Serum Phosphorus as a Risk Factor of Metabolic Syndrome in the Elderly in Taiwan: A Large-Population Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
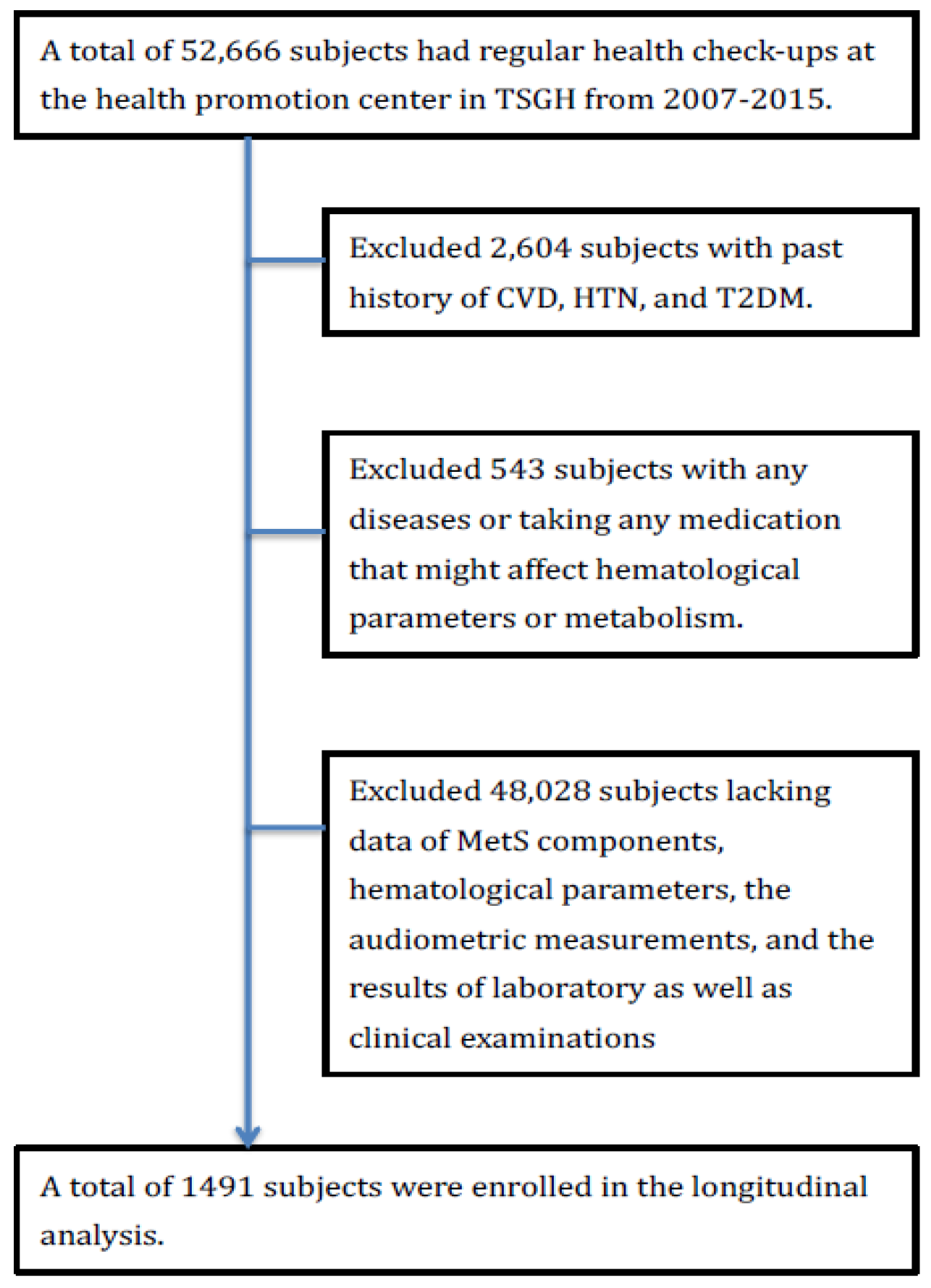
2.1. Study Subjects
2.2. Measurement of Serum Phosphorus
2.2.1. Covariates
2.2.2. The Definition of Hypertension (HTN), Diabetes Mellitus (DM), and Metabolic Syndrome (MetS)
- (1)
- Triglycerides ≥150 mg/dL (≥1.7 mmol/L)
- (2)
- HDL cholesterol <40 mg/dL (<1.03 mmol/L) in men or <50 mg/dL (<1.29 mmol/L) in women
- (3)
- Systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥85 mmHg
- (4)
- Fasting glucose ≥100 mg/dL (≥5.6 mmol/L) or be diagnosed of T2DM
2.3. Statistical analysis
3. Results
3.1. Participant Characteristics
3.2. Association between the Metabolic Diseases and Serum Phosphorus Level
3.3. Association between the Metabolic Syndrome Components and Serum Phosphorus Level
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isakova, T.; Ix, J.H.; Sprague, S.M.; Raphael, K.L.; Fried, L.; Gassman, J.J.; Raj, D.; Cheung, A.K.; Kusek, J.W.; Flessner, M.F.; et al. Rationale and Approaches to Phosphate and Fibroblast Growth Factor 23 Reduction in CKD. J. Am. Soc. Nephrol. JASN 2015, 26, 2328–2339. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Komaba, H.; Fukagawa, M. FGF23-parathyroid interaction: Implications in chronic kidney disease. Kidney Int. 2010, 77, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B.; Viaene, L.; Bammens, B.; Claes, K.; Kuypers, D.; Vanderschueren, D.; Vanrenterghem, Y. Fibroblast growth factor-23 in early chronic kidney disease: Additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Mandreoli, M.; Baldrati, L.; Corradini, M.; Di Nicolo, P.; Malmusi, G.; Santoro, A. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 883–891. [Google Scholar] [CrossRef]
- Eddington, H.; Hoefield, R.; Sinha, S.; Chrysochou, C.; Lane, B.; Foley, R.N.; Hegarty, J.; New, J.; O’Donoghue, D.J.; Middleton, R.J.; et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. JASN 2005, 16, 520–528. [Google Scholar] [CrossRef]
- Lee, H.; Oh, S.W.; Heo, N.J.; Chin, H.J.; Na, K.Y.; Kim, S.; Chae, D.W. Serum phosphorus as a predictor of low-grade albuminuria in a general population without evidence of chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Renal Assoc. 2012, 27, 2799–2806. [Google Scholar] [CrossRef]
- Lau, W.L.; Pai, A.; Moe, S.M.; Giachelli, C.M. Direct effects of phosphate on vascular cell function. Adv. Chronic Kidney Dis. 2011, 18, 105–112. [Google Scholar] [CrossRef]
