Hypocaloric Diet Prevents the Decrease in FGF21 Elicited by High Phosphorus Intake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Diets
2.3. Experimental Design
2.4. Blood Chemistries
2.5. RNA Extraction and Quantitative Real-Time PCR(RT-PCR)
2.6. Protein Extraction and Western Blot
2.7. Statistical Analysis
3. Results
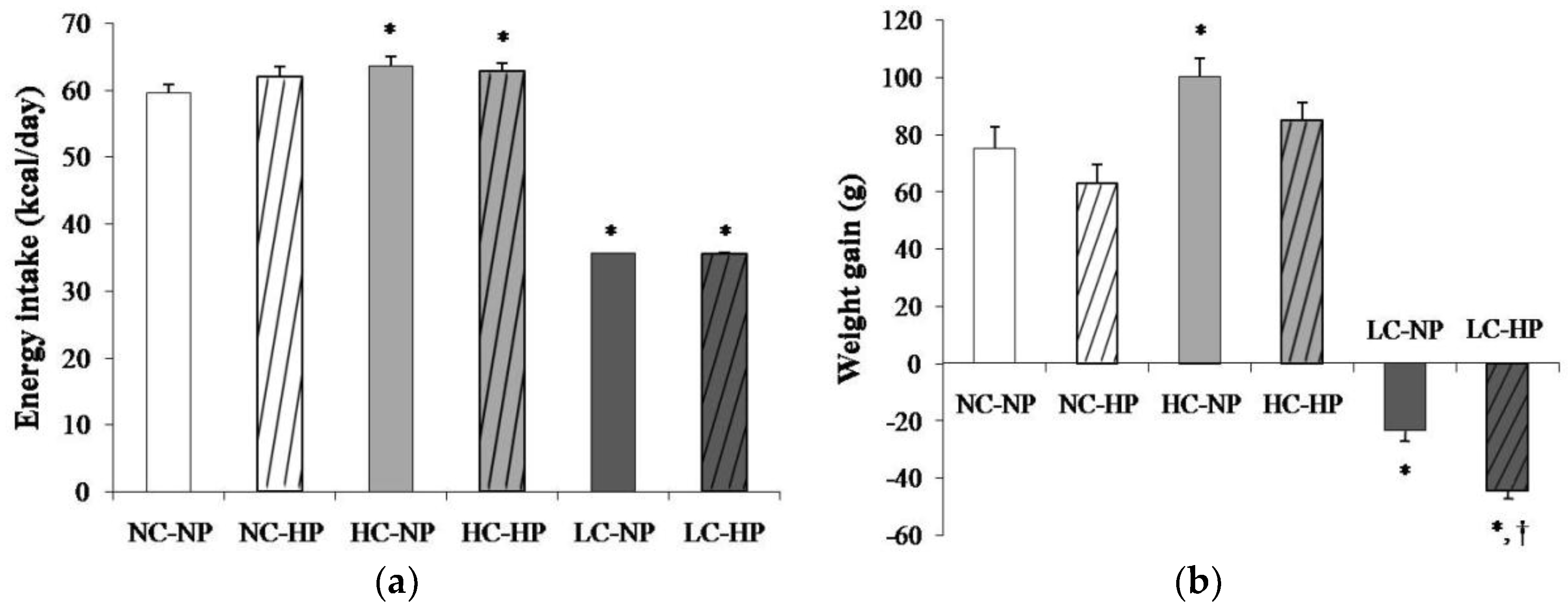
3.1. Energy Intake and Body Weight
3.2. Biochemical Data
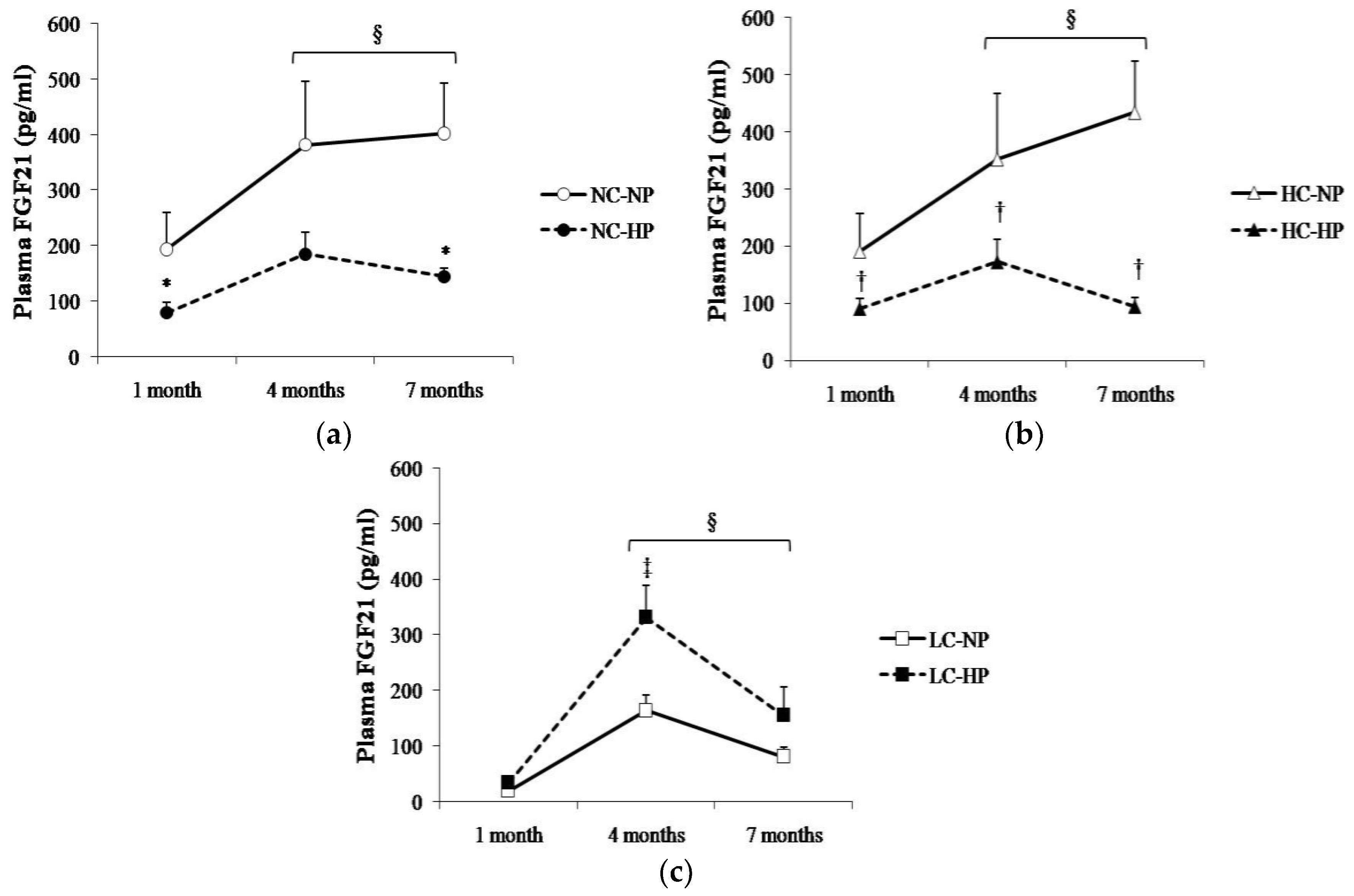
3.3. RT-PCR and Western Blot Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Migliaccio, S.; Greco, E.A.; Fornari, R.; Donini, L.M.; Lenzi, A. Is obesity in women protective against osteoporosis? Diabetes Metab. Syndr. Obes. 2011, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Gilbert, J.A. Human obesity: Is insufficient calcium/dairy intake part of the problem? J. Am. Coll. Nutr. 2011, 30, 449S–453S. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.P.; Doherty, G.M.; Chang, Y.C.; Liu, C.L. Leptin: The link between overweight and primary hyperparathyroidism. Med. Hypotheses 2011, 76, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.; Belyaeva, O.; Jude, E.B.; Tsiberkin, A.; Andreeva, A.; Grineva, E.; Pludowski, P. Serum 25(OH)D and adipokines levels in people with abdominal obesity. J. Steroid Biochem. Mol. Biol. 2018, 175, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Maeda, T.; Kawane, T.; Matsunuma, A.; Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 2010, 25, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Pineda, C.; Raya, A.I.; Rodriguez-Ortiz, M.E.; Diaz-Tocados, J.M.; Rios, R.; Rodriguez, J.M.; Aguilera-Tejero, E.; Almaden, Y. Leptin directly stimulates parathyroid hormone secretion. Endocrine 2017, 56, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Rascón-Pacheco, R.A.; Rodríguez-Morán, M.; de la Peña, J.E.; Wacher, N. Hypomagnesaemia and risk for metabolic glucose disorders: A 10-year follow-up study. Eur. J. Clin. Investig. 2008, 38, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M. Biochemical relationships between bone turnover markers and blood glucose in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 2017, 11, S369–S372. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Lamberg-Allardt, C.J. Phosphorus. Adv. Nutr. 2015, 6, 860–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaría, R.; Díaz-Tocados, J.M.; Pendón-Ruiz de Mier, M.V.; Robles, A.; Salmerón-Rodríguez, M.D.; Ruiz, E.; Vergara, N.; Aguilera-Tejero, E.; Raya, A.; Ortega, R.; et al. Increased phosphaturia accelerates the decline in renal function: A search for mechanisms. Sci. Rep. 2018, 8, 13701. