Plasma Retinol Levels and High-Sensitivity C-Reactive Protein in Prepubertal Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Data Collection
2.3. Anthropometric Variables
2.4. Biochemical Data
2.5. Statistical Analysis
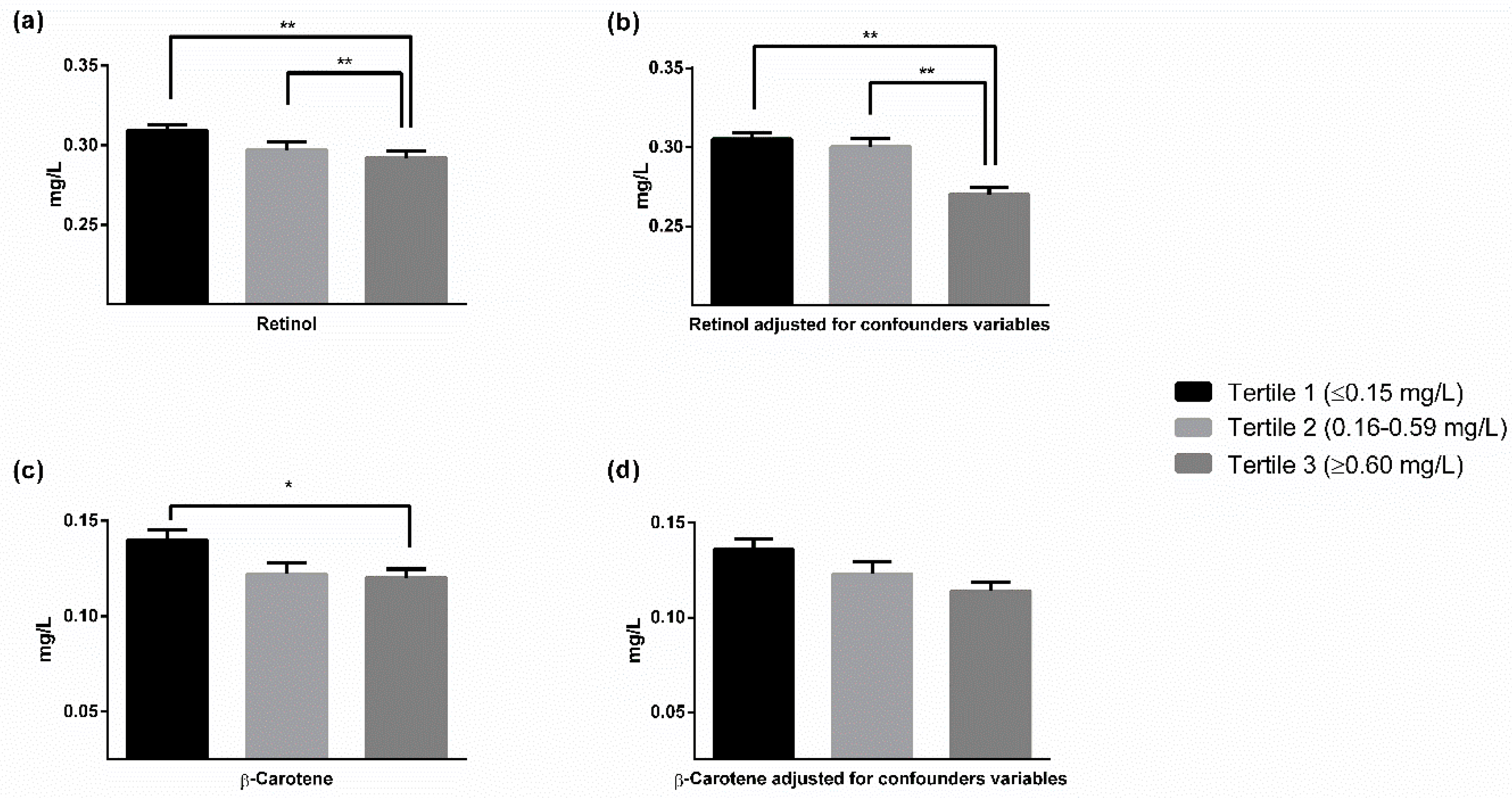
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J.; Tipping, R.W.; Ford, C.E.; Pressel, S.L.; et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [PubMed]
- Kobayashi, S.; Inoue, N.; Ohashi, Y.; Terashima, M.; Matsui, K.; Mori, T.; Fujita, H.; Awano, K.; Kobayashi, K.; Azumi, H.; et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: Important role of C-reactive protein. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Louw, J.A.; Werbeck, A.; Louw, M.E.J.; Kotze, T.J.V.W.; Cooper, R.; Labadarios, D. Blood vitamin concentrations during the acute-phase response. Crit. Care Med. 1992, 20, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Saboori, S.; Shab-Bidar, S.; Speakman, J.R.; Yousefi Rad, E.; Djafarian, K. Effect of Vitamin E supplementation on serum C-reactive protein level: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2015, 69, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Zierer, A.; Schneider, A.; Heier, M.; Koenig, W.; Kastenmüller, G.; Waldenberger, M.; Peters, A.; Thorand, B. Vitamin e supplementation is associated with lower levels of C-reactive protein only in higher dosages and combined with other antioxidants: The Cooperative Health Research in the Region of Augsburg (KORA) F4 study. Br. J. Nutr. 2015, 113, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Erlinger, T.P.; Guallar, E.; Miller, E.R.; Stolzenberg-Solomon, R.; Appel, L.J. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch. Intern. Med. 2001, 161, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, S.B.; Bush, A.J.; Pahor, M.; Gross, M.D. Serum carotenoids and markers of inflammation in nonsmokers. Am. J. Epidemiol. 2000, 152, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Il’Yasova, D.; Ivanova, A.; Morrow, J.D.; Cesari, M.; Pahor, M. Correlation between two markers of inflammation, serum C-reactive protein and interleukin 6, and indices of oxidative stress in patients with high risk of cardiovascular disease. Biomarkers 2008, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.D.; Strachan, A.A.; Thies, F.; Aucott, L.S.; Reid, D.M.; Hardcastle, A.C.; Mavroeidi, A.; Simpson, W.G.; Duthie, G.G.; Macdonald, H.M. