Medium-Chain Triglycerides Lower Blood Lipids and Body Weight in Streptozotocin-Induced Type 2 Diabetes Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.3. Assay of Serum and Hepatic Lipids
2.4. Enzyme Assay
2.5. Histology
2.6. Statistical Analysis
3. Results
3.1. Weight Gain and Organ Weights
3.2. Blood Glucose, Insulin Concentrations, Intraperitoneal Glucose Tolerance Test (IPGTT) and Lipid Levels
3.3. Liver Lipid Levels
3.4. Liver Enzyme Activity Assay
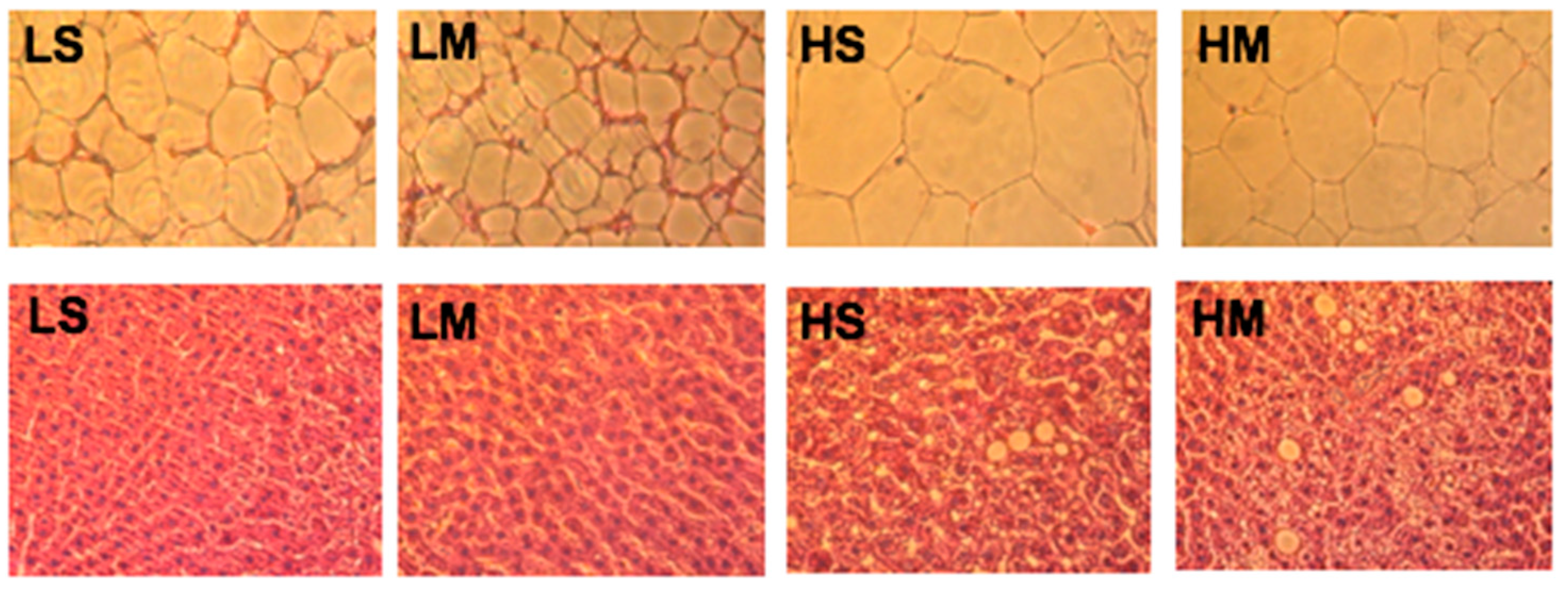
3.5. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections from 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, D.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.C.; Yang, H.C.; Chang, H.Y.; Yeh, C.J.; Chen, H.H.; Huang, K.C.; Pan, W.H. Morbid obesity in Taiwan: Prevalence, trends, associated social demographics, and lifestyle factors. PLoS ONE 2017, 12, e0169577. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C.; Young, S.K.; Cotter, R.; Lin, L.; Rowe, W.B. Medium-chain-triglyceride lipid emulsion: Metabolism and tissue distribution. Am. J. Clin. Nutr. 1990, 52, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Crozier, G.; Bois-Joyeux, B.; Chanez, M.; Girard, J.; Peret, J. Metabolic effects induced by long-term feeding of medium-chain triglycerides in the rat. Metabolism 1987, 36, 807–814. [Google Scholar] [CrossRef]
- Lavau, M.M.; Hashim, S.A. Effect of medium chain triglyceride on lipogenesis and body fat in the rat. J. Nutr. 1978, 108, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Birk, R.Z.; Brannon, P.M. Regulation of pancreatic lipase by dietary medium chain triglycerides in the weanling rat. Pediatr. Res. 2004, 55, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Geliebter, A.; Torbay, N.; Bracco, E.F.; Hashim, S.A.; Van Itallie, T.B. Overfeeding with medium-chain triglyceride diet results in diminished deposition of fat. Am. J. Clin. Nutr. 1983, 37, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Peters, J.C.; Yang, D.; Sharp, T.; Kaler, M.; Abumrad, N.N.; Greene, H.L. Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metabolism 1989, 38, 641–648. [Google Scholar] [CrossRef]
- Geelen, M.J.; Schoots, W.J.; Bijleveld, C.; Beynen, A.C. Dietary medium-chain fatty acids raise and (n-3) polyunsaturated fatty acids lower hepatic triacylglycerol synthesis in rats. J. Nutr. 1995, 125, 2449–2456. [Google Scholar] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Erickson, R.H.; Zakim, D.; Vessey, D.A. Preparation and properties of a phospholipid-free form of microsomal UDP-glucuronyltransferase. Biochemistry 1978, 17, 3706–3711. [Google Scholar] [CrossRef] [PubMed]
- Nepokroeff, C.M.; Lakshmanan, M.R.; Porter, J.W. Fatty-acid synthase from rat liver. Methods Enzymol. 1975, 35, 37–44. [Google Scholar] [PubMed]
- Small, G.M.; Burdett, K.; Connock, M.J. A sensitive spectrophotometric assay for peroxisomal acyl-CoA oxidase. Biochem. J. 1985, 227, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numa, S.; Ringelmann, E.; Lynen, F. On inhibition of acetyl-CoA-carboxylase by fatty acid-coenzyme A compounds. Biochem. Z. 1965, 343, 243–257. [Google Scholar] [PubMed]