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Calvo, M.S. Hidden sources of phosphorus in the typical American diet: Does it matter in nephrology? Semin. Dial. 2003, 16, 186–188. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; DiMarchi, R. Fibroblast growth factor 21 night watch: Advances and uncertainties in the field. J. Intern. Med. 2017, 281, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Overview of the FGF23-Klotho axis. Pediatr. Nephrol. 2010, 25, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Raya, A.I.; Rios, R.; Pineda, C.; Rodriguez-Ortiz, M.E.; Diez, E.; Almaden, Y.; Muñoz-Castañeda, J.R.; Rodriguez, M.; Aguilera-Tejero, E.; Lopez, I. Energy-dense diets increase FGF23, lead to phosphorus retention and promote vascular calcifications in rats. Sci. Rep. 2016, 6, 36881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, R.; Pineda, C.; Lopez, I.; Muñoz-Castañeda, J.; Rodriguez, M.; Aguilera-Tejero, E.; Raya, A.I. Phosphorus restriction does not prevent the increase in fibroblast growth factor 23 elicited by high fat diet. PLoS ONE 2018, 13, e0198481. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, J.R.; Herencia, C.; Pendón-Ruiz de Mier, M.V.; Rodriguez-Ortiz, M.E.; Diaz-Tocados, J.M.; Vergara, N.; Martínez-Moreno, J.M.; Salmerón, M.D.; Richards, W.G.; Felsenfeld, A. Differential regulation of renal Klotho and FGFR1 in normal and uremic rats. FASEB J. 2017, 31, 3858–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claycombe, K.J.; Vomhof-DeKrey, E.E.; Garcia, R.; Johnson, W.T.; Uthus, E.; Roemmich, J.N. Decreased beige adipocyte number and mitochondrial respiration coincide with increased histone methyl transferase (G9a) and reduced FGF21 gene expression in Sprague-Dawley rats fed prenatal low protein and postnatal high-fat diets. J. Nutr. Biochem. 2016, 31, 113–121. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Lang, R. Hypophosphatemia and glucose intolerance: Evidence for tissue insensitivity to insulin. N. Engl. J. Med. 1980, 303, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Fliser, D.; Fode, P.; Ritz, E. Changes in plasma phosphate levels influence insulin sensitivity under euglycemic conditions. J. Clin. Endocrinol. Metab. 