng, Q.; Siu, W.; Li, L.; Jin, Y.; Liang, S.; Cao, M.; Ma, M.; Wu, Z. Autophagy in Alzheimer’s disease and promising modulatory effects of herbal medicine. Exp. Gerontol. 2019, 119, 100–110. [Google Scholar] [CrossRef]
- Qiu, H.; Li, J.H.; Yin, S.B.; Ke, J.Q.; Qiu, C.L.; Zheng, G.Q. Dihuang Yinzi, a Classical Chinese Herbal Prescription, for Amyotrophic Lateral Sclerosis: A 12-Year Follow-up Case Report. Medicine (Baltimore) 2016, 95, e3324. [Google Scholar] [CrossRef]
- Pan, W.; Su, X.; Bao, J.; Wang, J.; Zhu, J.; Cai, D.; Yu, L.; Zhou, H. Open Randomized Clinical Trial on JWSJZ Decoction for the Treatment of ALS Patients. Evid. Based Complement Alternat. Med. 2013, 2013, 347525. [Google Scholar] [CrossRef]
- Yae, S.; Takahashi, F.; Yae, T.; Yamaguchi, T.; Tsukada, R.; Koike, K.; Minakata, K.; Murakami, A.; Nurwidya, F.; Kato, M.; et al. Hochuekkito (TJ-41), a Kampo Formula, Ameliorates Cachexia Induced by Colon 26 Adenocarcinoma in Mice. Evid. Based Complement Alternat. Med. 2012, 2012, 976926. [Google Scholar] [CrossRef] [PubMed]
- Dan, K.; Akiyoshi, H.; Munakata, K.; Hasegawa, H.; Watanabe, K. A Kampo (traditional Japanese herbal) medicine, Hochuekkito, pretreatment in mice prevented influenza virus replication accompanied with GM-CSF expression and increase in several defensin mRNA levels. Pharmacology 2013, 91, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Kao, T.I.; Chiang, B.L.; Chen, H.Y.; Chen, K.H.; Chen, J.L. Immune-modulatory effects of bu-zhong-yi-qi-tang in ovalbumin-induced murine model of allergic asthma. PLoS ONE 2015, 10, e0127636. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, L.; Yao, Z.; Qin, Z.; Idehara, M.; Dai, Y.; Kiyohara, H.; Yamada, H.; Yao, X. Mucosal immunomodulatory evaluation and chemical profile elucidation of a classical traditional Chinese formula, Bu-Zhong-Yi-Qi-Tang. J. Ethnopharmacol. 2019, 228, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Shinozuka, N.; Nakayama, K.; Sekiya, N.; Kuriyama, T.; Fukuchi, Y. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary disease. J. Am. Geriatr. Soc. 2009, 57, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Utsuyama, M.; Seidlar, H.; Kitagawa, M.; Hirokawa, K. Immunological restoration and anti-tumor effect by Japanese herbal medicine in aged mice. Mech. Ageing Dev. 2001, 122, 341–352. [Google Scholar] [CrossRef]
- Suzuki, T.; Takano, I.; Nagai, F.; Fujitani, T.; Ushiyama, K.; Okubo, T.; Seto, T.; Ikeda, S.; Kano, I. Suppressive effects of Hochu-ekki-to, a traditional Chinese medicine, on IgE production and histamine release in mice immunized with ovalbumin. Biol. Pharm. Bull. 1999, 22, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Kawakita, T.; Yamaoka, Y.; Nomoto, K. Development of the susceptibility to oral tolerance induction in infant mice administered a herbal drug, Hochu-ekki-to (Bu-Zhong-Yi-Qi-Tang). Int. Immunopharmacol. 2001, 1, 219–227. [Google Scholar] [CrossRef]
- Kiyohara, H.; Nagai, T.; Munakata, K.; Nonaka, K.; Hanawa, T.; Kim, S.J.; Yamada, H. Stimulating effect of Japanese herbal (kampo) medicine, hochuekkito on upper respiratory mucosal immune system. Evid. Based Complement Alternat. Med. 2006, 3, 459–467. [Google Scholar] [CrossRef]
- Jiang, J.H.; Yang, E.J.; Baek, M.G.; Kim, S.H.; Lee, S.M.; Choi, S.M. Anti-inflammatory effects of electroacupuncture in the respiratory system of a symptomatic amyotrophic lateral sclerosis animal model. Neurodegener. Dis. 2011, 8, 504–514. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Lee, S.H.; Yang, E.J. Bojungikgi-tang Improves Muscle and Spinal Cord Function in an Amyotrophic Lateral Sclerosis Model. Mol. Neurobiol. 2019, 56, 2394–2407. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Jiang, J.H.; Lee, S.M.; Yang, S.C.; Hwang, H.S.; Lee, M.S.; Choi, S.M. Bee venom attenuates neuroinflammatory events and extends survival in amyotrophic lateral sclerosis models. J. Neuroinflammation 2010, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Choi, S.M.; Yang, E.J. The effects of bee venom acupuncture on the central nervous system and muscle in an animal hSOD1G93A mutant. Toxins (Basel) 2015, 7, 846–858. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, E.J. Anti-Neuroinflammatory Effect of Jaeumganghwa-Tang in an Animal Model of Amyotrophic Lateral Sclerosis. Evid. Based Complement Alternat. Med. 2019, 2019, 1893526. [Google Scholar] [CrossRef]
