Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
3. Results
3.1. Experimental Cataract Models
3.2. In Vitro Models
3.2.1. Oxidative Stress Model
H2O2-Induced Cataract
3.2.2. Diabetic Cataract
Aldose Reductase (AR) Activity
Xylose-Induced Lens Opacity
Galactose-Induced Lens Opacity
Formation of Advanced Glycation End (AGE) Products
3.3. In Vivo Models
3.3.1. Diabetic Cataract
3.3.2. Selenite-Induced Cataract
3.3.3. UV-Induced Cataract
3.3.4. Steroid-Induced Cataract
3.4. Anti-Cataract Phytoconstituents
3.4.1. 1-O-Galloyl-β-d-glucose (β-Glucogallin)
3.4.2. 1,3-Di-O-caffeoylquinic Acid
3.4.3. 1,5-Di-hydroxy-1,5-di-[(E)-3-(4-hydroxyphenyl)-2-propenoic]-3-pentanonyl Ester (DHDP)
3.4.4. 1,5-Di-O-caffeoylquinic Acid
3.4.5. 1,3,6-Trihydroxy-2-methoxymethylanthraquinone
3.4.6. 1,2,3,6-Tetra-O-galloyl-β-d-glucose
3.4.7. 1,3,5,8-Tetrahydroxyxanthone
3.4.8. 2″,4″-O-Diacetylquercitrin
3.4.9. 3-Isomangostin
3.4.10. 3′,4-Dihydroxy-3,5′-dimethoxy-bibenzyl (Gigantol)
3.4.11. 3′,5′-Dimethoxy-(1,1′-biphenyl)-3,4-diol 3-O-β-d-glucopyranoside
3.4.12. 3,5-Di-O-caffeoylquinic Acid
3.4.13. 4-O-Butylpaeoniflorin and Palbinone
3.4.14. 4,5-Di-O-trans-caffeoyl-d-quinic Acid
3.4.15. 5-O-Feruloly Quinic Acid
3.4.16. 5,7,4′ Trihydroxyisoflavone (Genistein)
3.4.17. 20(S)-Ginsenoside Rh2
3.4.18. Acteoside
3.4.19. Basilicumin [7-(3-hydroxypropyl)-3-methyl-8-β-O-d-glucoside-2H-chromen-2-one]
3.4.20. Caffeic Acid
3.4.21. Canangafruiticoside E
3.4.22. Capsofulvesin A [((2S)-l-O-(6Z,9Z,12Z,15Zoctadecatetraenoyl)-2-O-(4Z,10Z,13Zhexadecatetraenoyl)-3-O-β-d-galactopyranosyl Glycerol)]
3.4.23. Caryatin-3′ methyl ether-7-O-β-d-glucoside
3.4.24. C-Phycocyanin (C-PC)
3.4.25. Davallialactone
3.4.26. Delphinidin 3-O-β-galactopyranoside-3′-O-β-glucopyranoside
3.4.27. Desmethylanhydroicaritin
3.4.28. Ellagic Acid
3.4.29. Epiberberine
3.4.30. Geraniin
3.4.31. Hipolon
3.4.32. Hirsutrin
3.4.33. Hopeafuran
3.4.34. Hypolaetin 7-O-[6‴-O-acetyl-β-d-allopyranosyl-(1→2)]-6″-O-acetyl-β-d-glucopyranoside
3.4.35. Isocampneoside II
3.4.36. Isorhamnetin-3-glucoside
3.4.37. Kaempferol
3.4.38. Kakkalide
3.4.39. Lucidumol A [(24S)-24,25-Dihydroxylanost-8-ene-3,7-dione]
3.4.40. Lupeol
3.4.41. Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromomenone)
3.4.42. Luteolin-7-O-β-d-glucopyranoside
3.4.43. Magnoflorine
3.4.44. Methyl-3,5-di-O-caffeoylquinate
3.4.45. Mumeic Acid-A
3.4.46. Puerariafuran
3.4.47. Quercetin-3-O-β-d-glucoside
3.4.48. Quercitrin (Quercetin 3-O-α-l-rhamnoside)
3.4.49. Rhetsinine
3.4.50. Rosmarinic Acid
3.4.51. Scopoletin
3.4.52. Semilicoisoflavone B
3.4.53. Sulfuretin and Butein
3.4.54. Syringic Acid
3.4.55. Swertisin
3.4.56. Valoneic Acid Dilactone
4. Discussion and Outlook
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end-product |
| AR | Aldose reductase |
| ARI | Aldose reductase inhibition |
| BLAR | Bovine lens aldose reductase |
| GC | Glucocorticoid |
| GSH | Glutathione |
| HLAR | Human lens aldose reductase |
| LEC | Lens epithelial cell |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| RHAR | Recombinant human aldose reductase |
| RLAR | Rat lens aldose reductase |
| ROS | Reactive oxygen species |
| SDH | Sorbitol dehydrogenase |
| STZ | Streptozotocin |
| UV | Ultraviolet |
References
- Lou, M.F. Redox regulation in the lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Fischel, J.D.; Lipton, J.R. Cataract surgery and recent advances: A review. Nurs. Stand. 1996, 10, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Garner, W.H. Hydrogen peroxide and human cataract. Exp. Eye Res. 1981, 33, 673–681. [Google Scholar] [CrossRef]
- Cui, X.L.; Lou, M.F. The effect and recovery of long-term H2O2 exposure on lens morphology and biochemistry. Exp. Eye Res. 1993, 57, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zigler, J.S., Jr.; Jernigan, H.M., Jr.; Garland, D.; Reddy, V.N. The effects of “oxygen radicals” generated in the medium on lenses in organ culture: Inhibition of damage by chelated iron. Arch. Biochem. Biophys. 1985, 241, 163–172. [Google Scholar] [CrossRef]
- Caird, F.I.; Hutchinson, M.; Pirie, A. Cataract and Diabetes. Br. Med. J. 1964, 2, 665–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodos, J.B.; Habegger-Chodos, H.E. Cataract formation in human and experimental diabetes. Part I. Surv. Ophthalmol. 1960, 5, 129–159. [Google Scholar] [PubMed]
- Snow, A.; Shieh, B.; Chang, K.C.; Pal, A.; Lenhart, P.; Ammar, D.; Ruzycki, P.; Palla, S.; Reddy, G.B.; Petrash, J.M. Aldose reductase expression as a risk factor for cataract. Chem. Biol. Interact. 2015, 234, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayman, S.; Kinoshita, J.H. Isolation and Properties of Lens Aldose Reductase. J. Biol. Chem. 1965, 240, 877–882. [Google Scholar] [PubMed]
- Obazawa, H.; Merola, L.O.; Kinoshita, J.H. The effects of xylose on the isolated lens. Investig. Ophthalmol. 1974, 13, 204–209. [Google Scholar]