- Shuto, E.; Taketani, Y.; Tanaka, R.; Harada, N.; Isshiki, M.; Sato, M.; Nashiki, K.; Amo, K.; Yamamoto, H.; Higashi, Y.; et al. Dietary phosphorus acutely impairs endothelial function. J. Am. Soc. Nephrol. JASN 2009, 20, 1504–1512. [Google Scholar] [CrossRef]
- Meng, J.; Wassel, C.L.; Kestenbaum, B.R.; Collins, T.C.; Criqui, M.H.; Lewis, C.E.; Cummings, S.R.; Ix, J.H. Serum phosphorus levels and the spectrum of ankle-brachial index in older men: The Osteoporotic Fractures in Men (MrOS) study. Am. J. Epidemiol. 2010, 171, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Ix, J.H.; Targher, G.; Smits, G.; Chonchol, M. Relation of serum phosphorus levels to ankle brachial pressure index (from the Third National Health and Nutrition Examination Survey). Am. J. Cardiol. 2010, 106, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; De Boer, I.H.; Peralta, C.A.; Adeney, K.L.; Duprez, D.A.; Jenny, N.S.; Siscovick, D.S.; Kestenbaum, B.R. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Short, R.A. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, M.; Okaniwa, S.; Nakayama, T. Reduced Serum Phosphorus Levels Were Associated with Metabolic Syndrome in Men But Not in Women: A Cross-Sectional Study among the Japanese Population. Ann. Nutr. Metab. 2017, 71, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.; Shaw, J. International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome. Diabetes Voice 2005, 50, 31–33. [Google Scholar]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Mancini, F.R.; Affret, A.; Dow, C.; Balkau, B.; Clavel-Chapelon, F.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. High dietary phosphorus intake is associated with an increased risk of type 2 diabetes in the large prospective E3N cohort study. Clin. Nutr. (Edinb. Scotl.) 2018, 37, 1625–1630. [Google Scholar] [CrossRef]
- Mirza, M.A.; Alsio, J.; Hammarstedt, A.; Erben, R.G.; Michaelsson, K.; Tivesten, A.; Marsell, R.; Orwoll, E.; Karlsson, M.K.; Ljunggren, O.; et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 219–227. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry; American Association for Clinical Chemistry: Washington, DC, USA, 1994. [Google Scholar]
