Determinants of Behaviour Change in a Multi-Component Telemonitoring Intervention for Community-Dwelling Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Ethics Approval and Consent to Participate
2.3. Trial Registration
2.4. Participants
2.5. Intervention
2.5.1. Telemonitoring Measurements
2.5.2. Education
2.5.3. Follow-Up
2.6. Measurements
2.6.1. Baseline Characteristics
2.6.2. Frequency of Self-Monitoring and Goalsetting
2.6.3. Behavioural Determinants
2.6.4. Compliance with Dutch Dietary Guidelines and Guidelines for Physical Activity
2.7. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Changes in Self-Monitoring and Goalsetting and Effects on Behavioural Determinants
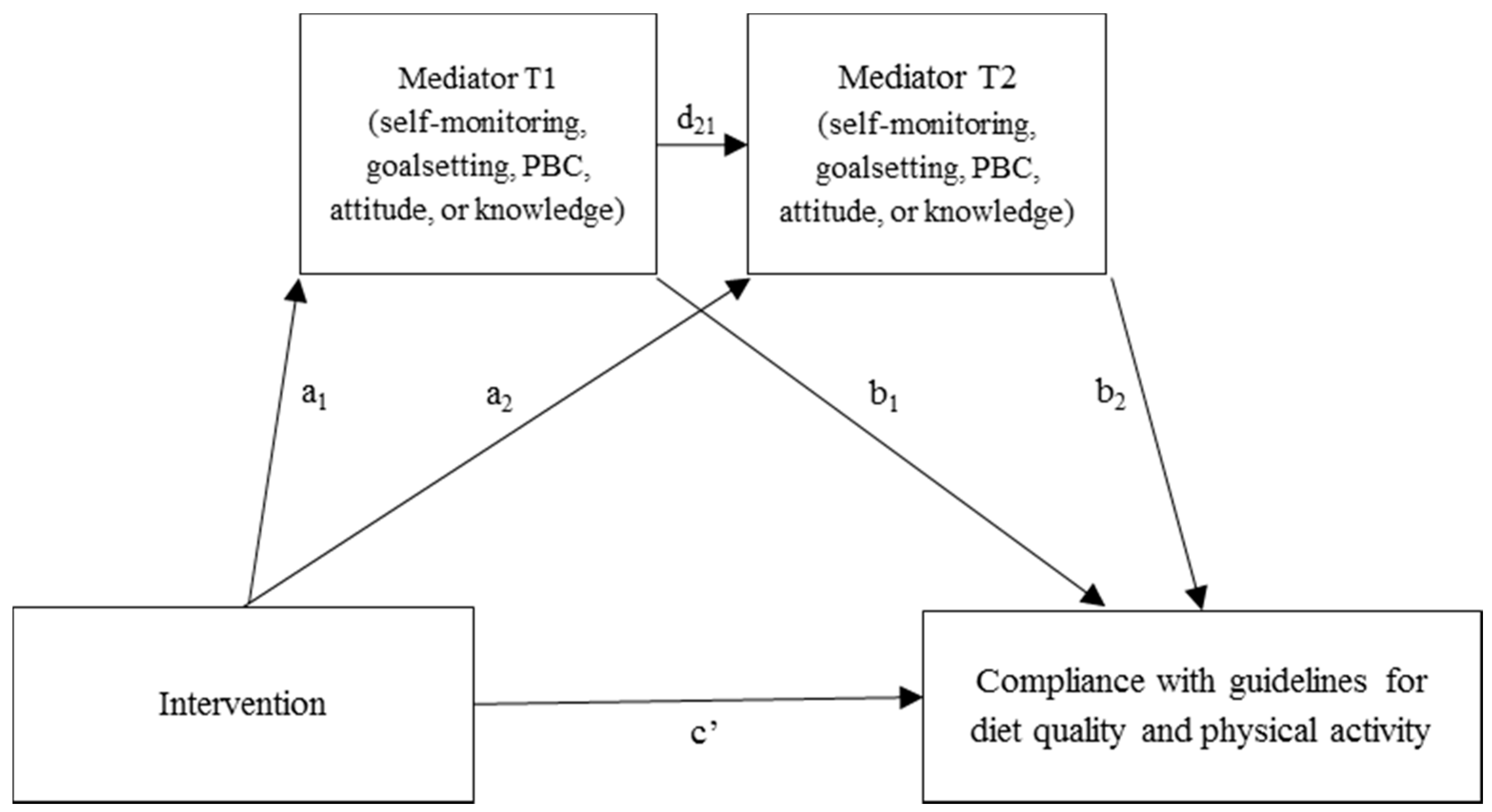
3.3. Effect Mediation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Indirect Effect 1 a,b (a1 × b1) | Indirect effect 2 a,c (a1 × d21 × b2) | Indirect Effect 3 a,d (a2 × b2) | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | (95% CI) | β (SE) | (95% CI) | N | |
| T0–T2 Total DHD-FFQ score | |||||||
| Self-monitoring | 0.79 (0.50) | 0.09, 2.11 | 0.07 (0.21) | −0.27, 0.65 | 0.16 (0.47) | −0.61, 1.35 | 141 |
| Goalsetting | 0.07 (0.30) | −0.24, 1.06 | 0.04 (0.16) | −0.15, 0.65 | 0.11 (0.26) | −0.15, 1.06 | 140 |
| Knowledge | 0.23 (0.37) | −0.28, 1.32 | −0.17 (0.19) | −0.74, 0.07 | −0.18 (0.27) | −1.10, 0.11 | 139 |
| PBC HE | 0.05 (0.23) | −0.28, 0.76 | 0.01 (0.11) | −0.20, 0.27 | −0.01 (0.16) | −0.44, 0.28 | 136 |
| Attitude HE | −0.03 (0.16) | −0.55, 0.19 | −0.01 (0.06) | −0.19, 0.05 | 0.05 (0.24) | −0.30, 0.80 | 137 |
| T0–T2 Fish | |||||||
| Self-monitoring | −0.07 (0.11) | −0.36, 0.10 | 0.01 (0.06) | −0.09, 0.14 | 0.02 (0.12) | −0.20, 0.30 | 141 |
| Goalsetting | −0.01 (0.07) | −0.23, 0.07 | −0.00 (0.03) | −0.08, 0.06 | −0.00 (0.05) | −0.14, 0.09 | 140 |
| Knowledge | 0.09 (0.10) | −0.04, 0.38 | −0.02 (0.06) | −0.17, 0.08 | −0.02 (0.07) | −0.24, 0.08 | 139 |
| PBC HE | 0.05 (0.09) | −0.04, 0.35 | −0.02 (0.04) | −0.18, 0.02 | 0.02 (0.06) | −0.04, 0.22 | 136 |
| Attitude HE | 0.00 (0.04) | −0.08, 0.09 | −0.00 (0.01) | −0.04, 0.01 | 0.01 (0.05) | −0.06, 0.17 | 137 |
| T0–T2 Saturated fatty acids | |||||||
| Self-monitoring | 0.31 (0.21) | 0.02, 0.86 | 0.06 (0.09) | −0.05, 0.32 | 0.14 (0.18) | −0.14, 0.59 | 141 |
| Goalsetting | 0.05 (0.16) | −0.17, 0.51 | −0.00 (0.06) | −0.18, 0.10 | −0.01 (0.10) | −0.30, 0.15 | 140 |
| Knowledge | 0.03 (0.15) | −0.22, 0.40 | −0.03 (0.08) | −0.24, 0.09 | −0.03 (0.10) | −0.35, 0.09 | 139 |
| PBC HE | −0.01 (0.10) | −0.29, 0.16 | 0.04 (0.06) | −0.03, 0.27 | −0.04 (0.10) | −0.39, 0.08 | 136 |
| Attitude HE | −0.03 (0.12) | −0.46, 0.12 | −0.01 (0.03) | −0.12, 0.02 | 0.03 (0.12) | −0.15, 0.37 | 137 |
| T0–T2 Salt | |||||||
| Self-monitoring | 0.05 (0.13) | −0.17, 0.37 | 0.02 (0.07) | −0.10, 0.20 | 0.04 (0.15) | −0.23, 0.40 | 141 |
| Goalsetting | −0.00 (0.07) | −0.18, 0.12 | 0.01 (0.05) | −0.04, 0.19 | 0.03 (0.08) | −0.05, 0.34 | 140 |