- Ai, Y.; Zheng, Z.; O’Brien-Jenkins, A.; Bernard, D.J.; Wynshaw-Boris, T.; Ning, C.; Reynolds, R.; Segal, S.; Huang, K.; Stambolian, D. A mouse model of galactose-induced cataracts. Hum. Mol. Genet. 2000, 9, 1821–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, S.; Dawczynski, J.; Strobel, J.; Niwa, T.; Stahl, P.; Stein, G. Increased levels of advanced glycation end products in human cataractous lenses. J. Cataract Refract. Surg. 2003, 29, 998–1004. [Google Scholar] [CrossRef]
- Ramalho, J.S.; Marques, C.; Pereira, P.C.; Mota, M.C. Role of glycation in human lens protein structure change. Eur. J. Ophthalmol. 1996, 6, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Abiko, T.; Abiko, A.; Ishiko, S.; Takeda, M.; Horiuchi, S.; Yoshida, A. Relationship between autofluorescence and advanced glycation end products in diabetic lenses. Exp. Eye Res. 1999, 68, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Joon, T.L.; Foo, K.; Panagiotopoulos, S.; Jerums, G.; Taylor, H.R. In vivo assessment of an animal model of diabetic cataract: Medical intervention studies. Dev. Ophthalmol. 1994, 26, 57–62. [Google Scholar] [PubMed]
- Patil, M.A.; Suryanarayana, P.; Putcha, U.K.; Srinivas, M.; Reddy, G.B. Evaluation of neonatal streptozotocin induced diabetic rat model for the development of cataract. Oxid. Med. Cell Longev. 2014, 2014, 463264. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Chung, S.S. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999, 13, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.R.; David, L.L.; Anderson, R.S. Selenite cataract: A review. Curr. Eye Res. 1987, 6, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.R.; David, L.L.; Anderson, R.S.; Azuma, M. Review of selenite cataract. Curr. Eye Res. 1992, 11, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.R.; Ma, H.; Fukiage, C.; Azuma, M. Selenite nuclear cataract: Review of the model. Mol. Vis. 1997, 3, 8. [Google Scholar] [PubMed]
- Dillon, J. UV-B as a pro-aging and pro-cataract factor. Doc. Ophthalmol. 1994, 88, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P. Cataract and UV radiation. Doc. Ophthalmol. 1994, 88, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Kronschlager, M.; Lofgren, S.; Yu, Z.; Talebizadeh, N.; Varma, S.D.; Soderberg, P. Caffeine eye drops protect against UV-B cataract. Exp. Eye Res. 2013, 113, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, E.R. The etiology of steroid cataract. J. Ocul. Pharmacol. Ther. 2007, 23, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Shui, Y.B.; Kojima, M.; Sasaki, K. A new steroid-induced cataract model in the rat: Long-term prednisolone applications with a minimum of X-irradiation. Ophthalmic Res. 1996, 28, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Shui, Y.B.; Vrensen, G.F.; Kojima, M. Experimentally induced steroid cataract in the rat: A scanning electron microscopic study. Surv. Ophthalmol. 1997, 42, S127–S132. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016, 111, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Puppala, M.; Ponder, J.; Suryanarayana, P.; Reddy, G.B.; Petrash, M.; LaBarbera, D.V. The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS ONE 2012, 7, e31399. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Laffin, B.; Ponder, J.; Énzsöly, A.; Németh, J.; Labarbera, D.V.; Petrash, J.M. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem. Biol. Interact. 2013, 202, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Hong, E.Y.; Whang, W.K. Inhibitory Effect of Chemical Constituents Isolated from Artemisia iwayomogi on Polyol Pathway and Simultaneous Quantification of Major Bioactive Compounds. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.N.; Lee, M.Y.; Kim, J.K.; Suh, H.W.; Lim, S.S. Aldose reductase inhibitory compounds from Xanthium strumarium. Arch. Pharm. Res. 2013, 36, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hwang, S.H.; Lim, S.S. Characterization of DHDP, a novel aldose reductase inhibitor isolated from Lysimachia christinae. J. Funct. Foods 2017, 37, 241–248. [Google Scholar] [CrossRef]
- Paek, J.H.; Lim, S.S. Preparative isolation of aldose reductase inhibitory compounds from Nardostachys chinensis by elution–extrusion counter-current chromatography. Arch. Pharm. Res. 2014, 37, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.H.; Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Cho, J.H.; Kim, J.H.; Kim, J.S. Anthraquinones from the Roots of Knoxia valerianoides inhibit the formation of advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Li, Z.; Luo, H.; Zhang, W.; Chen, L.; Xu, X. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2004, 14, 6041–6044. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, D.S.; Kim, N.H.; Lee, Y.M.; Kim, J.; Kim, J.S. Galloyl glucoses from the seeds of Cornus officinalis with inhibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo. Biol. Pharm. Bull. 2011, 34, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.H.; Luo, C.T.; Chen, H.; Lin, J.N.; Ye, C.L.; Mao, S.S.; Li, Y.L. Xanthones from Swertia mussotii as multitarget-directed antidiabetic agents. Chem. Med. Chem. 2014, 9, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kim, I.S.; Lee, Y.M.; Lee, Y.; Kim, J.H.; Kim, J.S. 2″,4″-O-diacetylquercitrin, a novel advanced glycation end-product formation and aldose reductase inhibitor from Melastoma sanguineum. Chem. Pharm. Bull. (Tokyo) 2013, 61, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Ersam, T.; Shimizu, K. The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn. Phytomedicine 2015, 22, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Han, H.; He, C.; Yang, L.; Wang, Z. Identification of the metabolites of gigantol in rat urine by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Biomed. Chromatogr. 