- Bieber, L.L.; Abraham, T.; Helmrath, T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal. Biochem. 1972, 50, 509–518. [Google Scholar] [CrossRef]
- Edwards, P.A.; Gould, R.G. Turnover rate of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase as determined by use of cycloheximide. J. Biol. Chem. 1972, 247, 1520–1524. [Google Scholar] [PubMed]
- Edwards, P.A.; Lemongello, D.; Fogelman, A.M. Improved methods for the solubilization and assay of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Lipid Res. 1979, 20, 40–46. [Google Scholar] [PubMed]
- Heller, R.A.; Gould, R.G. Solubilization and partial purification of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem. Biophys. Res. Commun. 1973, 50, 859–865. [Google Scholar] [CrossRef]
- Fernando-Warnakulasuriya, G.J.; Staggers, J.E.; Frost, S.C.; Wells, M.A. Studies on fat digestion, absorption, and transport in the suckling rat. I. Fatty acid composition and concentrations of major lipid components. J. Lipid Res. 1981, 22, 668–674. [Google Scholar] [PubMed]
- Mascioli, E.A.; Lopes, S.; Randall, S.; Porter, K.A.; Kater, G.; Hirschberg, Y.; Babayan, V.K.; Bistrian, B.R.; Blackburn, G.L. Serum fatty acid profiles after intravenous medium chain triglyceride administration. Lipids 1989, 24, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Muurling, M.; Jong, M.C.; Mensink, R.P.; Hornstra, G.; Dahlmans, V.E.; Pijl, H.; Voshol, P.J.; Havekes, L.M. A low-fat diet has a higher potential than energy restriction to improve high-fat diet-induced insulin resistance in mice. Metabolism 2002, 51, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Monzillo, L.U.; Hamdy, O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr. Rev. 2003, 61, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, G.T.; Ahmann, A.; Meeuws, K.; McMurry, M.P.; Duell, P.B.; Connor, W.E. Effects of a low-fat diet compared with those of a high-monounsaturated fat diet on body weight, plasma lipids and lipoproteins, and glycemic control in type 2 diabetes. Am. J. Clin. Nutr. 2004, 80, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kissebah, A.H. Low density lipoprotein metabolism in non-insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 1987, 3, 619–651. [Google Scholar] [CrossRef] [PubMed]
- Streicher, R.; Kotzka, J.; Müller-Wieland, D.; Siemeister, G.; Munck, M.; Avci, H.; Krone, W. SREBP-1 mediates activation of the low density lipoprotein receptor promoter by insulin and insulin-like growth factor-I. J. Biol. Chem. 1996, 271, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; West, K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. Diabetes, lipoprotein metabolism and atherosclerosis. Br. Med. Bull. 1989, 45, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Breslow, J.L.; Hennekens, C.