1996, 81, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, Y.; Mechin, M.C.; van de Werve, G. Upregulation of liver glucose-6-phosphatase in rats fed with a Pi-deficient diet. Biochem. J. 1999, 343, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tran, T.L.; Finegood, D.T.; van de Werve, G. Dietary Pi deprivation in rats affects liver cAMP, glycogen, key steps of gluconeogenesis and glucose production. Biochem. J. 2000, 352, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yamamoto, H.; Nakahashi, O.; Kagawa, T.; Ishiguro, M.; Masuda, M.; Kozai, M.; Ikeda, S.; Taketani, Y.; Takeda, E. Dietary phosphate restriction induces hepatic lipid accumulation through dysregulation of cholesterol metabolism in mice. Nutr. Res. 2013, 33, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Abuduli, M.; Ohminami, H.; Otani, T.; Kubo, H.; Ueda, H.; Kawai, Y.; Masuda, M.; Yamanaka-Okumura, H.; Sakaue, H.; Yamamoto, H.; et al. Effects of dietary phosphate on glucose and lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E526–E538. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.W.; Myers, M.G., Jr. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 2018, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, D.A.; Proietto, J.; Fam, B.C.; Thorburn, A.W. Short-term, high-fat diets lower circulating leptin concentrations in rats. Am. J. Clin. Nutr. 2000, 71, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, K.; Li, H.; Zhang, M.; Jiang, S.; Chen, S.; Zhou, J.; Dai, Z.; Fang, Q.; Jia, W. Endoplasmic reticulum stress induces up-regulation of hepatic β-klotho expression through ATF4 signalling pathway. Biochem. Biophys. Res. Commun. 2015, 459, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Escuredo, J.M.; Gómez-Ambrosi, J.; Catalan, V.; Domingo, P.; Giralt, M.; Frühbeck, G.; Villarroya, F. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes. (Lond.) 2015, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.A.; Meers, G.M.; Laughlin, M.H.; Ibdah, J.A.; Thyfault, J.P.; Rector, R.S. Modulating fibroblast growth factor 21 in hyperphagic OLETF rats with daily exercise and caloric restriction. Appl. Physiol. Nutr. Metab. 2012, 37, 1054–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, C.; Chen, M.M.; Stanislaus, S.; Chinookoswong, N.; Hager, T.; Wang, M.; Véniant, M.M.; Xu, J. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology 2012, 153, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rühlmann, C.; Wölk, T.; Blümel, T.; Stahn, L.; Vollmar, B.; Kuhla, A. Long-term caloric restriction in ApoE-deficient mice results in neuroprotection via Fgf21-induced AMPK/mTOR pathway. Aging (Albany NY) 2016, 8, 2777–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, A.C.; Bruss, M.D.; Nag, N.; Kharitonenkov, A.; Adams, A.C.; Hellerstein, M.K. Fibroblast growth factor 21 is not required for the reductions in circulating insulin-like growth factor-1 or global cell proliferation rates in response to moderate calorie restriction in adult mice. PLoS ONE 2014, 9, e111418. [Google Scholar] [CrossRef] [PubMed]