2014, 28, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Hu, X.; Wang, M.; Wan, W.; Yang, Q.; Sun, X.; Gu, Q.; Gao, X.X.; Wang, Z.T.; Gu, L.Q.; et al. Anti-osmotic and antioxidant activities of gigantol from Dendrobium aurantiacum var. denneanum against cataractogenesis in galactosemic rats. J. Ethnopharmacol. 2015, 172, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.; Xu, L. Phenols and a triterpene from Dendrobium aurantiacum var. denneanum (Orchidaceae). Biochem. Syst. Ecol. 2006, 34, 658–660. [Google Scholar] [CrossRef]
- Hu, J.; Fan, W.; Dong, F.; Miao, Z.; Zhou, J. Chemical components of Dendrobium chrysotoxum. Chin. J. Chem. 2012, 30, 1327–1330. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Wan, W.; Yang, Q.; Ma, W.; Chen, D.; Hu, J.M.; Chen, C.Y.O.; Wei, X.Y. Gigantol from Dendrobium chrysotoxum Lindl. binds and inhibits aldose reductase gene to exert its anti-cataract activity: An in vitro mechanistic study. J. Ethnopharmacol. 2017, 198, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Jung, S.H.; Lee, Y.M.; Choi, S.J.; Sun, H.; Kim, J.S. Phenolic Compounds from the Leaves and Twigs of Osteomeles schwerinae That Inhibit Rat Lens Aldose Reductase and Vessel Dilation in Zebrafish Larvae. J. Nat. Prod. 2015, 78, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Yoo, N.H.; Kim, N.H.; Lee, Y.M.; Kim, C.S.; Kim, J.; Kim, J.H.; Kim, J.S. 3,5-Di-O-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis. Biol. Pharm. Bull. 2010, 33, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Poetsch, F.; Nahrstedt, A. Structure assignment of natural quinic acid derivatives using proton nuclear magnetic resonance techniques. Phytochem. Anal. 1998, 9, 177–185. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.S. Identification of new dicaffeoylquinic acids from Chrysanthemum morifolium and their antioxidant activities. Planta Med. 2005, 71, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.M.; Lee, B.W.; Kim, J.H.; Kim, J.S. Chemical constituents from the aerial parts of Aster koraiensis with protein glycation and aldose reductase inhibitory activities. J. Nat. Prod. 2012, 75, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Islam, M.D.N.; Kwon, Y.S.; Jin, S.E.; Son, Y.K.; Park, J.J.; Sohn, H.S.; Choi, J.S. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem. Toxicol. 2011, 49, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Tran, M.N.; Lee, I.S.; Yun, M.L.; Jin, S.K.; Jung, H.J.; Lee, S.M.; Na, M.K.; Bae, K. Inhibitors of aldose reductase and formation of advanced glycation end-products in Moutan cortex (Paeonia suffruticosa). J. Nat. Prod. 2009, 72, 1465–1470. [Google Scholar]
- Kadota, S.; Terashima, S.; Basnet, P.; Kikuchi, T.; Namba, T.; Palbinone, A. Novel Terpenoid from Peaonia albiflora; Potent Inhibitory Activity on 3α-Hydroxysteroid Dehydrogenase. Chem. Pharm. Bull. 1993, 41, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nakamura, S.; Zhuang, Y.; Yoshikawa, M.; Mohamed, G.; Hussein, E.; Matsuo, K.; Matsuda, H. Medicinal Flowers. XXXX. 1) Structures of Dihydroisocoumarin Glycosides and Inhibitory Effects on Aldose Reducatase from the Flowers of Hydrangea macrophylla var. thunbergii. Chem. Pharm. Bull. 2013, 61, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Jung, H.A.; Kang, S.S.; Lee, J.H.; Cho, Y.S.; Moon, K.H.; Choi, J.S. Inhibitory activity of aralia continentalis roots on protein tyrosine phosphatase 1B and rat lens aldose reductase. Arch. Pharm. Res. 2012, 35, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Kim, J.M.; Lee, Y.M.; Kim, Y.S.; Kim, J.H.; Kim, J.S. Puerariafuran, a new inhibitor of advanced glycation end products (AGEs) isolated from the roots of Pueraria lobata. Chem. Pharm. Bull. 2006, 54, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.E.; Furusawa, J.I.; Baba, J.; Takeshita, T.; Yamasaki, M.; Nohara, T. Studies on the constituents of Pueraria lobata. III. Isoflavonoids and related compounds in the roots and the voluble stems. Chem. Pharm. Bull. (Tokyo) 1987, 35, 4846–4850. [Google Scholar] [CrossRef]
