Effects of Insect Protein Supplementation during Resistance Training on Changes in Muscle Mass and Strength in Young Men
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Protocol
2.3. Training
2.4. Dietary Supplementation
2.5. Dual-Energy X-ray Absorptiometry (DXA)
2.6. Strength Testing
2.7. Dietary Analysis
2.8. Statistics
3. Results
3.1. Subjects
3.2. Body Composition
3.3. Dietary Data
3.4. Strength
3.5. Training Volume
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1 RM | One repetition maximum |
| AA | Amino acid |
| ANOVA | Analysis of variance |
| BMC | Bone mineral content |
| BW | Body weight |
| CMJ | Counter-movement jump |
| Con | Control group |
| DXA | Dual-energy X-ray absorptiometry |
| EAA | Essential amino acid |
| FBFM | Fat- and bone- free mass |
| FM | Fat mass |
| LB | Lower body |
| MPS | Muscle protein synthesis |
| MVC | Maximal voluntary contraction |
| Post | Post-intervention |
| Pre | Pre-intervention |
| Pro | Protein group |
| UB | Upper body |
References
- Van Huis, A.; Klunder, H.; Itterbeeck, J.V.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security. Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, alphitobius diaperinus, and hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; West, D.W.; Moore, D.R.; Atherton, P.J.; Staples, A.W.; Prior, T.; Tang, J.E.; Rennie, M.J.; Baker, S.K.; Phillips, S.M. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J. Nutr. 2011, 141, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 1997, 273, E99–E107. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Ferrando, A.A.; Phillips, S.M.; Doyle, D., Jr.; Wolfe, R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. 1999, 276, E628–E634. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.D.; Wolfe, R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol, 1995, 268, E514–E520. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance Training-Induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Res, P.T.; Smeets, J.S.; van Vliet, S.; van Kranenburg, J.; Maase, K.; Kies, A.K.; Verdijk, L.B.; van Loon, L.J. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J. Nutr. 2015, 145, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; van Loon, L.J. Pre-sleep protein ingestion to improve the skeletal muscle adaptive response to exercise training. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.; Metges, C.C.; Gaudichon, C.; Petzke, K.J.; Pueyo, M.E.; Morens, C.; Everwand, J.; Benamouzig, R.; Tome, D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80. [Google Scholar] [CrossRef] [PubMed]
