The Divergent Effect of Maternal Protein Restriction during Pregnancy and Postweaning High-Fat Diet Feeding on Blood Pressure and Adiposity in Adult Mouse Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Dietary Challenges
2.2. Adiposity
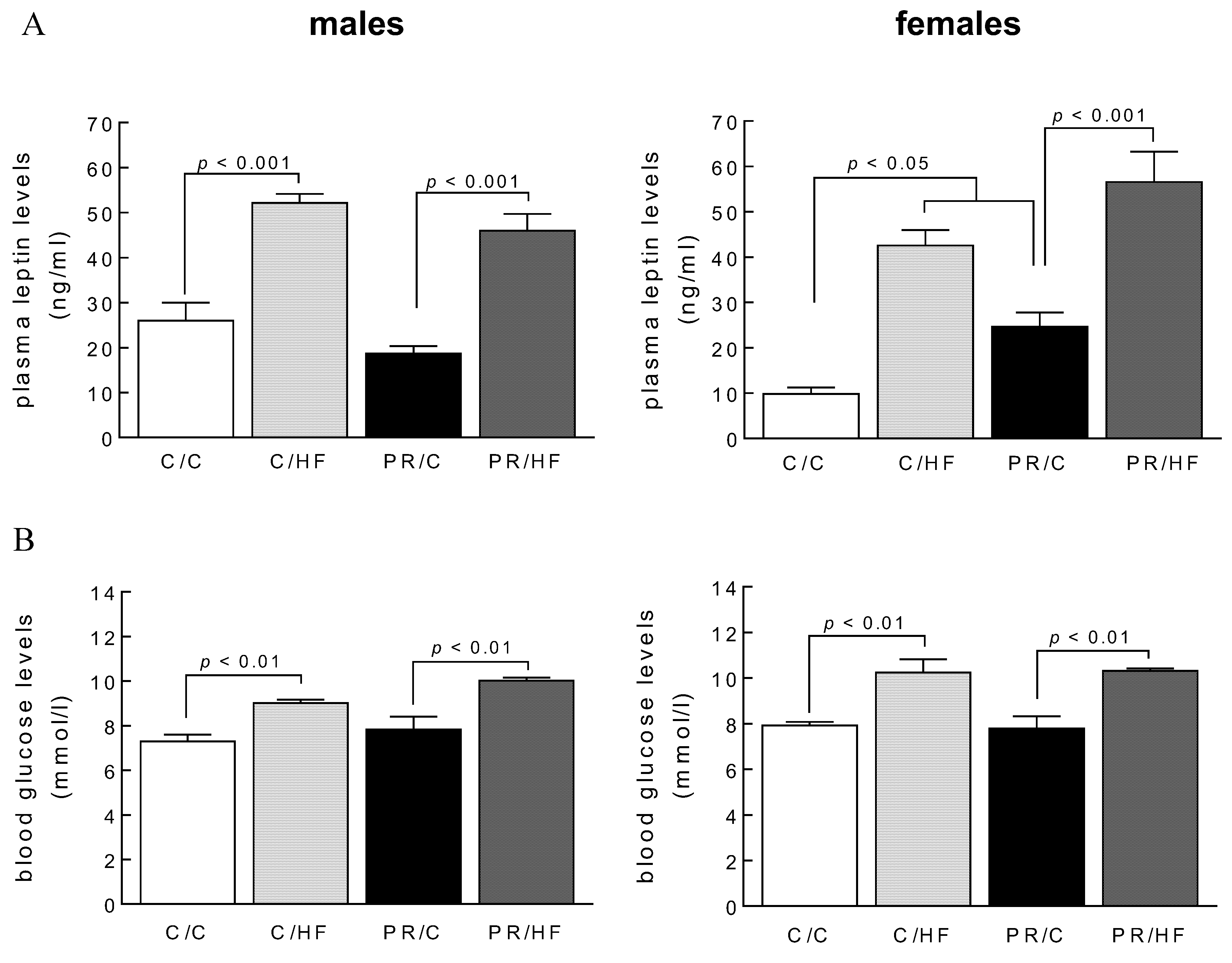
2.3. Plasma Leptin Levels
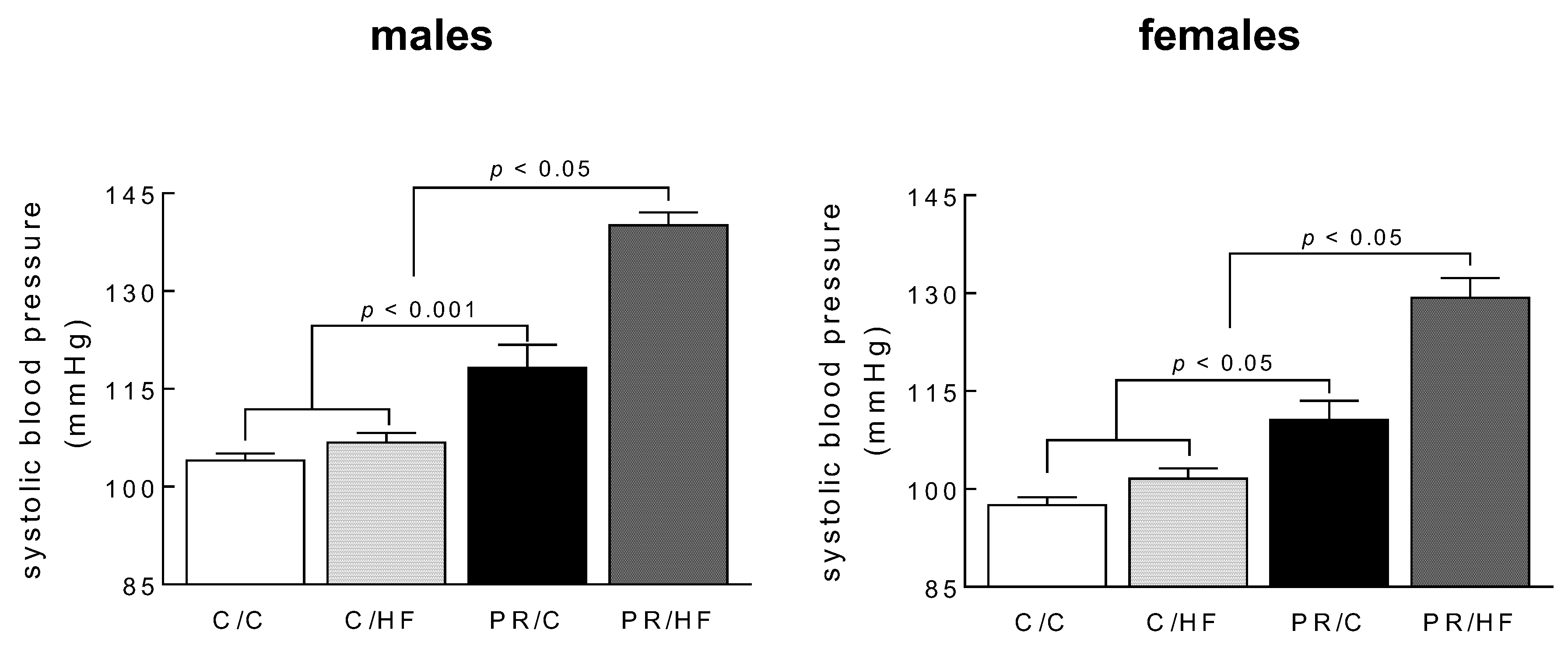
2.4. Systolic Blood Pressure and Fasting Blood Glucose
2.5. Statistical Analysis
3. Results
3.1. Adiposity and Plasma Leptin Levels
3.2. Plasma Leptin Levels and Fasting Blood Glucose
3.3. Systolic Blood Pressure and Heart Weights
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Arterburn, D.E.; Maciejewski, M.L.; Tsevat, J. Impact of morbid obesity on medical expenditures in adults. Int. J. Obes. (Lond.) 2005, 29, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Armitage, J.A.; Poston, L.; Taylor, P.D. Developmental origins of obesity and the metabolic syndrome: The role of maternal obesity. Front. Horm. Res. 2008, 36, 73–84. [Google Scholar] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Harrison, C.J.; Patel, R.; Gray, C.; Reynolds, C.M. Maternal high-fat and high-salt diets have differential programming effects on metabolism in adult male rat offspring. Front. Nutr. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.; Ethirajan, P.L.; Burdge, G.C.; Bateman, A.C.; Clough, G.F.; Poston, L.; Hanson, M.A.; et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 2009, 50, 1796–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Rodriguez-Gonzalez, G.L.; Guzman, C.; Garcia-Becerra, R.; Boeck, L.; Diaz, L.; Menjivar, M.; Larrea, F.; Nathanielsz, P. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J. Physiol. 2005, 563, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A.P.; Palou, M.; Sánchez, J.; Priego, T.; Palou, A.; Picó, C. Moderate caloric restriction during gestation in rats alters adipose tissue sympathetic innervation and later adiposity in offspring. PLoS ONE 2011, 6, e17313. [Google Scholar] [CrossRef] [PubMed]