- Vart, P.; Nigatu, Y.T.; Jaglan, A.; van Zon, S.K.; Shafique, K. Joint Effect of Hypertension and Elevated Serum Phosphorus on the Risk of Mortality in National Health and Nutrition Examination Survey-III. J. Am. Heart Assoc. 2015, 4, e001706. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Park, J.; Choi, S.H.; Ann, S.H.; Singh, G.B.; Shin, E.S.; Lee, J.S.; Chung, H.C. Serum Phosphorus Concentration and Coronary Artery Calcification in Subjects without Renal Dysfunction. PLoS ONE 2016, 11, e0151007. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.; Kasper, D.; Jameson, J.; Faucy, A.; Hauser, S.; Loscalzo, J. Harrison’s Principle of Internal Medicine. Edisi ke-18; McGraw-Hill Professional: New York, NY, USA, 2011. [Google Scholar]
| Group 1 Age < 40 | Group 2 40 ≤ Age < 60 | Group 3 60 ≤ Age | |
|---|---|---|---|
| Continuous Variables | |||
| Sample size | 698 | 549 | 244 |
| Age (years) | 29.26 (5.57) | 49.21 (5.64) | 69.21 (8.05) |
| BMI (kg/m2) | 22.99 (4.10) | 25.05(41.88) | 28.83(115.74) |
| Waist circumference (cm) | |||
| Male | 83.95 (10.20) | 86.30 (8.95) | 87.56 (8.39) |
| Female | 71.36 (10.01) | 77.17 (9.53) | 81.76 (9.10) |
| Systolic BP (mmHg) | 111.49 (20.95) | 115.96 (18.59) | 126.27 (20.56) |
| Diastolic BP (mmHg) | 69.54 (10.52) | 74.71 (11.84) | 77.10 (11.98) |
| Serum fast glucose | 119.46 (33.65) | 137.79 (43.63) | 157.62 (55.40) |
| Serum HDL-cholesterol (mg/dL) | 58.14 (15.77) | 56.85 (16.31) | 55.49 (14.80) |
| Serum triglycerides (mg/dL) | 88.92 (68.76) | 127.37 (103.23) | 122.32 (76.22) |
| Serum total cholesterol (mg/dL) | 174.78 (31.67) | 194.83 (34.92) | 192.47 (34.25) |
| Serum LDL-cholesterol (mg/dL) | 109.32 (29.04) | 125.90 (33.09) | 119.17 (31.07) |
| Serum creatinine (mg/dL) | 0.81 (0.28) | 0.83 (0.45) | 0.87 (0.23) |
| Serum phosphorus (mg/dL) | 3.80 (0.63) | 4.01 (2.34) | 4.17 (1.389) |
| Serum uric acid (mg/dL) | 5.50 (2.13) | 5.52 (1.48) | 5.69 (1.34) |
| Serum Hs-CRP (mg/L) | 0.19 (0.30) | 0.24 (0.54) | 0.24 (0.50) |
| Categorical Variables | |||
| Male, n (%) | 419 (50) | 330 (50) | 146 (50) |
| Family history of CVD, n (%) | 324 (46.5) | 222 (40.6) | 31 (12.9) |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
|---|---|---|---|---|
| Group Age 1–39 | Group Age 40–59 | Group Age 60– | ||
| Serum phosphorus concentration | 3.80 (0.63) | 4.01 (2.34) | 4.17 (1.389) | |
| Model 1 | Metabolic syndrome | 0.78 (0.49, 1.26) | 1.00 (0.91, 1.09) | 1.36 (1.12, 1.65) ** |
| Diabetes mellitus | 0.53 (0.13, 2.17) | 1.01 (0.92, 1.11) | 1.55 (1.22, 1.96) ** | |
| Hypertension | 0.78 (0.51, 1.20) | 1.03 (0.99, 1.07) | 1.10 (0.90, 1.33) | |
| Model 2 | Metabolic syndrome | 0.82 (0.55, 1.22) | 1.00 (0.89, 1.12) | 1.34 (1.08, 1.65) ** |
| Diabetes mellitus | 0.58 (0.16, 2.11) | 1.01 (0.92, 1.13) | 1.50 (1.17, 1.91) ** | |
| Hypertension | 0.85 (0.59, 1.22) | 1.03 (1.00, 1.07) | 1.00 (0.81, 1.23) | |