| Knowledge | 0.00 (0.11) | −0.21, 0.27 | −0.08 (0.07) | −0.32, 0.01 | −0.08 (0.10) | −0.42, 0.03 | 139 |
| PBC HE | −0.06 (0.09) | −0.37, 0.04 | 0.02 (0.04) | −0.02, 0.15 | −0.02 (0.06) | −0.23, 0.04 | 136 |
| Attitude HE | 0.01 (0.05) | −0.05, 0.16 | −0.00 (0.02) | −0.05, 0.02 | 0.01 (0.07) | −0.10, 0.20 | 137 |
| T0–T2 Alcohol | |||||||
| Self-monitoring | 0.04 (0.04) | −0.02, 0.17 | 0.01 (0.04) | −.0.05, 0.11 | 0.02 (0.08) | −0.13, 0.20 | 141 |
| Goalsetting | −0.00 (0.03) | −0.10, 0.05 | 0.00 (0.02) | −0.01, 0.06 | 0.01 (0.02) | −0.01, 0.09 | 140 |
| Knowledge | −0.00 (0.03) | −0.08, 0.05 | 0.01 (0.03) | −0.03, 0.11 | 0.01 (0.04) | −0.03, 0.15 | 139 |
| PBC HE | 0.01 (0.02) | −0.01, 0.07 | 0.01 (0.02) | −0.01, 0.11 | −0.01 (0.04) | −0.18, 0.03 | 136 |
| Attitude HE | 0.00 (0.03) | −0.02, 0.10 | −0.00 (0.02) | −0.08, 0.01 | 0.01 (0.08) | −0.07, 0.31 | 137 |
| T0–T2 Vitamin D | |||||||
| Self-monitoring | 0.00 (0.04) | −0.07, 0.08 | −0.01 (0.03) | −0.06, 0.06 | −0.01 (0.06) | −0.14, 0.11 | 141 |
| Goalsetting | −0.00 (0.02) | −0.07, 0.03 | −0.00 (0.01) | −0.05, 0.01 | −0.01 (0.02) | −0.09, 0.01 | 140 |
| Knowledge | 0.01 (0.03) | −0.05, 0.08 | 0.03 (0.04) | −0.01, 0.16 | 0.04 (0.05) | −0.01, 0.24 | 139 |
| PBC HE | 0.02 (0.0.3) | −0.01, 0.12 | −0.02 (0.03) | −0.12, 0.01 | 0.02 (0.05) | −0.03, 0.17 | 136 |
| Attitude HE | 0.00 (0.02) | −0.03, 0.08 | −0.00 (0.00) | −0.02, 0.01 | 0.00 (0.02) | −0.03, 0.06 | 137 |
References
- Morley, J.E. Undernutrition in Older Adults. Fam. Pract. 2012, 29, i89–i93. [Google Scholar] [CrossRef] [PubMed]
- Schilp, J.; Kruizenga, H.M.; Wijnhoven, H.A.; Leistra, E.; Evers, A.M.; Van Binsbergen, J.J.; Deeg, D.J.; Visser, M. High Prevalence of Undernutrition in Dutch Community-Dwelling Older Individuals. Nutrition 2012, 28, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Ocke, M.C.; Buurma-Rethans, E.J.M.; De Boer, E.J.; Wilson-van den Hooven, C.; Etemad-Ghameslou, Z.; Drijvers, J.J.M.M.; Van Rossum, C.T.M. Diet of Community-Dwelling Older Adults: Dutch National Food Consumption Survey Older Adults 2010–2012. Available online: http://rivm.openrepository.com/rivm/handle/10029/305649 (accessed on 8 August 2018).
- Ziylan, C.; Janssen, N.; De Roos, N.M.; De Groot, L.C.P.G.M. Undernutrition: Who Cares? Perspectives of Dietitians and Older Adults on Undernutrition. BMC Nutr. 2017, 3, 24. [Google Scholar]
- Ziylan, C.; Haveman-Nies, A.; Van Dongen, E.J.I.; Kremer, S.; De Groot, C.P.G.M. Dutch Nutrition and Care Professionals’ Experiences with Undernutrition Awareness, Monitoring, and Treatment among Community-Dwelling Older Adults: A. Qualitative Study. BMC Nutr. 2015, 1, 38. [Google Scholar] [CrossRef]
- Parmenter, K.; Waller, J.; Wardle, J. Demographic Variation in Nutrition Knowledge in England. Health Educ. Res. 2000, 15, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.A.; Crockett, S.J.; Heller, K.E.; Skauge, L.H. Nutrition Knowledge, Attitudes, and Practices of Older and Younger Elderly in Rural Areas. J. Am. Diet. Assoc. 1991, 91, 1398–1401. [Google Scholar] [PubMed]