- da Silva Aragao, R.; Guzmán-Quevedo, O.; Pérez-García, G.; Manhaes-de-Castro, R.; Bolanos-Jiménez, F. Maternal protein restriction impairs the transcriptional metabolic flexibility of skeletal muscle in adult rat offspring. Br. J. Nutr. 2014, 112, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qasem, R.J.; Li, J.; Tang, H.M.; Pontiggia, L.; D’mello, A.P. Maternal protein restriction during pregnancy and lactation alters central leptin signalling, increases food intake, and decreases bone mass in 1 year old rat offspring. Clin. Exp. Pharmacol. Physiol. 2016, 43, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Zimanyi, M.A.; Black, M.J. Effect of maternal protein restriction during pregnancy and lactation on the number of cardiomyocytes in the postproliferative weanling rat heart. Anat. Rec. (Hoboken) 2010, 293, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.C.; Seakins, M.; Grimble, R.F.; Jackson, A.A. The acute phase response of adult rats is altered by in utero exposure to maternal low protein diets. J. Nutr. 1994, 124, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Lucas, E.S.; Wilkins, A.; Cagampang, F.R.; Fleming, T.P. Maternal periconceptional and gestational low protein diet affects mouse offspring growth, cardiovascular and adipose phenotype at 1 year of age. PLoS ONE 2011, 6, e28745. [Google Scholar] [CrossRef] [PubMed]
- McMullen, S.; Gardner, D.S.; Langley-Evans, S.C. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br. J. Nutr. 2004, 91, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Erhuma, A.; Salter, A.M.; Sculley, D.V.; Langley-Evans, S.C.; Bennett, A.J. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1702–E1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brawley, L.; Itoh, S.; Torrens, C.; Barker, A.; Bertram, C.; Poston, L.; Hanson, M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr. Res. 2003, 54, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sellayah, D.; Dib, L.; Anthony, F.W.; Watkins, A.J.; Fleming, T.P.; Hanson, M.A.; Cagampang, F.R. Effect of maternal protein restriction during pregnancy and postweaning high-fat feeding on diet-induced thermogenesis in adult mouse offspring. Eur J. Nutr. 2014, 53, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Spencer, H.G. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 2005, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Breier, B.H.; Cutfield, W.S.; Hofman, P.L.; Gluckman, P.D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E83–E87. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Babu, J.; Ross, M.G. Programmed metabolic syndrome: Prenatal undernutrition and postweaning overnutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2306–R2314. [Google Scholar] [CrossRef] [PubMed]
