6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Conditioned Medium
2.3. Measurement of Secreted Factors
2.4. Isolation of CD14+ Monocytes and Osteoclast Differentiation
2.5. Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. PTHrP Knockdown
2.7. Statistical Analysis
3. Results
3.1. 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b]pyridine (PhIP) Induced Parathyroid Hormone-Related Protein (PTHrP) Secretion In Human 786-O Renal Cell Carcinoma Cells
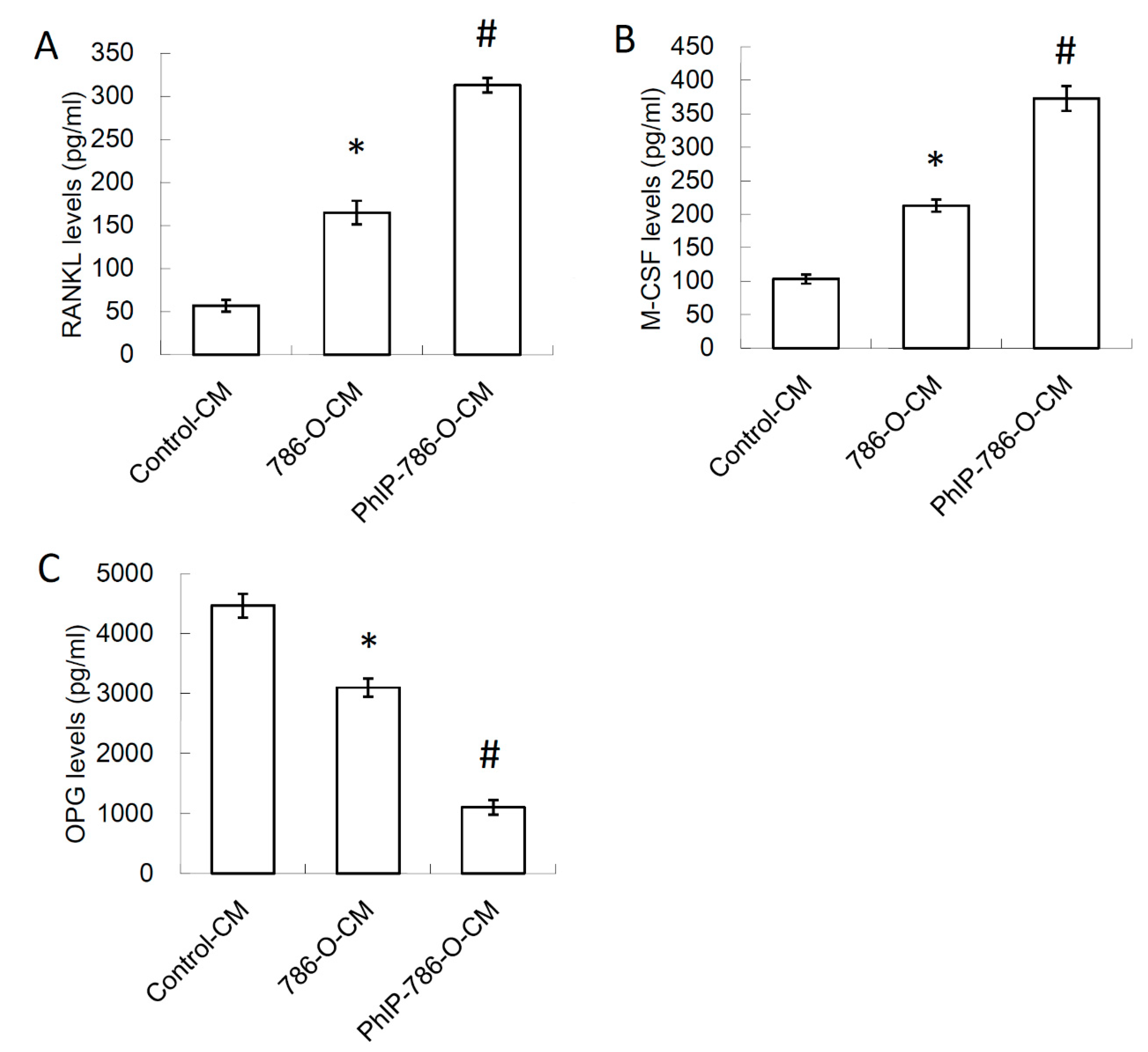
3.2. Conditioned Medium (CM) of PhIP-Treated 786-O Increased Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) and Macrophage Colony-Stimulating Factor (M-CSF) Expression, and Decreased Osteoprotegerin (OPG) Expression in Osteoblasts
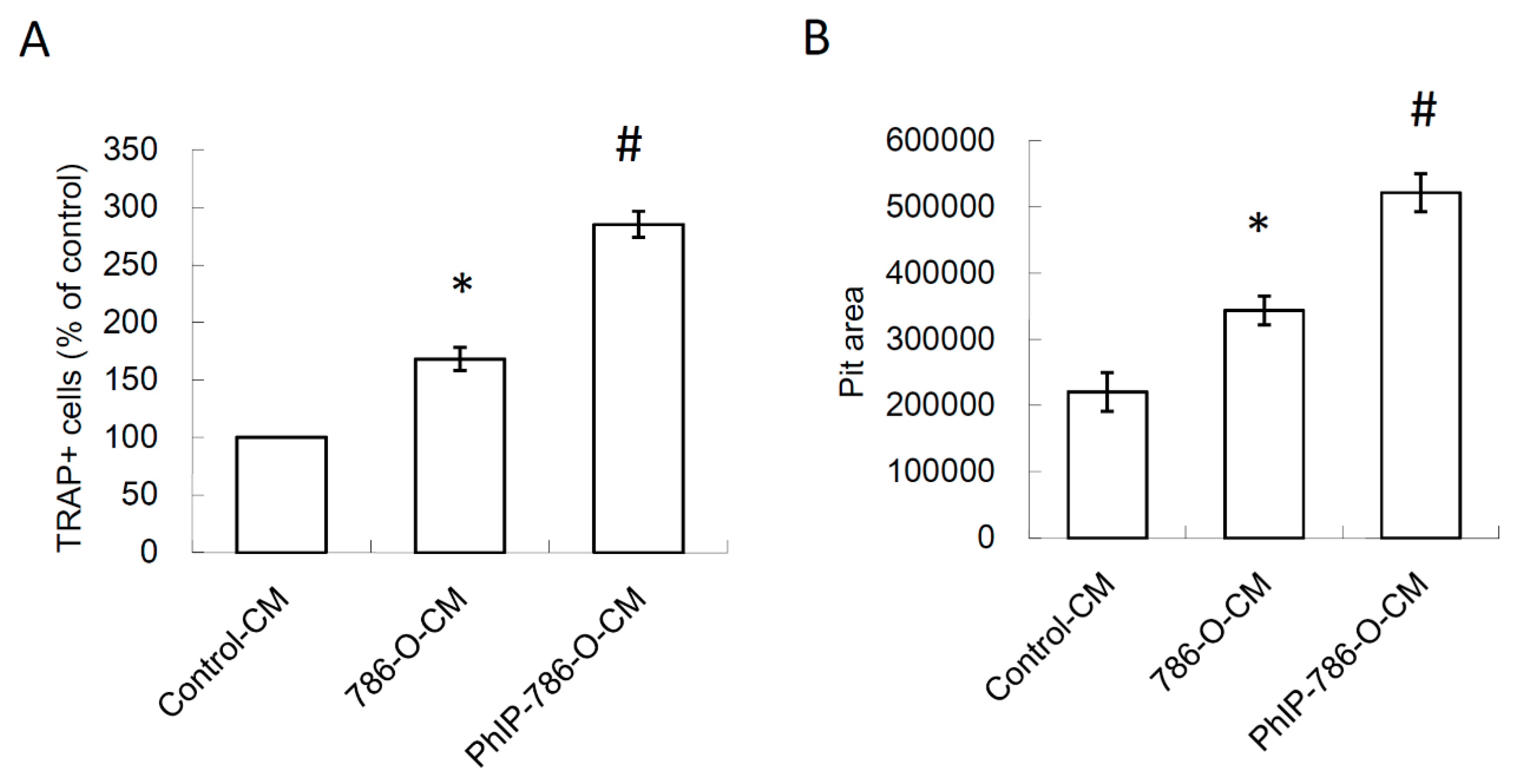
3.3. PhIP Increased Human 786-O Renal Cell Carcinoma Cell-Mediated Osteoclastogenesis and Bone Resorption
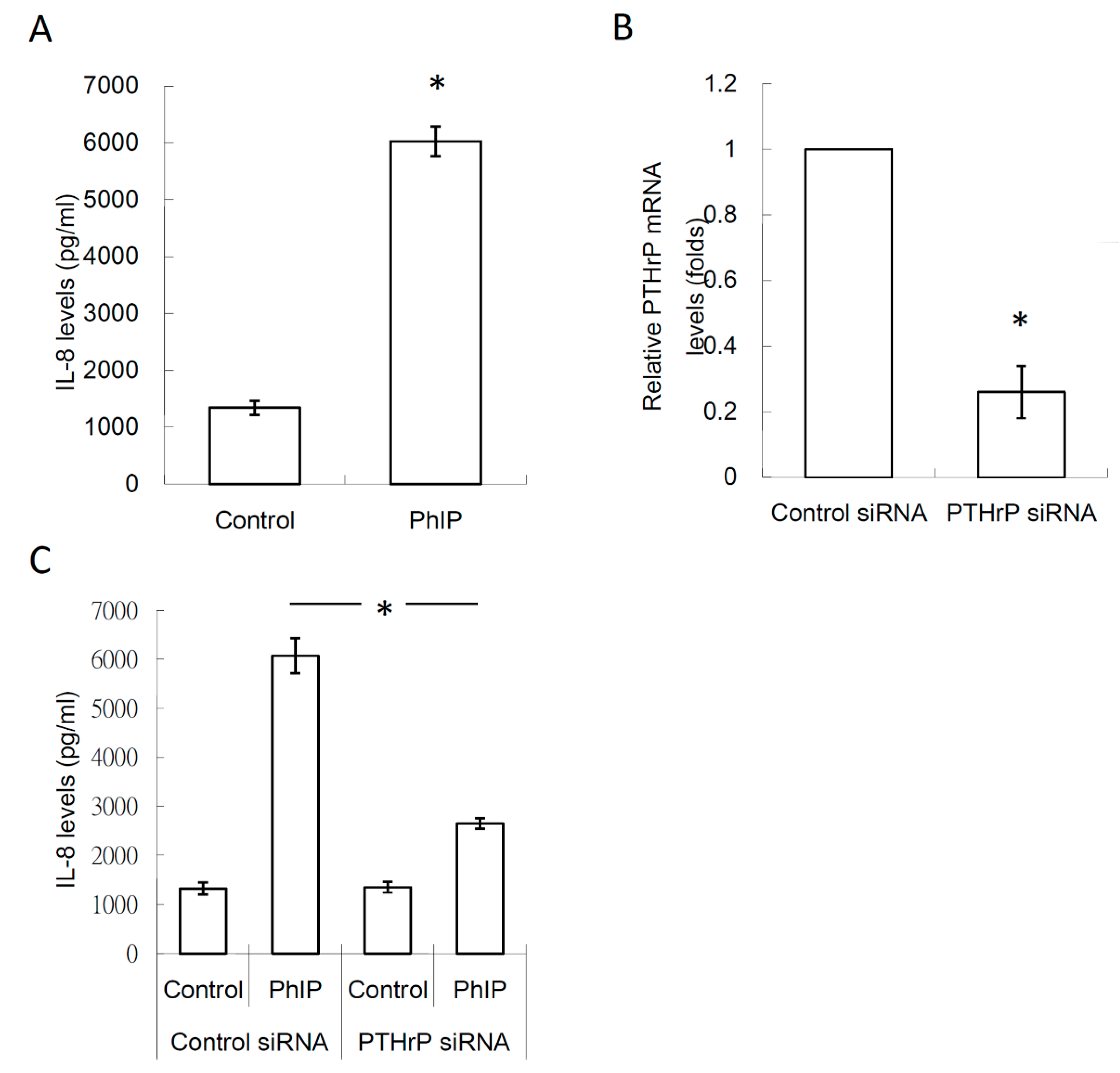
3.4. PTHrP/IL-8 Autocrine Loop was Involved in the Stimulation of PhIP on Renal Cell Carcinoma-Mediated Osteoclastogenesis
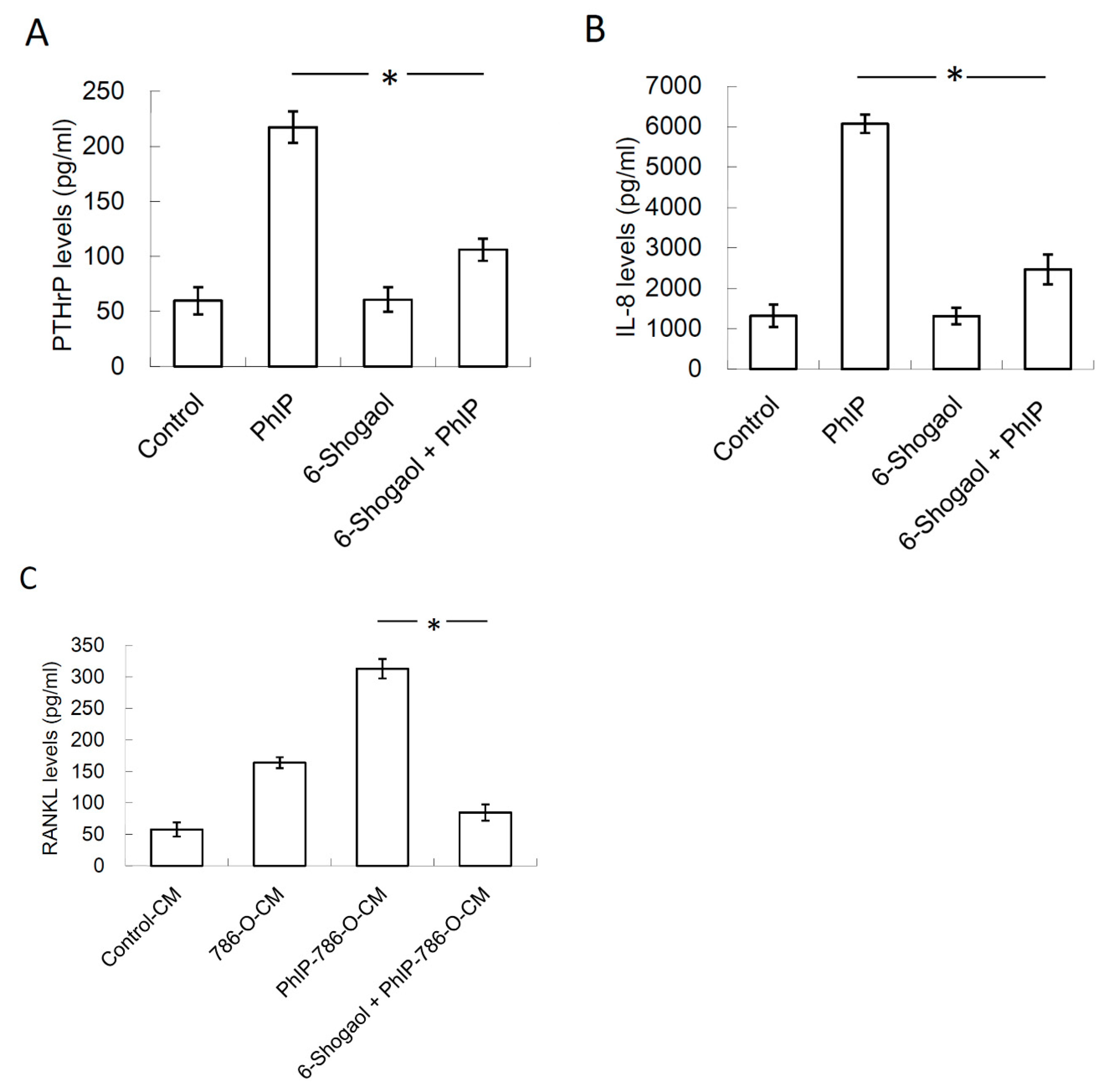
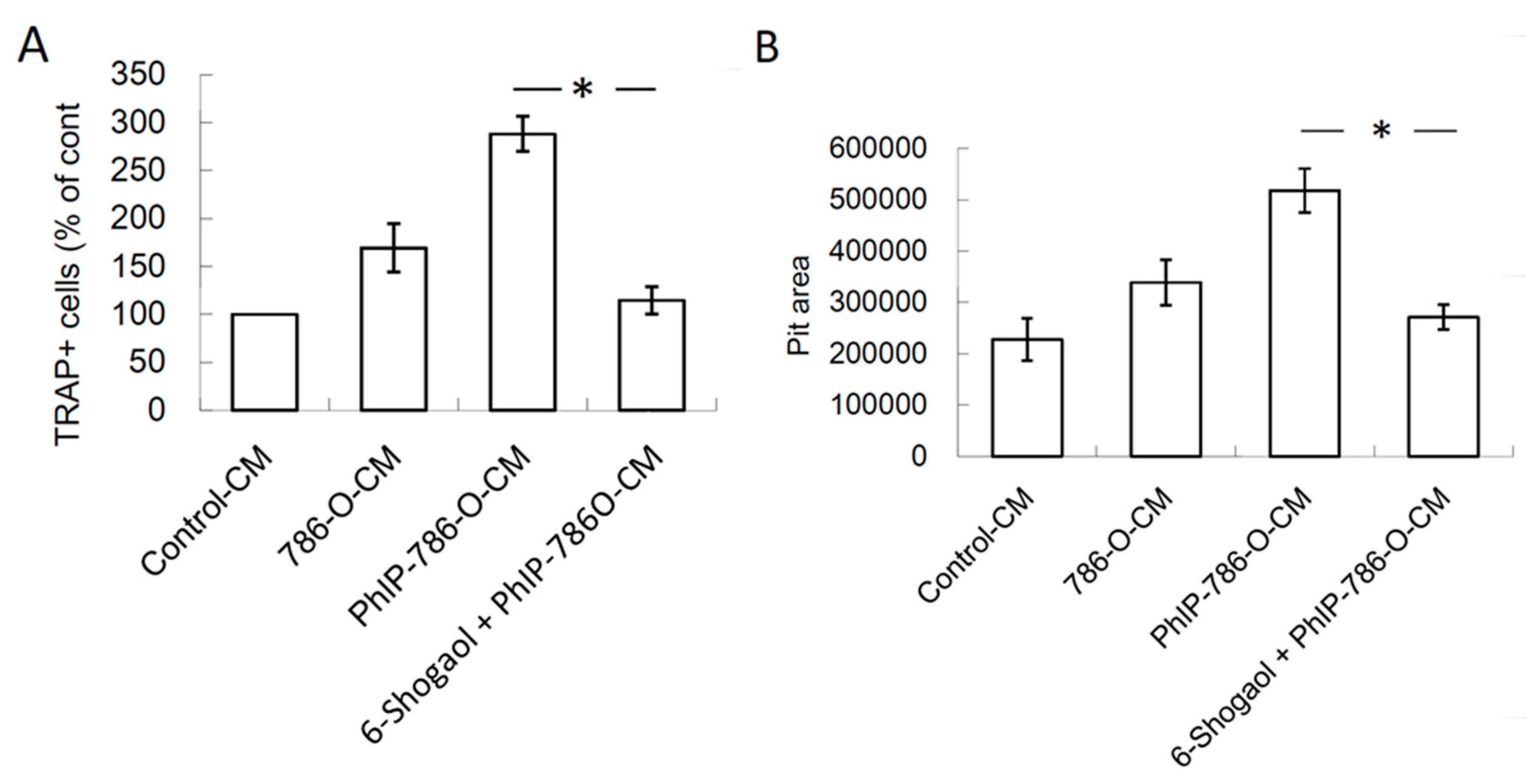
3.5. 6-Shogaol Suppressed PhIP-Mediated Bone Resorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahuman, A.A.; Gopalakrishnan, G.; Venkatesan, P.; Geetha, K.; Bagavan, A. Mosquito larvicidal activity of isolated compounds from the rhizome of Zingiber officinale. Phytother. Res. 2008, 22, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Marzec, Z.; Kasperek, E.; Wyszogrodzka-Koma, L.; Szwerc, W.; Asakawa, Y. Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations. Int. J. Mol. Sci. 2017, 18, 452. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef]