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Zhang, Y.L.; Hernandez-Ono, A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 2005, 36, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Baliarsingh, S.; Beg, Z.H.; Ahmad, J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005, 182, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Raz, I.; Eldor, R.; Cernea, S.; Shafrir, E. Diabetes: Insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes Metab. Res. Rev. 2005, 21, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Takase, S.; Morimoto, A.; Nakanishi, M.; Muto, Y. Long-term effect of medium-chain triglyceride on hepatic enzymes catalyzing lipogenesis and cholesterogenesis in rats. J. Nutr. Sci. Vitaminol. 1977, 23, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Trapani, L.; Segatto, M.; Pallottini, V. Regulation and deregulation of cholesterol homeostasis: The liver as a metabolic “power station”. World J. Hepatol. 2012, 4, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xue, C.; Zhang, Y.; Liu, Y.; Wang, J.; Yu, X.; Zhang, X.; Zhang, R.; Yang, X.; Guo, C. Medium-chain fatty acids enhanced the excretion of fecal cholesterol and cholic acid in C57BL/6J mice fed a cholesterol-rich diet. Biosci. Biotechnol. Biochem. 2013, 77, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.I.; Petro, A.E.; Tiller, J.M.; Feinglos, M.N.; Surwit, R.S. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism 1998, 47, 1089–1096. [Google Scholar] [CrossRef]




| LS | LM | HS | HM | |
|---|---|---|---|---|
| 0 week-Weight (g) | 353.8 ± 11.5 | 354.1 ± 8.0 | 357.4 ± 18.7 | 350.6 ± 12.81 |
| 8 week-Weight (g) | 373.1 ± 18.4 ab | 340.3 ± 14.3 b | 435.4 ± 12.0 a | 392.8 ± 19.2 ab |
| RDWAT (g) | 5.1 ± 1.0 | 4.9 ± 0.9 | 9.3 ± 0.9 | 8.4 ± 1.2 |
| RDWAT % | 1.2 ± 0.2 ab | 1.2 ± 0.2 b | 2.1 ± 0.2 a | 1.9 ± 0.2 ab |
| BAT (g) | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.33 ± 0.0 | 0.34 ± 0.0 |
| BAT % | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Liver (g) | 11.5 ± 0.6 | 12.5 ± 0.6 | 12.6 ± 0.4 | 12.1 ± 0.4 |
| Liver % | 3.2 ± 0.1 ab | 3.4 ± 0.1 a | 3.0 ± 0.1 b | 3.1 ± 0.1 ab |
| Kidney (g) | 3.0 ± 0.1 | 3.2 ± 0.1 | 3.7 ± 0.2 | 3.1 ± 0.1 |
| LS | LM | HS | HM | |
|---|---|---|---|---|
| Blood glucose (mg/dL) | 302.5 ± 15.4 | 287.9 ± 22.7 | 368.2 ± 32.8 | 297.9 ± 22.1 |
| Insulin (ug/L) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Triglyceride (mg/dL) | 55.9 ± 2.0 | 52.2 ± 1.8 | 69.2 ± 5.0 | 57.4 ± 3.6 |
| Total cholesterol (mg/dL) | 63.8 ± 3.3 | 63.3 ± 3.6 | 70.6 ± 1.6 | 64.2 ± 2.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.-H.; Liao, F.-H.; Chien, Y.-W. Medium-Chain Triglycerides Lower Blood Lipids and Body Weight in Streptozotocin-Induced Type 2 Diabetes Rats. Nutrients 2018, 10, 963. https://doi.org/10.3390/nu10080963
Sung M-H, Liao F-H, Chien Y-W. Medium-Chain Triglycerides Lower Blood Lipids and Body Weight in Streptozotocin-Induced Type 2 Diabetes Rats. Nutrients. 2018; 10(8):963. https://doi.org/10.3390/nu10080963
Chicago/Turabian StyleSung, Ming-Hua, Fang-Hsuean Liao, and Yi-Wen Chien. 2018. "Medium-Chain Triglycerides Lower Blood Lipids and Body Weight in Streptozotocin-Induced Type 2 Diabetes Rats" Nutrients 10, no. 8: 963. https://doi.org/10.3390/nu10080963