- Sellayah, D.; Sek, K.; Anthony, F.W.; Watkins, A.J.; Osmond, C.; Fleming, T.P.; Hanson, M.A.; Cagampang, F.R. Appetite regulatory mechanisms and food intake in mice are sensitive to mismatch in diets between pregnancy and postnatal periods. Brain Res. 2008, 1237, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Wilkins, A.; Cunningham, C.; Perry, V.H.; Seet, M.J.; Osmond, C.; Eckert, J.J.; Torrens, C.; Cagampang, F.R.A.; Cleal, J.; et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J. Physiol. 2008, 586, 2231–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozanne, S.E.; Hales, C.N. The long-term consequences of intra-uterine protein malnutrition for glucose metabolism. Proc. Nutr. Soc. 1999, 58, 615–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayol, S.A.; Farrington, S.J.; Stickland, N.C. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br. J. Nutr. 2007, 98, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, Q.; Zhang, L.; Maricelli, J.W.; Rodgers, B.D.; Zhu, M.J.; Du, M. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 2016, 6, 34345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Brown, L.M.; Woods, S.C.; Benoit, S.C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006, 55, 978–987. [Google Scholar] [CrossRef] [PubMed]
- de Fougerolles Nunn, E.; Greenstein, B.; Khamashta, M.; Hughes, G.R. Evidence for sexual dimorphism of estrogen receptors in hypothalamus and thymus of neonatal and immature Wistar rats. Int J. Immunopharmacol. 1999, 21, 869–877. [Google Scholar] [CrossRef]
- Torrens, C.; Hanson, M.A.; Gluckman, P.D.; Vickers, M.H. Maternal undernutrition leads to endothelial dysfunction in adult male rat offspring independent of postnatal diet. Br. J. Nutr. 2009, 101, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.C.; Jackson, A.A. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin. Sci. (Lond.) 1994, 86, 217–222. [Google Scholar] [CrossRef] [PubMed]