- Michalsik, L.; Bangsbo, J. Opvarmning. In Aerob og Anaerob Træning; Danmarks Idræts-Forbund: Brøndby, Danmark, 2002; pp. 136–141. (In Danish) [Google Scholar]
- American College of Sports Medicine. American college of sports medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar]
- Fleck, S.J.; Kraemer, W.J. Developing the individualized resistance training program. In Designing Resistance Training Programs, 4th ed.; Human Kinetics: Champaign, IL, USA, 2014; p. 200. [Google Scholar]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Willardson, J.M.; Norton, L.; Wilson, G. Training to failure and beyond in mainstream resistance exercise programs. Strength Cond. J. 2010, 32, 21–29. [Google Scholar] [CrossRef]
- Maud, P.J.; Foster, C. Strength testing: Development and evaluation of methodology. In Physiological Assessment of Human Fitness; Human Kinetics: Champaign, IL, USA, 2006; p. 129. [Google Scholar]
- Blazevich, A.J.; Cannavan, D.; Coleman, D.R.; Horne, S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J. Appl. Physiol. 2007, 103, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar] [PubMed]
- Juul, S. Epidemiologi og Evidens, 2nd ed.; Munksgaard: Copenhagen, Denmark, 2013. (In Danish) [Google Scholar]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Stout, J.R.; Wilborn, C.D. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2007, 32, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Eliot, K.A.; Knehans, A.W.; Bemben, D.A.; Witten, M.S.; Carter, J.; Bemben, M.G. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J. Nutr. Health Aging 2008, 12, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.W.; Goldman, L.P.; Puglisi, M.J.; Nickols-Richardson, S.M.; Earthman, C.P.; Gwazdauskas, F.C. Effect of post-exercise supplement consumption on adaptations to resistance training. J. Am. Coll. Nutr. 2004, 23, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Jonkers, R.A.; Gleeson, B.G.; Beelen, M.; Meijer, K.; Savelberg, H.H.; Wodzig, W.K.; Dendale, P.; van Loon, L.J. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am. J. Clin. Nutr. 2009, 89, 608–616. [Google Scholar] [CrossRef] [PubMed]