- Pan, M.H.; Hsieh, M.C.; Hsu, P.C.; Ho, S.Y.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol. Nutr. Food Res. 2008, 52, 1467–1477. [Google Scholar] [CrossRef]
- Pan, M.H.; Hsieh, M.C.; Kuo, J.M.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol. Nutr. Food Res. 2008, 52, 527–537. [Google Scholar] [CrossRef]
- Wu, C.H.; Hong, B.H.; Ho, C.T.; Yen, G.C. Targeting cancer stem cells in breast cancer: Potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 2015, 63, 2432–2441. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hung, J.Y.; Tsai, Y.M.; Tsai, E.M.; Huang, M.S.; Hou, M.F.; Kuo, P.L. 6-shogaol, an active constituent of dietary ginger, impairs cancer development and lung metastasis by inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in tumor-associated dendritic cells. J. Agric. Food Chem. 2015, 63, 1730–1738. [Google Scholar] [CrossRef]
- Ilic, N.M.; Dey, M.; Poulev, A.A.; Logendra, S.; Kuhn, P.E.; Raskin, I. Anti-inflammatory activity of grains of paradise (Aframomum melegueta Schum) extract. J. Agric. Food Chem. 2014, 62, 10452–10457. [Google Scholar] [CrossRef]
- Yoshida, K.; Satsu, H.; Mikubo, A.; Ogiwara, H.; Yakabe, T.; Inakuma, T.; Shimizu, M. 6-shogaol, a major compound in ginger, induces aryl hydrocarbon receptor-mediated transcriptional activity and gene expression. J. Agric. Food Chem. 2014, 62, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Warin, R.F.; Chen, H.; Soroka, D.N.; Zhu, Y.; Sang, S. Induction of lung cancer cell apoptosis through a p53 pathway by [6]-shogaol and its cysteine-conjugated metabolite M2. J. Agric. Food Chem. 2014, 62, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.Y.; Hsu, Y.L.; Li, C.T.; Ko, Y.C.; Ni, W.C.; Huang, M.S.; Kuo, P.L. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J. Agric. Food Chem. 2009, 57, 9809–9816. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, T.Z.; Liu, Y.W.; Tseng, W.C.; Liu, R.H.; Lu, F.J.; Lin, Y.S.; Kuo, S.H.; Chen, C.H. 6-shogaol (alkanone from ginger) induces apoptotic cell death of human hepatoma p53 mutant Mahlavu subline via an oxidative stress-mediated caspase-dependent mechanism. J. Agric. Food Chem. 2007, 55, 948–954. [Google Scholar] [CrossRef]
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Hasegawa, R.; Sano, M.; Tamano, S.; Esumi, H.; Takayama, S.; Sugimura, T. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP). Carcinogenesis 1991, 12, 1503–1506. [Google Scholar] [CrossRef]
- Shirai, T.; Sano, M.; Tamano, S.; Takahashi, S.; Hirose, M.; Futakuchi, M.; Hasegawa, R.; Imaida, K.; Matsumoto, K.; Wakabayashi, K.; et al. The prostate: A target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997, 57, 195–198. [Google Scholar]
- Sinha, R.; Gustafson, D.R.; Kulldorff, M.; Wen, W.Q.; Cerhan, J.R.; Zheng, W. 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine, a carcinogen in high-temperature-cooked meat, and breast cancer risk. J. Natl. Cancer Inst. 2000, 92, 1352–1354. [Google Scholar] [CrossRef]
- Daniel, C.R.; Cross, A.J.; Graubard, B.I.; Park, Y.; Ward, M.H.; Rothman, N.; Hollenbeck, A.R.; Chow, W.H.; Sinha, R. Large prospective investigation of meat intake, related mutagens, and risk of renal cell carcinoma. Am. J. Clin. Nutr. 2012, 95, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, S.C.; Daniel, C.R.; Ye, Y.; Tannir, N.M.; Karam, J.A.; Matin, S.F.; Wood, C.G.; Wu, X. Gene-environment interaction of genome-wide association