- McMullen, S.; Langley-Evans, S.C. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R85–R90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley-Evans, S.C. Intrauterine programming of hypertension by glucocorticoids. Life Sci. 1997, 60, 1213–1221. [Google Scholar] [CrossRef]
- Torrens, C.; Brawley, L.; Barker, A.C.; Itoh, S.; Poston, L.; Hanson, M.A. Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J. Physiol. 2003, 547, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonds, M.E.; Pope, M.; Budge, H. Adipose tissue development during early life: Novel insights into energy balance from small and large mammals. Proc. Nutr. Soc. 2012, 71, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.T.; Macko, A.R.; Nearing, M.; Chen, X.; Rhoads, R.P.; Limesand, S.W. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J. Pregnancy 2012, 2012, 631038. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, K.; Cedrone, M.; Staples, J.; Regnault, T. Altered fetal skeletal muscle nutrient metabolism following an adverse in utero environment and the modulation of later life insulin sensitivity. Nutrients 2015, 7, 1202–1216. [Google Scholar] [CrossRef] [PubMed]


| Diet | Sugars (% w/w) | Fat (% w/w) | Fat (% kcal) | Protein (% w/w) | Energy (MJ/kg) |
|---|---|---|---|---|---|
| Maternal Diets | |||||
| Normal Protein Chow (C) | 21 | 10 | 21 | 18 | 18.39 |
| Protein-Restricted (PR) | 24 | 10 | 21 | 9 | 18.27 |
| Offspring Diets | |||||
| Standard Chow (C) | 7 | 3 | 7 | 15 | 14.74 |
| High-Fat (HF) | 10 | 22 | 45 | 26 | 18.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellayah, D.; Cagampang, F.R. The Divergent Effect of Maternal Protein Restriction during Pregnancy and Postweaning High-Fat Diet Feeding on Blood Pressure and Adiposity in Adult Mouse Offspring. Nutrients 2018, 10, 1832. https://doi.org/10.3390/nu10121832
Sellayah D, Cagampang FR. The Divergent Effect of Maternal Protein Restriction during Pregnancy and Postweaning High-Fat Diet Feeding on Blood Pressure and Adiposity in Adult Mouse Offspring. Nutrients. 2018; 10(12):1832. https://doi.org/10.3390/nu10121832
Chicago/Turabian StyleSellayah, Dyan, and Felino R. Cagampang. 2018. "The Divergent Effect of Maternal Protein Restriction during Pregnancy and Postweaning High-Fat Diet Feeding on Blood Pressure and Adiposity in Adult Mouse Offspring" Nutrients 10, no. 12: 1832. https://doi.org/10.3390/nu10121832