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br. J. Nutr. 2014, 112, 1341–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.; Rodríguez-Artalejo, F.; Lopes, C. The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: Sex and body mass index interactions. Eur. J. Clin. Nutr. 2009, 63, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chung, S.-J.; Floegel, A.; Song, W.O.; Koo, S.I.; Chun, O.K. Dietary antioxidant capacity is associated with improved serum antioxidant status and decreased serum C-reactive protein and plasma homocysteine concentrations. Eur. J. Nutr. 2013, 52, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Liu, S.; Mannino, D.M.; Giles, W.H.; Smith, S.J. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur. J. Clin. Nutr. 2003, 57, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.G.; Alshaarawy, O.; Cantave, M.D.; Anthony, J.C. Inverse association linking serum levels of potential antioxidant vitamins with C-reactive protein levels using a novel analytical approach. Br. J. Nutr. 2016, 116, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, E.C.; Schramm-Sapyta, N.L. Race differences in the relation of vitamins A, C, E, and β-carotene to metabolic and inflammatory biomarkers. Nutr. Res. 2014, 34, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.K.; Alvarez, J.O.; Wahed, M.A.; Fuchs, G.J.; Stephensen, C.B. Predictors of serum retinol in children with shigellosis. Am. J. Clin. Nutr. 1998, 68, 1088–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqsood, M.; Dancheck, B.; Gamble, M.V.; Palafox, N.A.; Ricks, M.O.; Briand, K.; Semba, R.D. Vitamin A deficiency and inflammatory markers among preschool children in the Republic of the Marshall Islands. Nutr. J. 2004, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenzel, A.P.; Carvalho, R.; Jesus, P.; Bull, A.; Pereira, S.; Saboya, C.; Ramalho, A. Serum antioxidant associations with metabolic characteristics in metabolically healthy and unhealthy adolescents with severe obesity: An observational study. Nutrients 2018, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, J.; Liu, Z.; Yun, C.; Li, Y.; Piao, J.; Yang, X. Association of vitamin a status with overnutrition in children and adolescents. Int. J. Environ. Res. Public Health 2015, 12, 15531–15539. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.; Coperías, J.L.; Castilla, P.; Gómez-Coronado, D.; Lasunción, M.A. Liquid chromatographic method for the simultaneous determination of different lipid-soluble antioxidants in human plasma and low-density lipoproteins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 803, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.; Castilla, P.; Gómez-Coronado, D.; Garcés, C.; Benavente, M.; Rodríguez-Artalejo, F.; De Oya, M.; Lasunción, M.A. Influence of apolipoprotein E genotype on fat-soluble plasma antioxidants in Spanish children. Am. J. Clin. Nutr. 2005, 81, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Beisel, W.R. Infection-induced depression of serum retinol—A component of the acute phase response or a consequence? Am. J. Clin. Nutr. 1998, 68, 993–994. [Google Scholar] [CrossRef] [PubMed]