study-identified susceptibility loci and meat-cooking mutagens in the etiology of renal cell carcinoma. Cancer 2016, 122, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Oya, M. Renal cell carcinoma: Etiology, incidence and epidemiology. Curr. Opin. Urol. 2004, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Rathmell, W.K.; Godley, P. Renal cell carcinoma. Curr. Opin. Oncol. 2008, 20, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Kuo, P.L. Bone Metastasis from Renal Cell Carcinoma. Int. J. Mol. Sci. 2016, 17, 987. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Bander, N.H.; Nanus, D.M. Renal-cell carcinoma. N. Engl. J. Med. 1996, 335, 865–875. [Google Scholar] [CrossRef]
- Woodward, E.; Jagdev, S.; McParland, L.; Clark, K.; Gregory, W.; Newsham, A.; Rogerson, S.; Hayward, K.; Selby, P.; Brown, J. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011, 48, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Reddington, J.A.; Mendez, G.A.; Ching, A.; Kubicky, C.D.; Klimo, P., Jr.; Ragel, B.T. Imaging characteristic analysis of metastatic spine lesions from breast, prostate, lung, and renal cell carcinomas for surgical planning: Osteolytic versus osteoblastic. Surg. Neurol. Int. 2016, 7, S361–S365. [Google Scholar] [CrossRef]
- Sourbier, C.; Massfelder, T. Parathyroid hormone-related protein in human renal cell carcinoma. Cancer Lett. 2006, 240, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.B.; Moniz, C.; Knight, D.E. Parathyroid hormone related peptide can function as an autocrine growth factor in human renal cell carcinoma. Biochem. Biophys. Res. Commun. 1990, 167, 1134–1138. [Google Scholar] [CrossRef]