- Stafleu, A.; Van Staveren, W.A.; De Graaf, C.; Burema, J.; Hautvast, J.G. Nutrition Knowledge and Attitudes Towards High-Fat Foods and Low-Fat Alternatives in Three Generations of Women. Eur. J. Clin. Nutr. 1996, 50, 33–41. [Google Scholar] [PubMed]
- Kamp, B.J.; Wellman, N.S.; Russell, C. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and Nutrition Programs for Community-Residing Older Adults. J. Nutr. Educ. Behav. 2010, 42, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.; Merom, D.; Bull, F.C.; Buchner, D.M.; Fiatarone Singh, M.A. Updating the Evidence for Physical Activity: Summative Reviews of the Epidemiological Evidence, Prevalence, and Interventions to Promote “Active Aging”. Gerontologist 2016, 56, S268–S280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baert, V.; Gorus, E.; Mets, T.; Geerts, C.; Bautmans, I. Motivators and Barriers for Physical Activity in the Oldest Old: A Systematic Review. Ageing Res. Rev. 2011, 10, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Gellert, P.; Witham, M.D.; Crombie, I.K.; Donnan, P.T.; McMurdo, M.E.; Sniehotta, F.F. The Role of Perceived Barriers and Objectively Measured Physical Activity in Adults Aged 65-100. Age Ageing 2015, 44, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Lara, J.; Mathers, J.C. Personalising Nutritional Guidance for More Effective Behaviour Change. Proc. Nutr. Soc. 2015, 74, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Felix, L.; Miners, A.; Murray, E.; Michie, S.; Ferguson, E.; Free, C.; Lock, K.; Landon, J.; Edwards, P. Adaptive E-Learning to Improve Dietary Behaviour: A Systematic Review and Cost-Effectiveness Analysis. Health Technol. Assess. 2011, 15, 1–160. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.J.; Zabinski, M.F.; Adams, M.A.; Rosenberg, D.E.; Yaroch, A.L.; Atienza, A.A. A Review of Ehealth Interventions for Physical Activity and Dietary Behavior Change. Am. J. Prev. Med. 2007, 33, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Neville, L.M.; O’Hara, B.; Milat, A.J. Computer-Tailored Dietary Behaviour Change Interventions: A Systematic Review. Health Educ. Res. 2009, 24, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Reidlinger, D.P.; Hoffmann, T.C.; Campbell, K.L. Telehealth Methods to Deliver Dietary Interventions in Adults with Chronic Disease: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2016, 104, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; O’Brien, N.; Godfrey, A.; Heaven, B.; Evans, E.H.; Lloyd, S.; Moffatt, S.; Moynihan, P.J.; Meyer, T.D.; Rochester, L.; et al. Pilot Randomised Controlled Trial of a Web-Based Intervention to Promote Healthy Eating, Physical Activity and Meaningful Social Connections Compared with Usual Care Control in People of Retirement Age Recruited from Workplaces. PLoS ONE 2016, 11, e0159703. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, M.; Bakx, J.C.; Akkermans, R.; Van den Hoogen, H.; Godwin, N.M.; Rosser, W.; Van Staveren, W.; Van Weel, C. Web-Based Targeted Nutrition Counselling and Social Support for Patients at Increased Cardiovascular Risk in General Practice: Randomized Controlled Trial. J. Med. Internet Res. 2004, 6, e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The Behavior Change Technique Taxonomy (V1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Joseph, J.; Yardley, L.; Michie, S. Using the Internet to Promote Health Behavior Change: A Systematic Review and Meta-Analysis of the Impact of Theoretical Basis, Use of Behavior Change Techniques, and Mode of Delivery on Efficacy. J. Med. Int. Res. 2010, 12, e4. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn-van Atten, M.N.; Haveman-Nies, A.; Van Bakel, M.M.; Ferry, M.; Franco, M.; De Groot, L.; De Vries, J.H.M. Effects of a Multi-Component Nutritional Telemonitoring Intervention on Nutritional Status, Diet Quality, Physical Functioning and Quality of Life of Community-Dwelling Older Adults. Br. J. Nutr. 2018, 119, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Michie, S. A Taxonomy of Behavior Change Techniques Used in Interventions. Health Psychol. 2008, 27, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carver, C.S.; Scheier, M.F. Control Theory: A Useful Conceptual Framework for Personality-Social, Clinical, and Health Psychology. Psychol. Bull. 1982, 92, 111–135. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Abraham, C.; Whittington, C.; McAteer, J.; Gupta, S. Effective Techniques in Healthy Eating and Physical Activity Interventions: A Meta-Regression. Health Psychol. 2009, 28, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, L.G.; Yardley, L.; Powell, J.; Michie, S. What Design Features Are Used in Effective E-Health Interventions? A Review Using Techniques from Critical Interpretive Synthesis. Telemed. J. E Health 2012, 18, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bodenheimer, T.; Lorig, K.; Holman, H.; Grumbach, K. Patient Self-Management of Chronic Disease in Primary Care. JAMA 2002, 288, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (Mna-Sf): A Practical Tool for Identification of Nutritional Status. J. Nutr Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite Assessment: Simple Appetite Questionnaire Predicts Weight Loss in Community-Dwelling Adults and Nursing Home Residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Van Lee, L.; Feskens, E.J.; Meijboom, S.; Hooft van Huysduynen, E.J.; Van’t Veer, P.; De Vries, J.H.; Geelen, A. Evaluation of a Screener to Assess Diet Quality in the Netherlands. Br. J. Nutr. 2016, 115, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Ondervoeding. Eten Bij Ondervoeding En Bij Herstel Na Ziekte. Available online: https://webshop.voedingscentrum.nl/pdf/D752-20.pdf (accessed on 8 August 2018).