- Kim, S.B.; Hwang, S.H.; Wang, Z.; Yu, J.M.; Lim, S.S. Rapid identification and isolation of inhibitors of rat lens aldose reductase and antioxidant in Maackia amurensis. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, N.H.; Jung, D.H.; Jang, D.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Genistein inhibits aldose reductase activity and high glucose-induced TGF-beta2 expression in human lens epithelial cells. Eur. J. Pharmacol. 2008, 594, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Ersam, T.; Yu, H.; Zhang, C.; Jin, F.; Shimizu, K. 20(S)-Ginsenoside Rh2 as aldose reductase inhibitor from Panax ginseng. Bioorg. Med. Chem. Lett. 2014, 24, 4407–4409. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Cui, C.B.; Lim, S.S. Analysis of the inhibitory activity of Abeliophyllum distichum leaf constituents against aldose reductase by using high-speed counter current chromatography. Arch. Pharm. Res. 2013, 36, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Lee, I.S.; Jung, S.H.; Lee, Y.M.; Lee, Y.R.; Kim, J.H.; Sun, H.; Kim, J.S. Caffeoylated phenylpropanoid glycosides from Brandisia hancei inhibit advanced glycation end product formation and aldose reductase in vitro and vessel dilation in larval zebrafish in vivo. Planta Med. 2013, 79, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Tehseen, Y.; Maryam, K.; Uroos, M.; Siddiqui, B.S.; Hameed, A.; Iqbal, J. Bioorganic Chemistry Identification of new potent inhibitor of aldose reductase from Ocimum basilicum. Bioorg. Chem. 2017, 75, 62–70. [Google Scholar]
- Koo, D.C.; Baek, S.Y.; Jung, S.H.; Shim, S.H. Aldose reductase inhibitory compounds from extracts of Dipsacus asper. Biotechnol. Bioprocess Eng. 2013, 18, 926–931. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, Y.S.; Kim, S.H.; Bae, Y.S.; Lim, S.S. Inhibition of aldose reductase by phenylethanoid glycoside isolated from the seeds of Paulownia coreana. Biol. Pharm. Bull. 2011, 34, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, Y.H.; Jung, J.Y.; Kang, I.J.; Han, S.N.; Chung, J.S.; Shin, H.K.; Lim, S.S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Yoo, N.H.; Lee, Y.M.; Yoo, J.L.; Kim, Y.S.; Kim, J.S. Constituents of the flowers of Erigeron annuus with inhibitory activity on the formation of advanced glycation end products (AGEs) and aldose reductase. Arch. Pharm. Res. 2008, 31, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.H.; Shin, K.H.; Kang, Y.; Lee, J.; Lim, S.S. Rapid Identification of Aldose Reductase Inhibitory Compounds from Perilla frutescens. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Kwon, S.O.; Cui, C.B.; Lim, S.S. The inhibitory effect of Prunella vulgaris L. on aldose reductase and protein glycation. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nakamura, S.; Fujimoto, K.; Ohta, T.; Ogawa, K.; Yoshikawa, M.; Matsuda, H. Structure of constituents isolated from the flower buds of Cananga odorata and their inhibitory effects on aldose reductase. J. Nat. Med. 2014, 68, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Choi, S.H.; Moon, H.E.; Park, J.J.; Jung, H.A.; Woo, M.H.; Woo, H.C.; Choi, J.S. The inhibitory activities of the edible green alga Capsosiphon fulvescens on rat lens aldose reductase and advanced glycation end products formation. Eur. J. Nutr. 2014, 53, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Salama, M.M.; Abd-Elrahman, E.H.; El-Maraghy, S.A. Antidiabetic activity of phenolic compounds from Pecan bark in streptozotocin-induced diabetic rats. Phytochem. Lett. 2011, 4, 337–341. [Google Scholar] [CrossRef]
- Chethan, S.; Dharmesh, S.M.; Malleshi, N.G. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorg. Med. Chem. 2008, 16, 10085–10090. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. (Tokyo) 2002, 50, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Hetta, M.H.; Samhan, F.A.; El Din, R.A.S.; Ali, G.H. Phytochemical and antibacterial study of five freshwater algal species. Asian J. Plant Sci. Res. 2012, 11, 109–116. [Google Scholar]
- Kumari, R.P.; Anbarasu, K. Protective role of C-phycocyanin against secondary changes during sodium selenite mediated cataractogenesis. Nat. Products Bioprospect. 2014, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.P.; Sivakumar, J.; Thankappan, B.; Anbarasu, K. C-phycocyanin modulates selenite-induced cataractogenesis in rats. Biol. Trace Elem. Res. 2013, 151, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid.-Based Complement Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Kumari, R.P.; Ramkumar, S.; Thankappan, B.; Natarajaseenivasan, K.; Balaji, S.; Anbarasu, K. Transcriptional regulation of crystallin, redox, and apoptotic genes by C-Phycocyanin in the selenite-induced cataractogenic rat model. Mol. Vis. 2015, 21, 26. [Google Scholar] [PubMed]
- Kothadia, A.D.; Shenoy, A.M.; Shabaraya, A.R.; Rajan, M.S.; Viradia, U.M.; Patel, N.H. Evaluation of Cataract Preventive Action of Phycocyanin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 42–44. [Google Scholar]
- Ou, Y.; Yuan, Z.; Li, K.; Yang, X. Phycocyanin may suppress d-galactose-induced human lens epithelial cell apoptosis through mitochondrial and unfolded protein response pathways. Toxicol. Lett. 2012, 215, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, W.H.; Park, S.D.; Moon, H.I. Aldose reductase inhibitors from Litchi chinensis Sonn. J. Enzyme Inhib. Med. Chem. 2009, 24, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Yoon, N.Y.; Kang, S.S.; Kim, Y.S.; Choi, J.S. Inhibitory activities of prenylated flavonoids from Sophora flavescens against aldose reductase and generation of advanced glycation endproducts. J. Pharm. Pharmacol. 2008, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, J.K.; Jang, J.M.; Lim, S.S. Inhibitory Effect of the Phenolic Compounds from Geranium thunbergii on Rat Lens Aldose Reductase and Galactitol Formation. Korean J. Med. Crop. Sci. 2012, 20, 222–230. [Google Scholar] [CrossRef]