- Chapnik, N.; Genzer, Y.; Froy, O. Relationship between FGF21 and UCP1 levels under time-restricted feeding and high-fat diet. J. Nutr. Biochem. 2017, 40, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Bamba, T.; Suyama, T.; Ishijima, T.; Fukusaki, E.; Abe, K.; Nakai, Y. A high phosphorus diet affects lipid metabolism in rat liver: A DNA microarray analysis. PLoS ONE 2016, 11, e0155389. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, O.; Yamamoto, H.; Tanaka, S.; Kozai, M.; Takei, Y.; Masuda, M.; Kaneko, I.; Taketani, Y.; Iwano, M.; Miyamoto, K.; et al. Short-term dietary phosphate restriction up-regulates ileal fibroblast growth factor 15 gene expression in mice. J. Clin. Biochem. Nutr. 2014, 54, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameters | NC-NP | NC-HP | HC-NP | HC-HP | LC-NP | LC-HP |
|---|---|---|---|---|---|---|
| Triglycerides (mg/dL) | 54.8 ± 11.1 | 34.9 ± 2.9 | 83.1 ± 11.4 * | 39.6 ± 3.4† | 22.3 ± 1.7 * | 23.8 ± 2.3 * |
| Total cholesterol (mg/dL) | 50.6 ± 7.7 | 35.3 ± 3.5 | 49.4 ± 6.1 | 37.9 ± 6.2 | 35.9 ± 3.3 | 52.8 ± 6.0‡ |
| Glucose (mg/dL) | 116.9 ± 6.1 | 94.1 ± 4.4 * | 100.4 ± 6.1 | 103.6 ± 4.4 | 109.0 ± 19.6 | 74.2 ± 5.7‡ |
| Leptin (ng/mL) | 4.2 ± 0.5 | 3.1 ± 0.2 | 5.3 ± 0.8 * | 4.1 ± 0.5 | 0.6 ± 0.2 * | 0.6 ± 0.1 * |
| Adiponectin (ng/mL) | 8.1 ± 1.0 | 11.3 ± 1.7 | 5.7 ± 0.5 | 7.7 ± 0.8 | 10.5 ± 0.6 | 16.6 ± 2.3 *,‡ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda, C.; Rios, R.; Raya, A.I.; Rodriguez, M.; Aguilera-Tejero, E.; Lopez, I. Hypocaloric Diet Prevents the Decrease in FGF21 Elicited by High Phosphorus Intake. Nutrients 2018, 10, 1496. https://doi.org/10.3390/nu10101496
Pineda C, Rios R, Raya AI, Rodriguez M, Aguilera-Tejero E, Lopez I. Hypocaloric Diet Prevents the Decrease in FGF21 Elicited by High Phosphorus Intake. Nutrients. 2018; 10(10):1496. https://doi.org/10.3390/nu10101496
Chicago/Turabian StylePineda, Carmen, Rafael Rios, Ana I. Raya, Mariano Rodriguez, Escolastico Aguilera-Tejero, and Ignacio Lopez. 2018. "Hypocaloric Diet Prevents the Decrease in FGF21 Elicited by High Phosphorus Intake" Nutrients 10, no. 10: 1496. https://doi.org/10.3390/nu10101496