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State” A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189. [Google Scholar] [CrossRef]
- Laan, W.; Zuithoff, N.P.; Drubbel, I.; Bleijenberg, N.; Numans, M.E.; De Wit, N.J.; Schuurmans, M.J. Validity and Reliability of the Katz-15 Scale to Measure Unfavorable Health Outcomes in Community-Dwelling Older People. J. Nutr. Health Aging 2014, 18, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.E.; Chumlea, W.; Salva, A.; Rubenstein, L.Z.; et al. Overview of the MNA—Its History and Challenges. J. Nutr. Health Aging 2006, 10, 456–463. [Google Scholar] [PubMed]
- Nothwehr, F.; Dennis, L.; Wu, H. Measurement of Behavioral Objectives for Weight Management. Health Educ. Behav. 2007, 34, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Nothwehr, F.; Yang, J. Goal Setting Frequency and the Use of Behavioral Strategies Related to Diet and Physical Activity. Health Educ. Res. 2007, 22, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.J.; Eccles, M.P.; Johnston, M.; Walker, A.; Grimshaw, J.; Foy, R.; Kaner, E.F.S.; Smith, L.; Bonetti, D. Constructing Questionnaires Based on the Theory of Planned Behaviour. Available online: http://openaccess.city.ac.uk/1735/1/TPB%20Manual%20FINAL%20May2004.pdf (accessed on 8 August 2018).
- Blue, C.L.; Marrero, D.G. Psychometric Properties of the Healthful Eating Belief Scales for Persons at Risk of Diabetes. J. Nutr. Educ. Behav. 2006, 38, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Richtlijnen Goede Voeding; Gezondheidsraad: Den Haag, The Netherland, 2006.
- Becker, W.; Lyhne, N.; Pedersen, A.N.; Aro, A.; Fogelholm, M.; Phorsdottir, I.; Alexander, J.; Anderssen, S.A.; Meltzer, H.M.; Pedersen, J.I. Nordic Nutrition Recommendations 2004-integrating nutrition and physical activity. Scand. J. Nutr. 2004, 48, 178–187. [Google Scholar] [CrossRef]
- Nijs, K.A.; De Graaf, C.; Siebelink, E.; Blauw, Y.H.; Vanneste, V.; Kok, F.J.; Van Staveren, W.A. Effect of Family-Style Meals on Energy Intake and Risk of Malnutrition in Dutch Nursing Home Residents: A Randomized Controlled Trial. J. Gerontol. A: Biol. Sci. Med. Sci. 2006, 61, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2013. [Google Scholar]
- Burke, L.E.; Wang, J.; Sevick, M.A. Self-Monitoring in Weight Loss: A Systematic Review of the Literature. J. Am. Diet. Assoc. 2011, 111, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieffers, J.R.; Hanning, R.M. Dietary Assessment and Self-Monitoring with Nutrition Applications for Mobile Devices. Can. J. Diet. Pract. Res. 2012, 73, e253–e260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sereika, S.M.; Chasens, E.R.; Ewing, L.J.; Matthews, J.T.; Burke, L.E. Effect of Adherence to Self-Monitoring of Diet and Physical Activity on Weight Loss in a Technology-Supported Behavioral Intervention. Patient Prefer. Adherence 2012, 6, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajzen, I. The Theory of Planned Behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Rhodes, R.E.; De Bruijn, G.J. What Predicts Intention-Behavior Discordance? A Review of the Action Control Framework. Exerc. Sport Sci. Rev. 2013, 41, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruijn, G.-J. Exercise Habit Strength, Planning and the Theory of Planned Behaviour: An Action Control Approach. Psychol. Sport Exerc. 2011, 12, 106–114. [Google Scholar] [CrossRef]