- García, O.P.; Ronquillo, D.; del Carmen Caamaño, M.; Martínez, G.; Camacho, M.; López, V.; Rosado, J.L. Zinc, iron and vitamins A, C and E are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients 2013, 5, 5012–5030. [Google Scholar] [CrossRef] [PubMed]
- Balagopal, P.; Graham, T.E.; Kahn, B.B.; Altomare, A.; Funanage, V.; George, D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: Association with subclinical inflammation. J. Clin. Endocrinol. Metab. 2007, 92, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.M.; Trpkovic, A.; Bajic, V.; Soskic, S.; Jovanovic, A.; Stanimirovic, J.; Panic, M.; Isenovic, E.R. Interrelatedness between C-reactive protein and oxidized low-density lipoprotein. Clin. Chem. Lab. Med. 2015, 53, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; de Dios, O.; Jois, A.; Gavela-Pérez, T.; Gorgojo, L.; Martín-Moreno, J.M.; Soriano-Guillen, L.; Garcés, C. Vegetable and fruit intakes are associated with hs-CRP levels in pre-pubertal girls. Nutrients 2017, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Egan, B.M.; Woolson, R.F.; Mainous, A.G.; Al-Solaiman, Y.; Jesri, A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch. Intern. Med. 2007, 167, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R.; Hurley, T.G.; Hsieh, J.; Rogers, E.; Stoddard, A.M.; Sorensen, G.; Nicolosi, R.J. Determinants of plasma vitamins and lipids: The working well study. Am. J. Epidemiol. 1994, 140, 132–147. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Kristal, A.R.; Shikany, J.M.; Wilson, A.C.; Chen, C.; Mares-Perlman, J.A.; Masaki, K.H.; Caan, B.J. Correlates of serum α- and γ-tocopherol in the women’s health initiative. Ann. Epidemiol. 2001, 11, 136–144. [Google Scholar] [CrossRef]
- Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Rimm, E.B.; Litin, L.; Willett, W.C. Correlations of Vitamin A and E Intakes with the Plasma Concentrations of Carotenoids and Tocopherols among American Men and Women. J. Nutr. 1992, 122, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Patterson, B.H.; Mangels, A.R.; Levander, O.A.; Gibson, T.; Taylor, P.R.; Block, G. Determinants of plasma vitamin E in healthy males. Cancer Epidemiol. Biomarkers Prev. 1993, 2, 473–479. [Google Scholar] [PubMed]