- Kim, J.M.; Jang, D.S.; Lee, Y.M.; Yoo, J.L.; Kim, Y.S.; Kim, J.H.; Kim, J.S. Aldose-reductase- and protein-glycation-inhibitory principles from the whole plant of Duchesnea chrysantha. Chem. Biodivers. 2008, 5, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Sawant, L.; Singh, V.K.; Dethe, S.; Bhaskar, A.; Balachandran, J.; Mundkinajeddu, D.; Agarwal, A. Aldose reductase and protein tyrosine phosphatase 1B inhibitory active compounds from Syzygium cumini seeds. Pharm. Biol. 2015, 53, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Yoon, N.Y.; Bae, H.J.; Min, B.S.; Choi, J.S. Inhibitory activities of the alkaloids from Coptidis Rhizoma against aldose reductase. Arch. Pharm. Res. 2008, 31, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]
- Huang, G.J.; Hsieh, W.T.; Chang, H.Y.; Huang, S.S.; Lin, Y.C.; Kuo, Y.H. α-glucosidase and aldose reductase inhibitory activities from the fruiting body of Phellinus merrillii. J. Agric. Food Chem. 2011, 59, 5702–5706. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, J.K.; Kang, Y.H.; Lee, J.Y.; Kang, I.J.; Lim, S.S. Aldose Reductase Inhibitory Activity of Compounds from Zea mays L. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar]
- Morikawa, T.; Chaipech, S.; Matsuda, H.; Hamao, M.; Umeda, Y.; Sato, H.; Tamura, H.; Kon’i, H.; Ninomiya, K.; Yoshikawa, M.; et al. Antidiabetogenic oligostilbenoids and 3-ethyl-4-phenyl-3,4-dihydroisocoumarins from the bark of Shorea roxburghii. Bioorg. Med. Chem. 2012, 20, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, A.; Okada, Y.; Akkol, E.K.; Duman, H.; Okuyama, T.; Çaliş, I. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem. 2010, 118, 686–692. [Google Scholar] [CrossRef]
- Rodriguez-Lyon, M.L.; Diaz-Lanza, A.M.; Bernabé, M.; Villaescusa-castillo, L. Flavone glycosides containing acetylated sugars from Sideritis hyssopifolia. Magn. Reson. Chem. 2000, 38, 684–687. [Google Scholar] [CrossRef]
- Lenherr, A.; Mabry, T.J. Acetylated allose-containing flavonoid glucosides from Stachys anisochila. Phytochemistry 1987, 26, 1185–1188. [Google Scholar] [CrossRef]
- Patel, D.K.; Prasand, S.K.; Kumar, R.; Hemalatha, S. Cataract: A major secondary complication of diabetes, its epidemiology and an overview on major medicinal plants screened for anticataract activity. Asian Pac. J. Trop. Dis. 2011, 1, 323–329. [Google Scholar] [CrossRef]
- Devi, V.G.; Rooban, B.N.; Sasikala, V.; Sahasranamam, V.; Abraham, A. Isorhamnetin-3-glucoside alleviates oxidative stress and opacification in selenite cataract in vitro. Toxicol. In Vitro 2010, 24, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kim, Y.J.; Jung, S.H.; Kim, J.H.; Kim, J.S. Flavonoids from Litsea japonica Inhibit AGEs Formation and Rat Lense Aldose Reductase in Vitro and Vessel Dilation in Zebrafish. Planta Med. 2017, 83, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, S.H.; Suh, H.W.; Lim, S.S. Phytochemical analysis of Agrimonia pilosa Ledeb, its antioxidant activity and aldose reductase inhibitory potential. Int. J. Mol. Sci. 2017, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Choi, S.J.; Yu, S.Y.; Ku, S.K.; Kim, M.H.; Kim, J.S. Effects of Allium victorialis leaf extracts and its single compounds on aldose reductase, advanced glycation end products and TGF-β1 expression in mesangial cells. BMC Complement Altern. Med. 2013, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, M.Y.; Park, W.H.; Moon, H.I. Aldose reductase inhibitors from Viola hondoensis W. Becker et H Boss. Am. J. Chin. Med. 2008, 36, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tian, J.; Zhang, J.; Wang, K.; Liu, L.; Yang, B.; Bao, L.; Liu, H. Triterpenes and meroterpenes from Ganoderma lucidum with inhibitory activity against HMGs reductase, aldose reductase and α-glucosidase. Fitoterapia 2017, 120, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, X.; Guan, S.; Xia, J.; Sun, J.; Guo, H.; Guo, D. Analysis of Triterpenoids in Ganoderma lucidum Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibitory activity from the fruiting body of Ganoderma lucidum. Fitoterapia 2010, 81, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Inhibition of aldose reductase in vitro by constituents of Ganoderma lucidum. Planta Med. 2010, 76, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Kondo, R.; Shimizu, K. Structure-activity relationships of lanostane-type triterpenoids from Ganoderma lingzhi as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5900–5903. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Ranganatha, L.V.; Prasad, M.N.N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S. Afr. J. Bot. 2014, 95, 54–63. [Google Scholar] [CrossRef]