- White, K.M.; Bauer, S.J.; Hartz, K.K.; Baldridge, M. Changes in body composition with yogurt consumption during resistance training in women. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Rasmussen, C.J.; Lancaster, S.L.; Magu, B.; Smith, P.; Melton, C.; Greenwood, M.; Almada, A.L.; Earnest, C.P.; Kreider, R.B. The effects of protein and amino acid supplementation on performance and training adaptations during ten weeks of resistance training. J. Strength Cond. Res. 2006, 20, 643–653. [Google Scholar] [PubMed]
- Walker, T.B.; Smith, J.; Herrera, M.; Lebegue, B.; Pinchak, A.; Fischer, J. The influence of 8 weeks of whey-protein and leucine supplementation on physical and cognitive performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Deschenes, M.R.; Fleck, S.J. Physiological adaptations to resistance exercise. Implications for athletic conditioning. Sports Med. 1988, 6, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Kosek, D.J.; Kim, J.S.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Efficacy of 3 days/week resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J. Appl. Physiol. 2006, 101, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, J.T.; Hurlbut, D.E.; Martel, G.F.; Tracy, B.L.; Ivey, F.M.; Metter, E.J.; Fozard, J.L.; Fleg, J.L.; Hurley, B.F. Age and gender responses to strength training and detraining. Med. Sci. Sports Exerc. 2000, 32, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.F.; Roth, S.M.; Ivey, F.M.; Lemmer, J.T.; Tracy, B.L.; Hurlbut, D.E.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Age and sex affect human muscle fibre adaptations to heavy-resistance strength training. Exp. Physiol. 2006, 91, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.W.; Housh, D.J.; Housh, T.J.; Malek, M.H.; Beck, T.W.; Cramer, J.T.; Johnson, G.O.; Donlin, P.E. Effects of leucine and whey protein supplementation during eight weeks of unilateral resistance training. J. Strength Cond. Res. 2006, 20, 284–291. [Google Scholar] [PubMed]
- Cribb, P.J.; Williams, A.D.; Hayes, A. A Creatine-protein-carbohydrate supplement enhances responses to resistance training. Med. Sci. Sports Exerc. 2007, 39, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Rozenek, R.; Ward, P.; Long, S.; Garhammer, J. Effects of high-calorie supplements on body composition and muscular strength following resistance training. J. Sports Med. Phys. Fit. 2002, 42, 340–347. [Google Scholar]