| Boys (n = 254) | Girls (n = 287) | |
|---|---|---|
| BMI | 17.2 ± 2.6 | 17.2 ± 2.7 |
| hs-CRP (mg/L) a | 0.90 ± 1.52 | 0.99 ± 1.71 |
| Total cholesterol (mg/dL) | 183.9 ± 27.1 | 183.5 ± 29.1 |
| Triglycerides (mg/dL) a | 70.7 ± 27.9 | 72.4 ± 24.0 |
| HDL cholesterol (mg/dL) | 59.4 ± 13.1 | 58.5 ± 13.5 |
| LDL cholesterol (mg/dL) | 110.3 ± 26.6 | 110.5 ± 26.9 |
| Apo A-I (mg/dL) | 136.0 ± 18.4 | 133.6 ± 18.1 |
| Apo B (mg/dL) | 70.2 ± 14.2 | 70.8 ± 15.0 |
| α-tocopherol (mg/L) a | 9.07 ± 1.61 | 9.37 ± 1.74 * |
| γ-tocopherol (mg/L) | 0.95 ± 0.40 | 0.95 ± 0.41 |
| Lycopene (mg/L) a | 0.187 ± 0.129 | 0.183 ± 0.129 |
| α-carotene (mg/L) a | 0.033 ± 0.023 | 0.031 ± 0.020 |
| β-carotene (mg/L) a | 0.129 ± 0.074 | 0.123 ± 0.066 |
| Retinol (mg/L) | 0.29 ± 0.06 | 0.30 ± 0.064 |
| hs-CRP a | BMI a | Triglycerides a | Cholesterol | LDL-C | HDL-C | Apo B | Apo A-I | |
|---|---|---|---|---|---|---|---|---|
| α-Tocopherola | −0.013 | −0.012 | 0.134 ** | 0.421 *** | 0.296 *** | 0.250 *** | 0.459 *** | 0.256 *** |
| γ-Tocopherol | 0.033 | 0.082 | 0.047 | 0.136 ** | 0.104 * | 0.058 | 0.141 *** | 0.059 |
| Lycopenea | −0.073 | 0.065 | −0.064 | 0.119 ** | 0.062 | 0.149 *** | 0.085 * | 0.216 *** |
| α-Carotenea | −0.066 | −0.085 | −0.074 | 0.151 *** | 0.112 ** | 0.129 ** | 0.144 *** | 0.133 ** |
| β-Carotenea | −0.144 *** | −0.074 | −0.065 | 0.219 *** | 0.172 *** | 0.147 *** | 0.226 *** | 0.173 *** |
| Retinol | −0.280 *** | 0.192 *** | 0.063 | 0.071 | −0.030 | 0.167 *** | 0.125 ** | 0.214 *** |
| Base Line Variable | β | p-Value |
|---|---|---|
| Retinol | −0.315 | <0.001 |
| BMI * | 0.287 | <0.001 |
| Apo A-I | −0.138 | 0.002 |
| Gender | 0.113 | 0.009 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Dios, O.; Navarro, P.; Ortega-Senovilla, H.; Herrero, L.; Gavela-Pérez, T.; Soriano-Guillen, L.; Lasunción, M.A.; Garcés, C. Plasma Retinol Levels and High-Sensitivity C-Reactive Protein in Prepubertal Children. Nutrients 2018, 10, 1257. https://doi.org/10.3390/nu10091257
De Dios O, Navarro P, Ortega-Senovilla H, Herrero L, Gavela-Pérez T, Soriano-Guillen L, Lasunción MA, Garcés C. Plasma Retinol Levels and High-Sensitivity C-Reactive Protein in Prepubertal Children. Nutrients. 2018; 10(9):1257. https://doi.org/10.3390/nu10091257
Chicago/Turabian StyleDe Dios, Olaya, Pilar Navarro, Henar Ortega-Senovilla, Leticia Herrero, Teresa Gavela-Pérez, Leandro Soriano-Guillen, Miguel A. Lasunción, and Carmen Garcés. 2018. "Plasma Retinol Levels and High-Sensitivity C-Reactive Protein in Prepubertal Children" Nutrients 10, no. 9: 1257. https://doi.org/10.3390/nu10091257