- Asha, R.; Devi, V.G.; Abraham, A. Chemico-Biological Interactions Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinerea attenuate selenite induced cataract formation in Sprague Dawley rat pups. Chem. Biol. Interact. 2016, 245, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Anticataractogenic Activity of Luteolin. Chem. Biodivers. 2016, 13, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Vit, P.; Jacob, T.J. Putative Anticataract Properties of Honey Studied by the Action of Flavonoids on a Lens Culture Model. J. Heal. Sci. 2008, 54, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Kim, J.S. Constituents of the flowers of Platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Rooban, B.N.; Sasikala, V.; Devi, V.G.; Sahasranamam, V.; Abraham, A. Prevention of selenite induced oxidative stress and cataractogenesis by luteolin isolated from Vitex negundo. Chem. Biol. Interact. 2012, 196, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, J.H.; Lee, M.H.; Lee, B.W.; Kwon, H.S.; Park, C.H.; Shim, K.B.; Kim, H.T.; Baek, I.Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rodriguez, J.P.; Quilantang, N.G.; Lee, M.H.; Cho, E.J.; Jacinto, S.D.; Lee, S. Determination of flavonoids from Perilla frutescens var. japonica seeds and their inhibitory effect on aldose reductase. Appl. Biol. Chem. 2017, 60, 155–162. [Google Scholar] [CrossRef]
- Morikawa, T.; Xie, H.; Wang, T.; Matsuda, H.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXXII. Aminopeptodase N and aldose reductase inhibitors from Sinocrassula indica: Structures of Sinocrassosides B4, B5, C1 and D1-D3. Chem. Pharm. Bull. (Tokyo) 2008, 56, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Tao, J.; Ueda, K.; Yoshikawa, M. Bioactive constituents of Chinese natural medicines. VII. Inhibitors of degranulation in RBL-2H3 cells and absolute stereostructures of three new diarylheptanoid glycosides from the bark of Myrica rubra. Chem. Pharm. Bull. (Tokyo) 2002, 50, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kwon, S.H.; Kim, S.B.; Lim, S.S. Inhibitory Activities of Stauntonia hexaphylla Leaf Constituents on Rat Lens Aldose Reductase and Formation of Advanced Glycation End Products and Antioxidant. BioMed Res. Int. 2017, 2017, 4273257. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Mishra, S. Isoquinoline alkaloids from Tinospora cordifolia inhibit rat lens aldose reductase. Phyther. Res. 2012, 26, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Fujimoto, K.; Matsumoto, T.; Nakashima, S.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Structures of Acylated Sucroses and Inhibitory Effects of Constituents on Aldose Reducatase from the Flower Buds of Prunus mume. Chem. Pharm. Bull. 2013, 61, 445–451. [Google Scholar]
- Nakamura, S.; Fujimoto, K.; Matsumoto, T.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Structures of acylated sucroses and an acylated flavonol glycoside and inhibitory effects of constituents on aldose reductase from the flower buds of Prunus mume. J. Nat. Med. 2013, 67, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, Y.S.; Lee, Y.M.; Jang, D.S.; Kim, J.S. Inhibition of aldose reductase and xylose-induced lens opacity by puerariafuran from the roots of Pueraria lobata. Biol. Pharm. Bull. 2010, 33, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Stobiecki, M.; Kachlicki, P. Isolation and identification of flavonoids. Sci. Flavonoids 2006, 27, 47–69. [Google Scholar]
- Lee, H.E.; Kim, J.A.; Whang, W.K. Chemical constituents of smilax China l. stems and their inhibitory activities against glycation, aldose reductase, α-glucosidase, and lipase. Molecules 2017, 22, 451. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Yasuko, H.; Goto, H.; Hollinshead, J.; Nash, R.J.; Adachi, I. Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine 2009, 16, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tang, Y.; Liu, Q.; Guo, N.; Zhang, J.; Xiao, Z.; Chen, R.; Shen, Z. Isolation, modification, and aldose reductase inhibitory activity of rosmarinic acid derivatives from the roots of Salvia grandifolia. Fitoterapia 2016, 112, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Hwang, S.H.; Kang, B.G.; Hong, J.S.; Lim, S.S. Inhibitory effects of Colocasia esculenta (L.) Schott constituents on aldose reductase. Molecules 2014, 19, 13212–13224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, N.H.; Nam, J.W.; Lee, Y.M.; Jang, D.S.; Kim, Y.S.; Nam, S.H.; Seo, E.K.; Yang, M.S.; Kim, J.S. Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis Ex Vivo. Arch. Pharm. Res. 2010, 33, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kwon, S.B.; Heo, N.K.; Chun, W.J.; Kim, M.J.; Kwon, Y.S. Constituents of the stem of Angelica gigas with rat lens aldose reductase inhibitory activity. J. Appl. Biol. Chem. 2011, 54, 194–199. [Google Scholar] [CrossRef]