- Josse, A.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Body composition and strength changes in women with milk and resistance exercise. Med. Sci. Sports Exerc. 2010, 42, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Moritani, T.; deVries, H.A. Neural factors versus hypertrophy in the time course of muscle strength gain. Am. J. Phys. Med. 1979, 58, 115–130. [Google Scholar] [PubMed]
- Pedersen, A.N.; Kondrup, J.; Börsheim, E.; Hambraeus, L.; Bosaeus, I. Protein. In Nordic Nutrition Recommendations 2012, 5th ed.; Narayana Press: Odder, Denmark, 2012; p. 281. [Google Scholar]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. The effect of protein timing on muscle strength and hypertrophy: A meta-analysis. J. Int. Soc. Sports Nutr. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.L.; Braun, B.; Pollack, M.; MacDonald, J.R.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Henderson, G.C.; Horning, M.A.; Brooks, G.A.; et al. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E446–E455. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.H.; Miller, J.C.; Petocz, P.; Farmakalidis, E. A satiety index of common foods. Eur. J. Clin.Nutr. 1995, 49, 675–690. [Google Scholar] [PubMed]
- Rindom, E.; Nielsen, M.H.; Kececi, K.; Jensen, M.E.; Vissing, K.; Farup, J. Effect of protein quality on recovery after intense resistance training. Eur. J. Appl. Physiol. 2016, 116, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.N.; Christensen, T.; Matthiessen, J.; Knudsen, V.K.; Sørensen, M.R.; Biltoft-Jensen, A.; Fagt, S. Dietary Habits in Denmark 2011-2013; Technical University of Denmark: Lyngby, Denmark, 2015; p. 8. [Google Scholar]
- Bemben, M.G.; Witten, M.S.; Carter, J.M.; Eliot, K.A.; Knehans, A.W.; Bemben, D.A. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J. Nutr. Health Aging 2010, 14, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Wolfe, R.R. Exercise, protein metabolism, and muscle growth. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.P.; Tarpenning, K.M.; Marino, F.E. Independent and combined effects of liquid carbohydrate/essential amino acid ingestion on hormonal and muscular adaptations following resistance training in untrained men. Eur. J. Appl. Physiol. 