| Model 3 | Metabolic syndrome | 0.75 (0.49, 1.16) | 0.99 (0.85, 1.15) | 1.39 (1.11, 1.74) ** |
| Diabetes mellitus | 0.31 (0.06, 1.75) | 1.01 (0.91, 1.13) | 1.49 (1.15, 1.92) ** | |
| Hypertension | 0.83 (0.57, 1.21) | 1.04 (1.00, 1.08) | 0.96 (0.77, 1.20) | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
|---|---|---|---|---|
| Group Age 1–39 | Group Age 40–59 | Group Age 60– | ||
| Serum phosphorus concentration | 3.80 (0.63) | 4.01 (2.34) | 4.17 (1.389) | |
| Model 1 | Blood Pressure | 0.96 (0.67, 1.39) | 0.99 (0.91, 1.08) | 1.27 (1.05, 1.54) * |
| Waist circumference | 0.90 (0.80, 1.01) | 1.01 (0.99, 1.04) | 1.28 (1.16, 1.41) | |
| HDL | 0.86 (0.66, 1.12) | 1.01 (0.97, 1.06) | 1.38 (1.18, 1.61) ** | |
| Triglyceride | 0.97 (0.69, 1.36) | 1.02 (0.98, 1.07) | 1.46 (1.22, 1.74) ** | |
| Fast glucose | 0.92 (0.60, 1.45) | 1.01 (0.95, 1.01) | 1.43 (1.25, 1.64) ** | |
| Model 2 | Blood Pressure | 1.06 (0.77, 1.46) | 0.99 (0.90, 1.09) | 1.12 (0.90, 1.38) |
| Waist circumference | 0.81 (0.71, 0.92) ** | 1.02 (0.99, 1.04) | 1.18 (1.06, 1.31) ** | |
| HDL | 0.80 (0.60, 1.06) | 1.02 (0.96, 1.07) | 1.26 (1.06, 1.48) ** | |
| Triglyceride | 1.04 (0.78, 1.38) | 1.03 (0.99, 1.07) | 1.36 (1.12, 1.65) ** | |
| Fast glucose | 0.98 (0.67, 1.44) | 1.00 (0.94, 1.08) | 1.33 (1.15, 1.54) ** | |
| Model 3 | Blood Pressure | 1.06 (0.76, 1.47) | 0.99 (0.89, 1.11) | 1.09 (0.87, 1.35) |
| Waist circumference | 0.81 (0.72, 0.93) ** | 1.01 (0.98, 1.04) | 1.18 (1.06, 1.31) ** | |
| HDL | 0.78 (0.59, 1.03) | 1.02 (0.97, 1.07) | 1.27 (1.07, 1.50) ** | |
| Triglyceride | 1.03 (0.76, 1.38) | 1.03 (0.99, 1.08) | 1.41 (1.15, 1.72) ** | |
| Fast glucose | 0.96 (0.64, 1.44) | 1.00 (0.93, 1.08) | 1.32 (1.14, 1.53) ** | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhuang, Y.-H.; Kao, T.-W.; Peng, T.-C.; Chen, W.-L.; Chang, P.-K.; Wu, L.-W. Serum Phosphorus as a Risk Factor of Metabolic Syndrome in the Elderly in Taiwan: A Large-Population Cohort Study. Nutrients 2019, 11, 2340. https://doi.org/10.3390/nu11102340
Jhuang Y-H, Kao T-W, Peng T-C, Chen W-L, Chang P-K, Wu L-W. Serum Phosphorus as a Risk Factor of Metabolic Syndrome in the Elderly in Taiwan: A Large-Population Cohort Study. Nutrients. 2019; 11(10):2340. https://doi.org/10.3390/nu11102340
Chicago/Turabian StyleJhuang, Yi-Han, Tung-Wei Kao, Tao-Chun Peng, Wei-Liang Chen, Pi-Kai Chang, and Li-Wei Wu. 2019. "Serum Phosphorus as a Risk Factor of Metabolic Syndrome in the Elderly in Taiwan: A Large-Population Cohort Study" Nutrients 11, no. 10: 2340. https://doi.org/10.3390/nu11102340