- Van Stralen, M.M.; De Vries, H.; Mudde, A.N.; Bolman, C.; Lechner, L. Determinants of Initiation and Maintenance of Physical Activity among Older Adults: A Literature Review. Health Psychol. Rev. 2009, 3, 147–207. [Google Scholar] [CrossRef]
- Hardeman, W.; Kinmonth, A.L.; Michie, S.; Sutton, S. Theory of Planned Behaviour Cognitions Do Not Predict Self-Reported or Objective Physical Activity Levels or Change in the Proactive Trial. Br. J. Health Psychol. 2011, 16, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Southgate, K.M.; Keller, H.H.; Reimer, H.D. Determining Knowledge and Behaviour Change: After Nutrition Screening among Older Adults. Can. J. Diet. Pract. Res. 2010, 71, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Racine, E.; Troyer, J.L.; Warren-Findlow, J.; McAuley, W.J. The Effect of Medical Nutrition Therapy on Changes in Dietary Knowledge and Dash Diet Adherence in Older Adults with Cardiovascular Disease. J. Nutr. Health Aging 2011, 15, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.P. Nutrition Education Intervention with Community-Dwelling Older Adults: Research Challenges and Opportunities. J. Community Health 2014, 39, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Spronk, I.; Kullen, C.; Burdon, C.; O’Connor, H. Relationship between Nutrition Knowledge and Dietary Intake. Br. J. Nutr. 2014, 111, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Worsley, A. Nutrition Knowledge and Food Consumption: Can Nutrition Knowledge Change Food Behaviour? Asia Pac. J. Clin. Nutr. 2002, 11, 579–585. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary Protein Recommendations and the Prevention of Sarcopenia: Protein, Amino Acid Metabolism and Therapy. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.J.; Conner, M. Efficacy of the Theory of Planned Behaviour: A Meta-Analytic Review. Br. J. Soc. Psychol. 2001, 40, 471–499. [Google Scholar] [CrossRef] [PubMed]
- French, D.P.; Olander, E.K.; Chisholm, A.; Mc Sharry, J. Which Behaviour Change Techniques Are Most Effective at Increasing Older Adults’ Self-Efficacy and Physical Activity Behaviour? A Systematic Review. Ann. Behav. Med. 2014, 48, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grady, A.; Yoong, S.; Sutherland, R.; Lee, H.; Nathan, N.; Wolfenden, L. Improving the Public Health Impact of Ehealth and Mhealth Interventions. Aust. N. Z. J. Public Health 2018, 42, 118–119. [Google Scholar] [CrossRef] [PubMed]

| Construct | Questionnaire Items | Answering Options (1–5) | Crohnbach’s Alpha T0 |
|---|---|---|---|
| Self-monitoring |
| Never/a single time/a couple of times/every week/everyday | 0.77 |
| Goalsetting |
| Never/a single time/a couple of times/every week/everyday | 0.67 |
| Perceived behavioural control healthy eating |
| Totally disagree–totally agree Totally disagree–totally agree Difficult–easy No control–complete control | 0.70 |
| Perceived behavioural control physical activity |
| Totally disagree–totally agree Totally disagree–totally agree Difficult–easy No control–complete control | 0.71 |
| Attitude healthy eating | Healthy eating in the coming month is for me... | foolish–wise pleasant–unpleasant bad–good harmful–helpful unnecessary–necessary unenjoyable–enjoyable | 0.70 |