2006, 97, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Peterson, M.D.; Ogborn, D.; Contreras, B.; Sonmez, G.T. Effects of low- vs. high-load resistance training on muscle strength and hypertrophy in well-trained men. J. Strength Cond. Res. 2015, 29, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annual Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]

| Weeks | Repetitions 1 | Sets 2 |
|---|---|---|
| 1–2 | 10–12 | 2 |
| 3–4 | 10–12 | 3 |
| 5–6 | 8–10 | 4 |
| 7–8 | 6–8 | 5 |
| DXA Results | Con | Pro | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ (95% CI) | p | Pre | Post | Δ (95% CI) | p | p | |
| BW (kg) * | 80.8 ± 9.1 | 83.6 ± 9.0 | 2.8 1 (1.4, 3.2) | <0.01 | 81.6 ± 8.7 | 84.0 ± 8.4 | 2.3 1 (1.4, 3.2) | <0.01 | 0.54 |
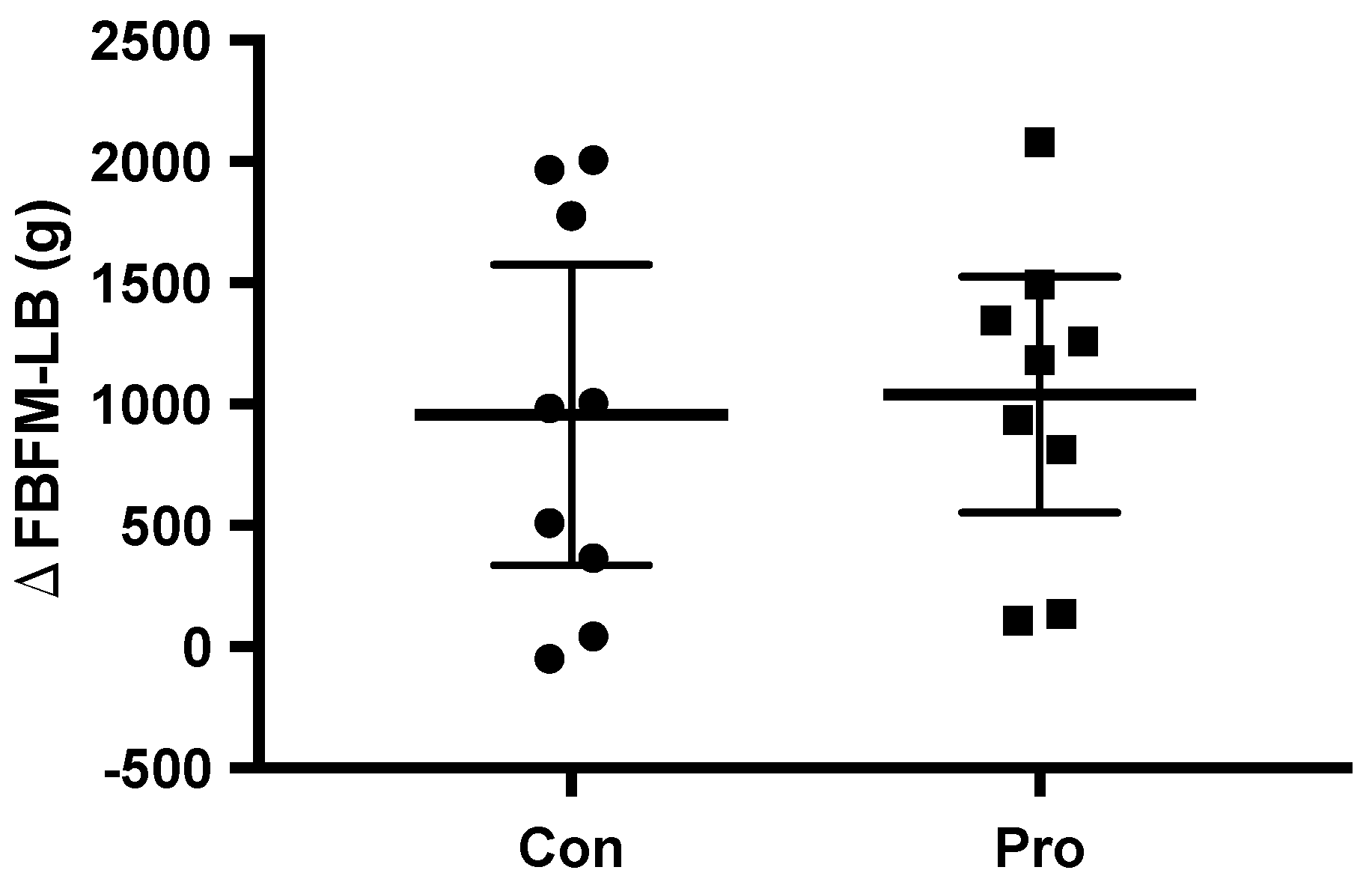
| FM-LB (kg) | 8.5 ± 3.1 | 8.7 ± 3.1 | 0.2 (0.0, 0.5) | 0.10 | 7.1 ± 1.6 | 7.0 ± 1.7 | −0.1 (−0.4, 0.3) | 0.73 | 0.18 |
| FM-UB (kg) * | 8.4 ± 4.3 | 8.4 ± 4.1 | 0.0 (−0.3, 0.3) | 0.96 | 6.4 ± 1.9 | 6.1 ± 2.2 | −0.3 (−0.7, 0.2) | 0.25 | 0.34 |
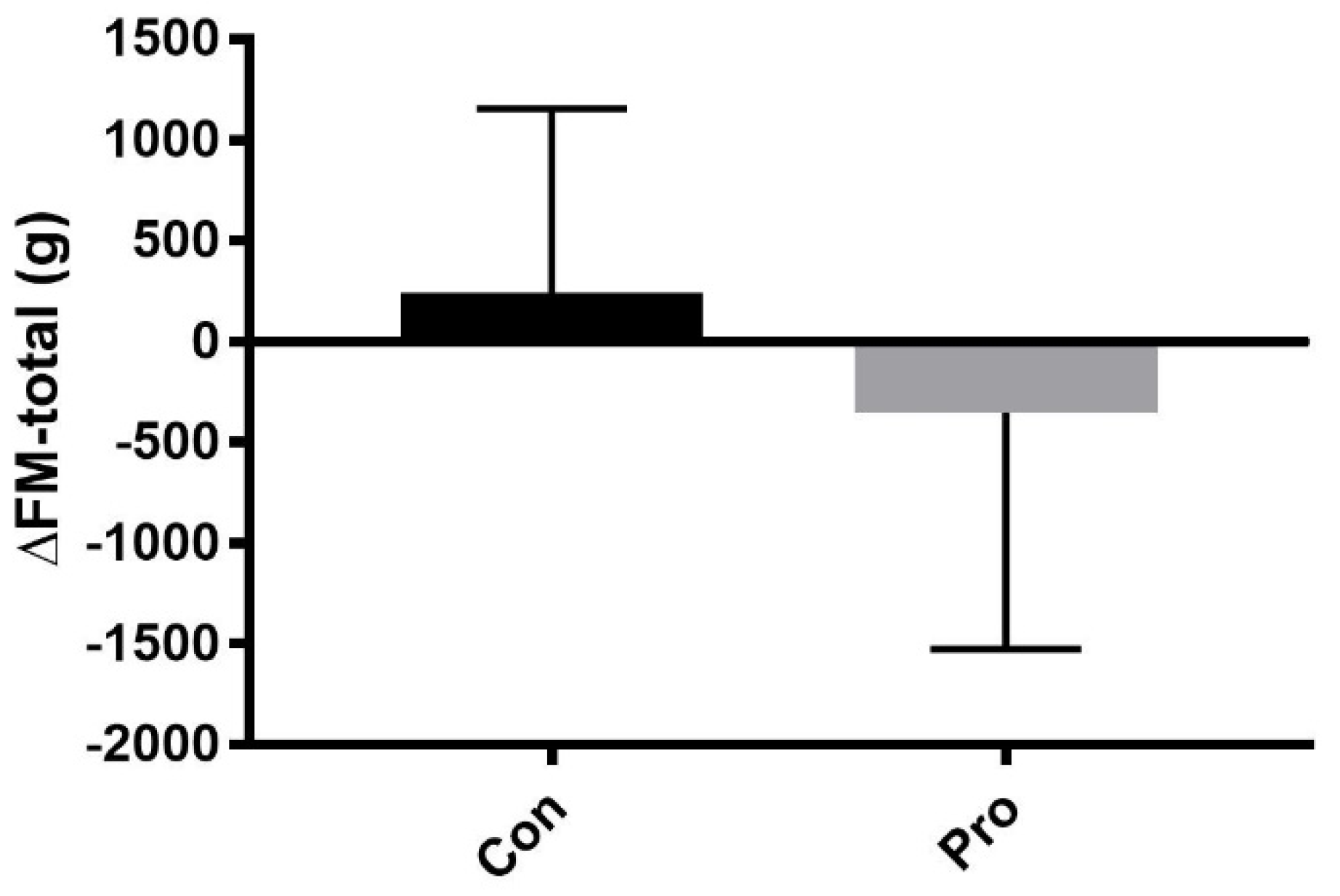
| FM-total (kg) * | 17.8 ± 7.4 | 18.1 ± 7.1 | 0.2 (−0.1, 0.8) | 0.44 | 14.6 ± 3.5 | 14.3 ± 3.8 | −0.4 (−1.2, 0.5) | 0.43 | 0.27 |
| FBFM-LB (kg) | 30.4 ± 2.3 | 31.4 ± 2.7 | 1.0 1 (0.4, 1.5) | <0.01 | 31.8 ± 4.0 | 33.0 ± 4.0 | 1.2 1 (0.8, 1.5) | <0.01 | 0.50 |
| FBFM-UB (kg) * | 25.7 ± 2.0 | 27.3 ± 2.5 | 1.6 1 (1.1, 2.0) | <0.01 | 27.6 ± 3.8 | 29.1 ± 3.7 | 1.5 1 (0.8, 2.2) | <0.01 | 0.91 |
| FBFM-total (kg) * | 59.7 ± 4.2 | 62.2 ± 5.2 | 2.5 1 (1.6, 3.5) | <0.01 | 63.5 ± 7.9 | 66.1 ± 7.7 | 2.7 1 (1.6, 3.8) | <0.01 | 0.86 |
| BMC (g) | 3299 ± 399 | 3317 ± 383 | 18 ± (−3, 39) | 0.09 | 3546 ± 378 | 3555 ± 371 | 8.5 (−10, 27) | 0.37 | 0.51 |
| Nutrition Data | Con (n = 6) | Pro (n = 9) | Interaction | ||||
|---|---|---|---|---|---|---|---|
| Pre | Mid * | p | Pre | Mid * | p | p | |
| Energy intake kcal/day | 2764 ± 312 | 3593 2 ± 621 | <0.01 | 3142 ± 747 | 3312 ± 441 | 0.56 | 0.10 |
| Protein intake g/day | 101.7 ± 16.9 | 128.2 2 ± 16.6 | 0.01 | 123.0 ± 24.4 | 182.5 2,3 ± 15.6 | <0.01 | 0.02 |
| Protein intake g/kg/day | 1.3 ± 0.3 | 1.7 ± 0.3 | 0.03 | 1.6 ± 0.3 | 2.3 2,3 ± 0.3 | <0.01 | <0.05 |
| Fat intake g/day | 104.3 ± 18.2 | 118.2 ± 39.7 | 0.44 | 114.5 ± 33.8 | 91.6 ± 53.3 | 0.28 | 0.18 |
| Fat intake g/kg/day | 1.4 ± 0.3 | 1.55 ± 0.5 | 0.47 | 1.4 ± 0.3 | 1.2 ± 0.7 | 0.33 | 0.23 |