- Abou Assi, R.; Darwis, Y.; Abdulbaqi, I.; Khan, A.A.; Lim, V.; Laghari, M.H. Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab. J. Chem. 2017, 10, 691–707. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, Y.; Wang, Q.; Yang, Y.F.; Ji, S.; Song, W.; Qiao, X.; Guo, D.; Liang, H.; Ye, M. Metabolites identification of bioactive licorice compounds in rats. J. Pharm. Biomed. Anal. 2015, 115, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, S.H.; Jung, S.H.; Kim, J.K.; Pan, C.H.; Lim, S.S. Aldose Reductase Inhibitory Compounds from Glycyrrhiza uralensis. Biol. Pharm. Bull. 2010, 33, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Hwang, J.K.; Koh, H.W.; Jang, K.Y.; Lee, J.H.; Park, J.W.; Park, B.H. Sulfuretin, a major flavonoid isolated from Rhus verniciflua, ameliorates experimental arthritis in mice. Life Sci. 2012, 90, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Song, D.G.; Lee, J.Y.; Pan, C.H.; Um, B.H.; Jung, S.H. Inhibitory Effect of the Compounds Isolated from Rhus verniciflua on Aldose Reductase and Advanced Glycation Endproducts. Biol. Pharm. Bull. 2008, 31, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Jung, S.H.; Ji, J.; Shin, K.H.; Keum, S.R. Inhibitory effects of 2′-hydroxychalcones on rat lens aldose reductase and rat platelet aggregation. Chem. Pharm. Bull. 2000, 48, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Yuan, H.; Yamazaki, Y.; Sasaki, T.; Oka, S. Alkaloids and Flavonoids from Peanut Skins. Planta Med. 2001, 67, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic acid extracted from Herba dendrobii prevents diabetic cataract pathogenesis by inhibiting aldose reductase activity. Evid.-Based Complement Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.Y.; Liu, H.L.; Zhang, J.Y.; Yang, B. Phenolic glycosides and other constituents from the bark of Magnolia officinalis. J. Asian Nat. Prod. Res. 2014, 16, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Mishra, S.M. Aldose Reductase Inhibitory Activity of a Cglycosidic Flavonoid Derived from Enicostemma hyssopifolium. Inf. J. Complement. Integr. Med. 2009, 6, 1553–3840. [Google Scholar]

| Active Ingredient | Structure | IC50 Values | ||||||
|---|---|---|---|---|---|---|---|---|
| AGE | ARI | GLWW | RHAR | BLAR | HLAR | RLAR | ||
| 1-O-galloyl-β-d-glucose (β-Glucogallin) | 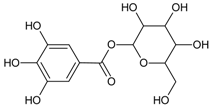 | NA | NA | NA | 17.00 µM [30] | NA | NA | NA |
| 1,3-di-O-caffeoylquinic acid | 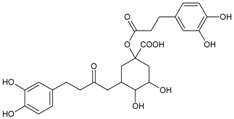 | 24.85 µM [32] | NA | NA | 0.810 µM [33] | NA | NA | 0.22 µM [32] |
| 1,5-Di-hydroxy-1,5-di-[(E)-3-(4-hydroxyphenyl)-2-propenoic]-3-pentanonyl ester (DHDP) | 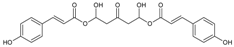 | NA | NA | NA | 194.67µM [34] | NA | NA | NA |
| 1,5-di-O-caffeoylquinic acid | 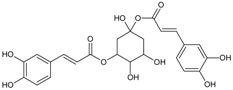 | NA | NA | NA | NA | NA | NA | 2.98 µM [35] |
| 1,3,6-trihydroxy-2-methoxymethylanthraquinone | 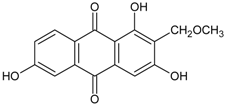 | 52.72 µM [36] | NA | NA | NA | NA | NA | 3.04 µM [36] |
| 1,2,3,6-tetra-O-galloyl-β-d-glucose | 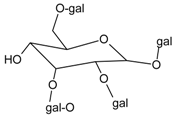 | 1.99 µM [38] | NA | NA | NA | NA | NA | 0.70 µM [38] |
| 1,3,5,8-Tetrahydroxyxanthone | 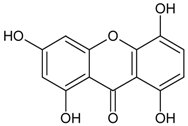 | NA | NA | NA | NA | NA | NA | 0.0886 μM [39] |
| 2″,4″-O-Diacetylquercitrin | 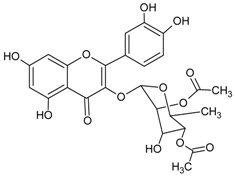 | 11.46 µM [40] | NA | NA | NA | NA | NA | 0.077 µM [40] |
| 3-Isomangostin | 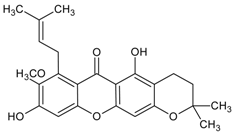 | NA | NA | NA | NA | NA | NA | 3.48 μM [41] |
| 3′,5′-dimethoxy-(1,1′-biphenyl)-3,4-diol 3-O-β-d-glucopyranoside |  | NA | NA | NA | NA | NA | NA | 3.80 µM [47] |
| 3,5-di-O-caffeoylquinic Acid | 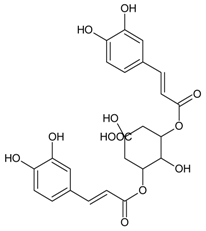 | 6.06 µM [48] | NA | 153 g [33] | 1.34 µM [33] | NA | NA | 0.19 µM [33] |
| 4-O-butylpaeoniflorin | 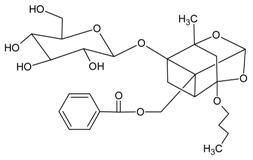 | 10.80 µM [53] | NA | NA | NA | NA | NA | 36.20 µM [53] |
| 4,5-Di-O-trans-caffeoyl-d-quinic acid |  | NA | NA | NA | NA | NA | NA | 0.29 µM [55] |
| 5-O-Feruloly quinic acid | 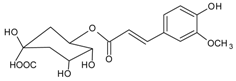 | NA | NA | NA | NA | NA | NA | 14.19 µM [56] |
| 5,7,4′-trihydroxyisoflavone (Genistein) |  | NA | NA | NA | NA | NA | NA | 9.48 µM [60] |
| 20(S)-Ginsenoside Rh2 | 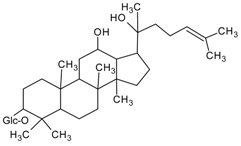 | NA | NA | NA | 147.40 µM [61] | NA | NA | NA |
| Acteoside |  | 5.11 µM [63] | NA | NA | NA | NA | NA | 0.83 µM [63] |
| Basilicumin [7-(3-hydroxypropyl)-3-methyl-8-β-O-d-glucoside-2H-chromen-2-one] | 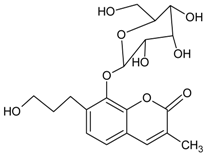 | NA | NA | NA | NA | 2.09 µM | NA | NA |
| Caffeic acid | 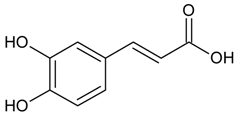 | 7.56 µM [68] | NA | NA | 210.28µM [66] | NA | NA | 16.71 µM [65] |
| Canangafruiticoside E | 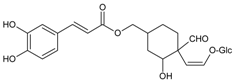 Glc=β-d-glucopyranoside | NA | NA | NA | NA | NA | NA | 0.80 µM [71] |
| Capsofulvesin A [((2S)-l-O-(6Z,9Z,12Z,15Zoctadecatetraenoyl)-2-O-(4Z,10Z,13Zhexadecatetraenoyl)-3-O-β-d-galactopyranosyl glycerol)] | 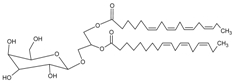 | NA | NA | NA | NA | NA | NA | 52.53 µM [72] |
| Davallialactone | 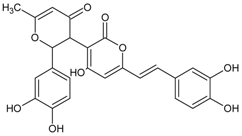 | NA | NA | NA | 0.56 µM [67] | NA | NA | 0.33 µM [67] |
| Delphinidin 3-O-β-galactopyranoside-3′-O-β-glucopyranoside | 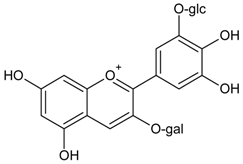 Glc= β-glucopyranoside, Gal= β-galactopyranoside | NA | NA | NA | NA | NA | NA | 0.37 µM [83] |
| Desmethylanhydroicaritin | 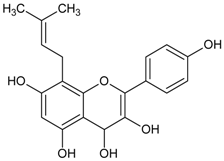 | 294.60 µM [84] | NA | NA | 0.45 µM [84] | NA | NA | 0.95 µM [84] |