- Massfelder, T.; Lang, H.; Schordan, E.; Lindner, V.; Rothhut, S.; Welsch, S.; Simon-Assmann, P.; Barthelmebs, M.; Jacqmin, D.; Helwig, J.J. Parathyroid hormone-related protein is an essential growth factor for human clear cell renal carcinoma and a target for the von Hippel-Lindau tumor suppressor gene. Cancer Res. 2004, 64, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, X.; Koh, A.J.; Berry, J.E.; Thudi, N.; Rosol, T.J.; Pienta, K.J.; McCauley, L.K. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int. J. Cancer 2008, 123, 2267–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballanti, P.; Minisola, S.; Pacitti, M.T.; Scarnecchia, L.; Rosso, R.; Mazzuoli, G.F.; Bonucci, E. Tartrate-resistant acid phosphate activity as osteoclastic marker: Sensitivity of cytochemical assessment and serum assay in comparison with standardized osteoclast histomorphometry. Osteoporos. Int. 1997, 7, 39–43. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Glass-Holmes, M.; Aguilar, B.J.; Gragg, R.D., 3rd; Darling-Reed, S.; Goodman, C.B. Characterization of 2-amino-1-methyl-6-phenylimidazo [4,5b] pyridine at androgen receptor: Mechanistic support for its role in prostate cancer. Am. J. Cancer Res. 2015, 5, 191–200. [Google Scholar]
- Bacon, J.R.; Williamson, G.; Garner, R.C.; Lappin, G.; Langouet, S.; Bao, Y. Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes. Carcinogenesis 2003, 24, 1903–1911. [Google Scholar] [CrossRef]
- Casimiro, S.; Mohammad, K.S.; Pires, R.; Tato-Costa, J.; Alho, I.; Teixeira, R.; Carvalho, A.; Ribeiro, S.; Lipton, A.; Guise, T.A.; et al. RANKL/RANK/MMP-1 molecular triad contributes to the metastatic phenotype of breast and prostate cancer cells in vitro. PLoS ONE 2013, 8, e63153. [Google Scholar] [CrossRef]