| CHO intake g/day | 309.8 ± 86.1 | 443.8 2 ± 56.7 | <0.01 | 366.6 ± 73.9 | 381.7 2,3 ± 16.5 | 0.55 | 0.02 |
| CHO intake g/kg/day | 4.0 ± 1.0 | 5.8 2 ± 0.4 | <0.01 | 4.6 ± 0.9 | 4.8 2,3 ± 0.2 | 0.62 | <0.01 |
| Strength Measures | Con n = 9 * | Pro n = 9 * | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ (95% CI) | p | Pre | Post | Δ (95% CI) | p | p | |
| 1 RM Leg press (kg) * | 175 ± 34 | 217 ± 41 | 42 1 (32, 52) | <0.01 | 181 ± 26 | 217 ± 22 | 37 1 (27, 46) | <0.01 | 0.92 |
| Leg press volume * | 1924 ± 491 | 3140 ± 1175 | 1216 1 (515, 1915) | <0.01 | 1957 ± 559 | 2465 ± 810 | 508 (−60, 1076) | 0.08 | 0.59 |
| 1 RM bench press (kg) * | 71 ± 14 | 85 ± 16 | 14 1 (10, 17) | <0.01 | 78 ± 22 | 86 ± 22 | 8 1 (5, 12) | <0.01 | 0.73 |
| Bench press volume * | 647 ± 136 | 648 ± 105 | 1 (−46, 48) | 0.97 | 645 ± 204 | 612 ± 173 | −33 (−139, 73) | 0.54 | 0.30 |
| MVC Ext. (Nm) | 341 ± 56 | 403 ± 61 | 62 1 (50, 75) | <0.01 | 356 ± 71 | 403 ± 77 | 47 1 (19, 75) | <0.01 | 0.84 |
| MVC Flex. (Nm) | 157 ± 16 | 176 ± 13 | 20 1 (10, 29) | <0.01 | 173 ± 26 | 183 ± 29 | 10 1 (3, 18) | 0.01 | 0.67 |
| CMJ (W) | 1027 ± 15 | 1075 ± 172 | 48 1 (26, 70) | <0.01 | 1068 ± 165 | 1092 ± 139 | 24 1 (2, 47) | 0.03 | 0.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vangsoe, M.T.; Joergensen, M.S.; Heckmann, L.-H.L.; Hansen, M. Effects of Insect Protein Supplementation during Resistance Training on Changes in Muscle Mass and Strength in Young Men. Nutrients 2018, 10, 335. https://doi.org/10.3390/nu10030335
Vangsoe MT, Joergensen MS, Heckmann L-HL, Hansen M. Effects of Insect Protein Supplementation during Resistance Training on Changes in Muscle Mass and Strength in Young Men. Nutrients. 2018; 10(3):335. https://doi.org/10.3390/nu10030335
Chicago/Turabian StyleVangsoe, Mathias T., Malte S. Joergensen, Lars-Henrik L. Heckmann, and Mette Hansen. 2018. "Effects of Insect Protein Supplementation during Resistance Training on Changes in Muscle Mass and Strength in Young Men" Nutrients 10, no. 3: 335. https://doi.org/10.3390/nu10030335
APA StyleVangsoe, M. T., Joergensen, M. S., Heckmann, L.-H. L., & Hansen, M. (2018). Effects of Insect Protein Supplementation during Resistance Training on Changes in Muscle Mass and Strength in Young Men. Nutrients, 10(3), 335. https://doi.org/10.3390/nu10030335