| Ellagic acid | 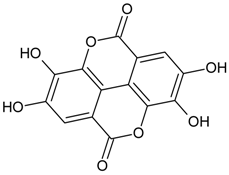 | 26.0 µM [86] | NA | 42.47% [85] | NA | NA | 1.37 µM [67] | 0.12 µM [87] |
| Epiberberine | 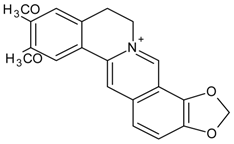 | NA | NA | NA | 168.10 µM [88] | NA | NA | 100.07 µM [88] |
| Geraniin |  | 21.00 µM 96% * [89] | 0.15 µM [89] | 39.87% [85] | NA | NA | NA | NA |
| Hipolon | 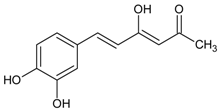 | NA | NA | NA | NA | NA | NA | 9.47 µM [90] |
| Hirsutrin | 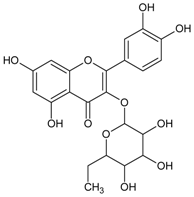 | NA | NA | 33.78% [91] | NA | NA | NA | 4.78 µM [91] |
| Hopeafuran | 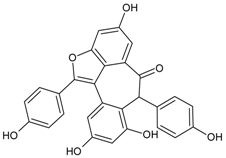 | NA | NA | NA | NA | NA | NA | 14.80 µM [92] |
| Hypolaetin 7-O-[6′′′-O-acetyl-β-d-allopyranosyl-(1→2)]-6′′-O-acetyl-β-d-glucopyranoside |  | NA | 0.66 µM [93] | NA | NA | NA | NA | NA |
| Isocampneoside II | 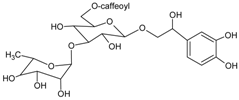 | NA | NA | NA | 9.72 µM [66] | NA | NA | NA |
| Kaempferol |  | 36.01 µM [100] | NA | NA | 45.58 µM [66] | NA | NA | 1.10 µM [98,100] |
| Kakkalide | 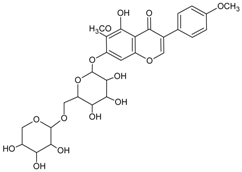 | NA | NA | NA | NA | NA | NA | 0.56 µM [101] |
| Lucidumol A [(24S)-24,25-Dihydroxylanost-8-ene-3,7-dione] |  | NA | NA | NA | NA | 19.10 µM [102] | NA | NA |
| Lupeol | 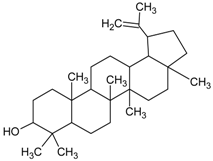 | NA | NA | NA | 3.60 µM [107] | NA | NA | NA |
| Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromomenone) |  | 16.60 µM [111] | NA | NA | 6.34 µM [52] | NA | NA | 0.087 µM [111] |
| Luteolin-7-O-β-d-glucopyranoside |  | 117.80 µM [117] | NA | NA | NA | NA | NA | 7.34 µM [117] |
| Magnoflorine |  | NA | NA | NA | NA | NA | NA | 3.60 µM [118] |
| Methyl-3,5-di-O-caffeoylquinate |  | NA | NA | 117 g [33] | 0.67 µM [33] | NA | NA | 0.30 µM [33] |
| Mumeic acid-A |  | NA | NA | NA | NA | NA | NA | 0.40 µM [119] |
| Palbinone |  | >500 µM [53] | NA | NA | NA | NA | NA | 11.40 µM [53] |
| Puerariafuran | 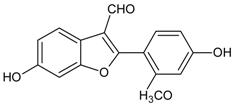 | NA | NA | NA | NA | NA | NA | 22.20 µM [57,121] |
| Quercetin-3-O-β-d-glucoside | 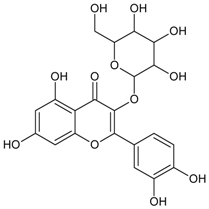 | >1000 µM [117] | NA | NA | NA | NA | NA | 2.21 µM [122] |
| Quercitrin (Quercetin 3-O-α-l-rhamnoside) |  | 4.20 µM [100] | NA | NA | NA | NA | NA | 0.17 µM [40] |
| Rhetsinine |  | NA | NA | NA | NA | NA | NA | 24.10 µM [124] |
| Rosmarinic acid | 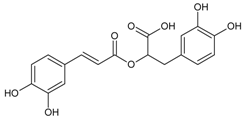 | NA | NA | 532.38g [70] | 2.77 µM [69] | NA | NA | 0.30 µM [125] |
| Scopoletin |  | 2.93 µM [127] | NA | NA | NA | NA | NA | 2.59 µM [128] |
| Semilicoisoflavone B | 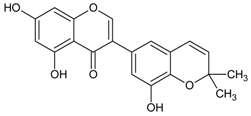 | NA | NA | NA | 10.60 µM [131] | NA | NA | 1.80 µM [131] |
| Sulfuretin | 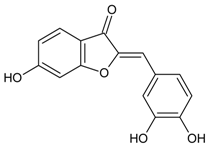 | 124.00 µM [133] | NA | NA | 1.30 µM [133] | NA | NA | NA |
| Syringic Acid | 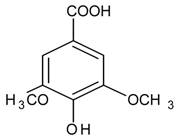 | NA | NA | NA | NA | NA | NA | 1081.1 µm [136] |
| Swertisin (C-glycosidic flavonoid) | 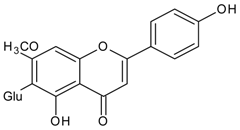 Glu=glucose | NA | NA | NA | NA | NA | NA | 1.60 µm [138] |
| Valoneic acid dilactone | 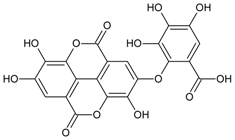 | NA | NA | NA | NA | NA | NA | 0.075 µM [87] |
| Constituent Name (Class of Constituent) | Structure | Doses (IC50/EC50) | |||||
|---|---|---|---|---|---|---|---|
| AR Transgenic Mice | Selenite-Induced | AR Rat Lens | Galactose-Induced Lens Opacity | Xylose-Induced Lens Opacity | Ref | ||
| 1-O-galloyl-β-d-glucose (β-Glucogallin) |  | Ex vivo: 30.00 µM | NA | NA | NA | NA | [29] |
| 1,2,3,6-Tetra-O-galloyl-β-d-glucose | 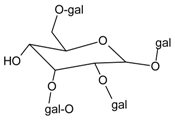 | NA | NA | NA | NA | Ex vivo: 80.00 µM | [38] |
| 3,5-di-O-caffeoyl-epi-quinic Acid | 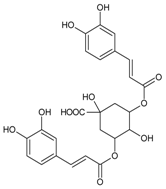 | NA | NA | NA | NA | Ex vivo: 10.00 μM | [48] |
| 5,7,4′-trihydroxyisoflavone (Genistein) | 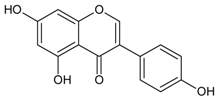 | NA | NA | NA | NA | Ex vivo: 18.50 µM | [60] |
| Isorhamnetin-3-glucoside |  | NA | Ex vivo: 52.25 µM | NA | NA | NA | [97] |
| Lupeol | 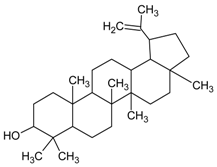 | NA | In vivo: 126.15 μM | NA | NA | NA | [108] |
| Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromomenone) | 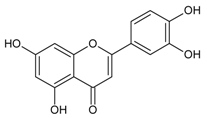 | NA | Ex vivo: 6.98 µM | NA | NA | NA | [112] |
| Puerariafuran | 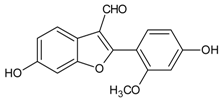 | NA | NA | NA | NA | Ex vivo: 15.00 µM | [121] |
| Scopoletin |  | NA | NA | NA | NA | Ex vivo: 25.00 µM | [127] |
| Syringic acid | 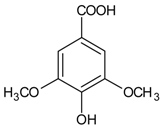 | NA | NA | NA | Ex vivo: 1075.70 μM In vivo: 2% syringic acid eye drop (131,197.80 μM) | NA | [136] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, V.; Schneider, E.; Wu, H.; Pang, I.-H. Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research. Nutrients 2018, 10, 1580. https://doi.org/10.3390/nu10111580
Lim V, Schneider E, Wu H, Pang I-H. Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research. Nutrients. 2018; 10(11):1580. https://doi.org/10.3390/nu10111580
Chicago/Turabian StyleLim, Vuanghao, Edward Schneider, Hongli Wu, and Iok-Hou Pang. 2018. "Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research" Nutrients 10, no. 11: 1580. https://doi.org/10.3390/nu10111580