| Attitude physical activity | Physical activity in the coming month is for me... | foolish–wise pleasant–unpleasant bad–good harmful–helpful unnecessary–necessary boring–interesting | 0.80 |
| Intervention Group (n = 97) | Control Group (n = 107) | p-Value a | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 78.4 | 7.2 | 81.0 | 7.9 | 0.02 |
| BMI (kg/m2) | 29.2 | 4.5 | 27.7 | 5.4 | 0.04 |
| Number of diagnoses | 1.5 | 1.5 | 1.3 | 1.3 | 0.26 |
| MMSE score | 28.6 | 1.5 | 25.8 | 1.9 | 0.69 |
| Katz-15 score | Mdn | IQR | Mdn | IQR | |
| 1.0 | 0–4 | 1.0 | 0–3 | 0.69 | |
| Percentage | Percentage | ||||
| Sex (male) | 35 | 23.4 | 0.09 | ||
| Education level b | 0.08 | ||||
| Low | 17.5 | 10.3 | |||
| Moderate | 55.7 | 49.5 | |||
| High | 26.8 | 40.2 | |||
| Civil status | 0.11 | ||||
| Married | 42.3 | 27.1 | |||
| Single | 7.2 | 13.1 | |||
| Divorced | 7.2 | 10.3 | |||
| Widowed | 43.3 | 49.5 | |||
| Living alone | 55.7 | 74.8 | 0.004 | ||
| Born in the Netherlands | 96.9 | 90.7 | 0.07 | ||
| Desire to lose weight | 52.7 | 39.4 | 0.07 | ||
| Nutritional status | 0.45 | ||||
| Normal nutritional status | 79.2 | 83.8 | |||
| At risk of undernutrition | 19.8 | 16.2 | |||
| Undernourished | 1.0 | 0.0 | |||
| Type of care | |||||
| Domestic care | 78.4 | 80.4 | 0.72 | ||
| Personal care | 32.0 | 29.9 | 0.75 | ||
| Nursing care | 9.3 | 2.8 | 0.05 | ||
| Individual support | 3.1 | 0.9 | 0.27 | ||
| Informal care | 32.0 | 11.2 | <0.001 | ||
| Intervention Group | Control Group | Linear Mixed Models | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | β T1 (95% CI) | β T2 (95% CI) | N | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Self-monitoring | 2.9 | 1.2 | 3.5 | 0.9 | 3.3 | 1.1 | 3.1 | 1.2 | 3.1 | 1.2 | 3.0 | 1.3 | 0.49 (0.19, 0.80) ** | 0.50 (0.20, 0.80) ** | 199 |
| Goalsetting | 2.7 | 1.2 | 3.0 | 1.1 | 2.8 | 1.2 | 3.0 | 1.1 | 3.0 | 1.3 | 2.9 | 1.2 | 0.25 (−0.05, 0.55) | 0.19 (−0.10, 0.48) | 199 |
| Knowledge a | 7.3 | 2.1 | 8.2 | 1.9 | 8.3 | 1.8 | 7.5 | 2.0 | 7.5 | 2.1 | 7.6 | 2.2 | 0.51 (−0.09, 1.12) | 0.51 (0.04, 0.99) * | 198 |
| PBC HE b | 4.1 | 0.7 | 4.2 | 0.7 | 4.2 | 0.6 | 4.3 | 0.6 | 4.1 | 0.8 | 4.3 | 0.7 | 0.16 (−0.02, 0.33) | 0.08 (−0.09, 0.25) | 188 |
| PBC PA | 3.7 | 0.8 | 3.8 | 0.9 | 3.9 | 0.9 | 4.0 | 0.9 | 3.8 | 1.0 | 3.9 | 0.9 | 0.19 (−0.03, 0.41) | 0.26 (0.08, 0.45) ** | 199 |
| Attitude HE c | 4.7 | 0.5 | 4.7 | 0.5 | 4.7 | 0.5 | 4.6 | 0.5 | 4.6 | 0.5 | 4.6 | 0.6 | −0.01 (−0.18, 0.16) | 0.00 (−0.17, 0.17) | 188 |
| Attitude PA d | 4.5 | 0.6 | 4.4 | 0.8 | 4.5 | 0.7 | 4.5 | 0.7 | 4.4 | 0.8 | 4.5 | 0.7 | −0.04 (−0.29, 0.22) | −0.05 (−0.26, 0.15) | 190 |
| Indirect Effect 1 a,b (a1 × b1) | Indirect Effect 2 a,c (a1 × d21 × b2) | Indirect Effect 3 a,d (a2 × b2) | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | (95% CI) | β (SE) | (95% CI) | N | |
| T0–T2 Fruit | |||||||
| Self-monitoring | 0.16 (0.10) | 0.02, 0.45 | 0.02 (0.04) | −0.04, 0.13 | 0.04 (0.08) | −0.11, 0.24 | 141 |
| Goalsetting | 0.03 (0.08) | −0.08, 0.27 | −0.00 (0.03) | −0.09, 0.03 | −0.01 (0.04) | −0.15, 0.03 | 140 |
| Knowledge | 0.17 (0.14) | −0.01, 0.57 | −0.02 (0.05) | −0.15, 0.05 | −0.02 (0.06) | −0.21, 0.04 | 139 |
| PBC HE | 0.01 (0.05) | −0.05, 0.18 | 0.02 (0.03) | −0.01, 0.13 | −0.02 (0.05) | −0.20, 0.04 | 136 |