- Cai, M.; Yang, E.J. Gamisoyo-San Ameliorates Neuroinflammation in the Spinal Cord of hSOD1(G93A) Transgenic Mice. Mediators Inflamm. 2018, 2018, 5897817. [Google Scholar] [CrossRef]
- Schafer, D.P.; Stevens, B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015, 7, a020545. [Google Scholar] [CrossRef]
- Weiss, A.; Attisano, L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Abeta in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef]
- Henkel, J.S.; Beers, D.R.; Zhao, W.; Appel, S.H. Microglia in ALS: The good, the bad, and the resting. J. Neuroimmune Pharmacol. 2009, 4, 389–398. [Google Scholar] [CrossRef]
- Gordon, P.H.; Moore, D.H.; Miller, R.G.; Florence, J.M.; Verheijde, J.L.; Doorish, C.; Hilton, J.F.; Spitalny, G.M.; MacArthur, R.B.; Mitsumoto, H.; et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: A phase III randomised trial. Lancet Neurol. 2007, 6, 1045–1053. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Son, Y.J.; Sanes, J.R.; Lichtman, J.W. Nerve terminals form but fail to mature when postsynaptic differentiation is blocked: In vivo analysis using mammalian nerve-muscle chimeras. J. Neurosci. 2000, 20, 6077–6086. [Google Scholar] [CrossRef] [PubMed]
- Halter, B.; Gonzalez de Aguilar, J.L.; Rene, F.; Petri, S.; Fricker, B.; Echaniz-Laguna, A.; Dupuis, L.; Larmet, Y.; Loeffler, J.P. Oxidative stress in skeletal muscle stimulates early expression of Rad in a mouse model of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2010, 48, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Dadon-Nachum, M.; Melamed, E.; Offen, D. The “dying-back” phenomenon of motor neurons in ALS. J. Mol. Neurosci. 2011, 43, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzo, S.; Zucca, I.; Mastropietro, A.; de Rosbo, N.K.; Cavalcante, P.; Tartari, S.; Bonanno, S.; Preite, L.; Mantegazza, R.; Bernasconi, P. Hind limb muscle atrophy precedes cerebral neuronal degeneration in G93A-SOD1 mouse model of amyotrophic lateral sclerosis: A longitudinal MRI study. Exp. Neurol. 2011, 231, 30–37. [Google Scholar] [CrossRef]
- Dupuis, L.; Pradat, P.F.; Ludolph, A.C.; Loeffler, J.P. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011, 10, 75–82. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. Overnutrition and undernutrition as modifiers of metabolic processes in disease states. Am. J. Clin. Nutr. 2000, 72, 533S–537S. [Google Scholar] [CrossRef]
- Dupuis, L.; Oudart, H.; Rene, F.; Gonzalez de Aguilar, J.L.; Loeffler, J.P. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: Benefit of a high-energy diet in a transgenic mouse model. Proc. Natl. Acad. Sci. USA 2004, 101, 11159–11164. [Google Scholar] [CrossRef] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Yang, E.J. Hochu-Ekki-To Improves Motor Function in an Amyotrophic Lateral Sclerosis Animal Model. Nutrients 2019, 11, 2644. https://doi.org/10.3390/nu11112644
Cai M, Yang EJ. Hochu-Ekki-To Improves Motor Function in an Amyotrophic Lateral Sclerosis Animal Model. Nutrients. 2019; 11(11):2644. https://doi.org/10.3390/nu11112644
Chicago/Turabian StyleCai, Mudan, and Eun Jin Yang. 2019. "Hochu-Ekki-To Improves Motor Function in an Amyotrophic Lateral Sclerosis Animal Model" Nutrients 11, no. 11: 2644. https://doi.org/10.3390/nu11112644