- Krishnan, V.; Vogler, E.A.; Sosnoski, D.M.; Mastro, A.M. In vitro mimics of bone remodeling and the vicious cycle of cancer in bone. J. Cell. Physiol. 2014, 229, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Manenti, G.; Peissel, B.; Gariboldi, M.; Falvella, F.S.; Zaffaroni, D.; Allaria, B.; Pazzaglia, S.; Rebessi, S.; Covelli, V.; Saran, A.; et al. A cancer modifier role for parathyroid hormone-related protein. Oncogene 2000, 19, 5324–5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David Roodman, G.; Silbermann, R. Mechanisms of osteolytic and osteoblastic skeletal lesions. BoneKEy Rep. 2015, 4, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, M.; Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mikami, S.; Oya, M.; Mizuno, R.; Kosaka, T.; Katsube, K.; Okada, Y. Invasion and metastasis of renal cell carcinoma. Med. Mol. Morphol. 2014, 47, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Goto, M.; Mochizuki, S.I.; Tsuda, E.; Morinaga, T.; Udagawa, N.; et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone 1999, 25, 109–113. [Google Scholar] [CrossRef]
- Jansen, I.D.; Vermeer, J.A.; Bloemen, V.; Stap, J.; Everts, V. Osteoclast fusion and fission. Calcif. Tissue Int. 2012, 90, 515–522. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Perut, F.; Cenni, E.; Unger, R.E.; Kirkpatrick, C.J.; Giunti, A.; Baldini, N. Immunogenic properties of renal cell carcinoma and the pathogenesis of osteolytic bone metastases. Int. J. Oncol. 2009, 34, 1387–1393. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Sterling, J.A.; Edwards, J.R.; Martin, T.J.; Mundy, G.R. Advances in the biology of bone metastasis: How the skeleton affects tumor behavior. Bone 2011, 48, 6–15. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs 2011, 71, 791–814. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, P.D. Bisphosphonate-associated adverse events. Hormones (Athens) 2009, 8, 96–110. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Yang, H.; Tan, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-kappaB activation. Br. J. Pharmacol. 2010, 161, 1763–1777. [Google Scholar] [CrossRef]
- Kakonen, S.M.; Mundy, G.R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 97, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Warin, R.F.; Soroka, D.N.; Chen, H.; Sang, S. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: Chemical synthesis and biological evaluation. PLoS ONE 2013, 8, e54677. [Google Scholar] [CrossRef]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef]
- Levita, J.; Syafitri, D.M.; Supu, R.D.; Mutakin, M.; Megantara, S.; Febrianti, M.; Diantini, A. Pharmacokinetics of 10-gingerol and 6-shogaol in the plasma of healthy subjects treated with red ginger (Zingiber officinale var. Rubrum) suspension. Biomed. Rep. 2018, 9, 474–482. [Google Scholar] [CrossRef]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Sun, C.; Zhu, Y.; Yang, Q.; Wei, Q.; Chen, J.; Deng, W.; Adu-Frimpong, M.; Yu, J.; et al. Enhanced Oral Bioavailability, Anti-Tumor Activity and Hepatoprotective Effect of 6-Shogaol Loaded in a Type of Novel Micelles of Polyethylene Glycol and Linoleic Acid Conjugate. Pharmaceutics 2019, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Shaw, Y.A.; Williams, L.A.; Junor, G.A.; Green, C.E.; Hibbert, S.L.; Salmon, C.N.; Smith, A.M. Changes in the contents of oleoresin and pungent bioactive principles of Jamaican ginger (Zingiber officinale Roscoe.) during maturation. J. Agric. Food Chem. 2008, 56, 5564–5571. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bernart, M.W.; Lian, L.; Lin, L. High-performance liquid chromatography–electrospray mass spectrometric analysis of pungent constituents of ginger. J. Chromatogr. A 1998, 796, 327–334. [Google Scholar] [CrossRef]
- Cheng, X.L.; Liu, Q.; Peng, Y.B.; Qi, L.W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Guo, J.B.; Zhang, W.J.; Wu, H.; Du, L.M. Microwave-assisted decomposition coupled with acidic food condiment as an efficient technology for ginger (Zingiber officinale Roscoe) processing. Sep. Purif. Technol. 2015, 146, 219–226. [Google Scholar] [CrossRef]
- Teng, H.; Seuseu, K.T.; Lee, W.Y.; Chen, L. Comparing the effects of microwave radiation on 6-gingerol and 6-shogaol from ginger rhizomes (Zingiber officinale Rosc). PLoS ONE 2019, 14, e0214893. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, I.-J.; Chen, S.-C.; Yen, M.-C.; Wu, Y.-H.; Hung, C.-H.; Kuo, P.-L. 6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients 2019, 11, 2306. https://doi.org/10.3390/nu11102306
Yeh I-J, Chen S-C, Yen M-C, Wu Y-H, Hung C-H, Kuo P-L. 6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients. 2019; 11(10):2306. https://doi.org/10.3390/nu11102306
Chicago/Turabian StyleYeh, I-Jeng, Szu-Chia Chen, Meng-Chi Yen, Yen-Hung Wu, Chih-Hsing Hung, and Po-Lin Kuo. 2019. "6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential" Nutrients 11, no. 10: 2306. https://doi.org/10.3390/nu11102306
APA StyleYeh, I.-J., Chen, S.-C., Yen, M.-C., Wu, Y.-H., Hung, C.-H., & Kuo, P.-L. (2019). 6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients, 11(10), 2306. https://doi.org/10.3390/nu11102306