| Attitude HE | −0.00 (0.04) | −0.09, 0.07 | 0.00 (0.01) | −0.01, 0.03 | −0.00 (0.05) | −0.12, 0.07 | 137 |
| T0–T2 Vegetables | |||||||
| Self–monitoring | −0.07 (0.11) | −0.11, 0.35 | −0.06 (0.05) | −0.22, 0.01 | −0.12 (0.11) | −0.39, 0.04 | 141 |
| Goalsetting | 0.01 (0.06) | −0.05, 0.22 | −0.00 (0.03) | −0.07, 0.05 | −0.00 (0.05) | −0.12, 0.08 | 140 |
| Knowledge | −0.07 (0.09) | −0.32, 0.06 | −0.01 (0.05) | −0.11, 0.08 | −0.01 (0.06) | −0.18, 0.08 | 139 |
| PBC HE | −0.01 (0.07) | −0.25, 0.06 | −0.01 (0.03) | −0.13, 0.02 | 0.02 (0.05) | −0.04, 0.21 | 136 |
| Attitude HE | −0.01 (0.07) | −0.23, 0.07 | 0.00 (0.01) | −0.01, 0.04 | −0.00 (0.05) | −0.13, 0.08 | 137 |
| T0–T2 Dietary fibre | |||||||
| Self-monitoring | −0.07 (0.07) | −0.27, 0.03 | 0.03 (0.04) | −0.02. 0.14 | 0.07 (0.07) | −0.05, 0.26 | 141 |
| Goalsetting | −0.00 (0.03) | −0.08, 0.05 | 0.01 (0.02) | −0.02, 0.09 | 0.02 (0.04) | −0.02, 0.15 | 140 |
| Knowledge | 0.02 (0.06) | −0.06, 0.22 | 0.01 (0.03) | −0.04, 0.10 | 0.1 (0.04) | −0.04, 0.14 | 139 |
| PBC HE | 0.05 (0.05) | −0.03, 0.20 | −0.01 (0.02) | −0.08, 0.01 | 0.01 (0.03) | −0.02, 0.13 | 136 |
| Attitude HE | 0.00 (0.03) | −0.05, 0.06 | 0.00 (0.01) | −0.00, 0.03 | −0.00 (0.02) | −0.09, 0.03 | 137 |
| T0–T2 Protein | |||||||
| Self-monitoring | −0.25 (0.19) | −0.76, 0.00 | 0.00 (0.08) | −0.16, 0.18 | 0.00 (0.17) | −0.37, 0.34 | 141 |
| Goalsetting | −0.04 (0.12) | −0.42, 0.12 | 0.01 (0.06) | −0.05, 0.23 | 0.04 (0.09) | −0.05, 0.37 | 140 |
| Knowledge | −0.07 (0.12) | −0.44, 0.07 | 0.12 (0.09) | 0.006, 0.41 | 0.12 (0.13) | −0.04, 0.52 | 139 |
| PBC HE | 0.06 (0.09) | −0.04, 0.40 | −0.02 (0.04) | −0.17, 0.02 | 0.02 (0.07) | −0.05, 0.28 | 136 |
| Attitude HE | 0.01 (0.07) | −0.08, 0.23 | 0.00 (0.01) | −0.01, 0.06 | −0.01 (0.06) | −0.20, 0.08 | 137 |
| T0–T2 Physical activity | |||||||
| Self-monitoring | −0.02 (0.16) | −0.40, 0.29 | −0.04 (0.08) | −0.27, 0.07 | −0.10 (0.17) | −0.52, 0.18 | 141 |
| Goalsetting | −0.02 (0.08) | −0.32, 0.07 | −0.00 (0.05) | −0.51, 0.07 | −0.01 (0.08) | −0.25, 0.10 | 140 |
| Knowledge | −0.10 (0.13) | −0.49, 0.07 | −0.04 (0.07) | −0.24, 0.05 | −0.04 (0.09) | −0.35, 0.05 | 139 |
| PBC PA | −0.04 (0.10) | −0.31, 0.10 | 0.07 (0.08) | −0.01, 0.34 | 0.16 (0.13) | −0.02, 0.51 | 137 |
| Attitude PA | −0.003 (0.05) | −0.14, 0.07 | 0.001 (0.02) | −0.04, 0.06 | 0.008 (0.05) | −0.06, 0.17 | 133 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Doorn-van Atten, M.N.; De Groot, L.C.P.G.M.; De Vries, J.H.M.; Haveman-Nies, A. Determinants of Behaviour Change in a Multi-Component Telemonitoring Intervention for Community-Dwelling Older Adults. Nutrients 2018, 10, 1062. https://doi.org/10.3390/nu10081062
Van Doorn-van Atten MN, De Groot LCPGM, De Vries JHM, Haveman-Nies A. Determinants of Behaviour Change in a Multi-Component Telemonitoring Intervention for Community-Dwelling Older Adults. Nutrients. 2018; 10(8):1062. https://doi.org/10.3390/nu10081062
Chicago/Turabian StyleVan Doorn-van Atten, Marije N., Lisette C. P. G. M. De Groot, Jeanne H. M. De Vries, and Annemien Haveman-Nies. 2018. "Determinants of Behaviour Change in a Multi-Component Telemonitoring Intervention for Community-Dwelling Older Adults" Nutrients 10, no. 8: 1062. https://doi.org/10.3390/nu10081062





