Impact of Blood or Erythrocyte Membrane Fatty Acids for Disease Risk Prediction: Focusing on Cardiovascular Disease and Chronic Kidney Disease
Abstract
:1. Introduction
2. Impact of Dietary FAs on CVD Risk
3. Impact of Dietary FAs on CKD Risk Prediction
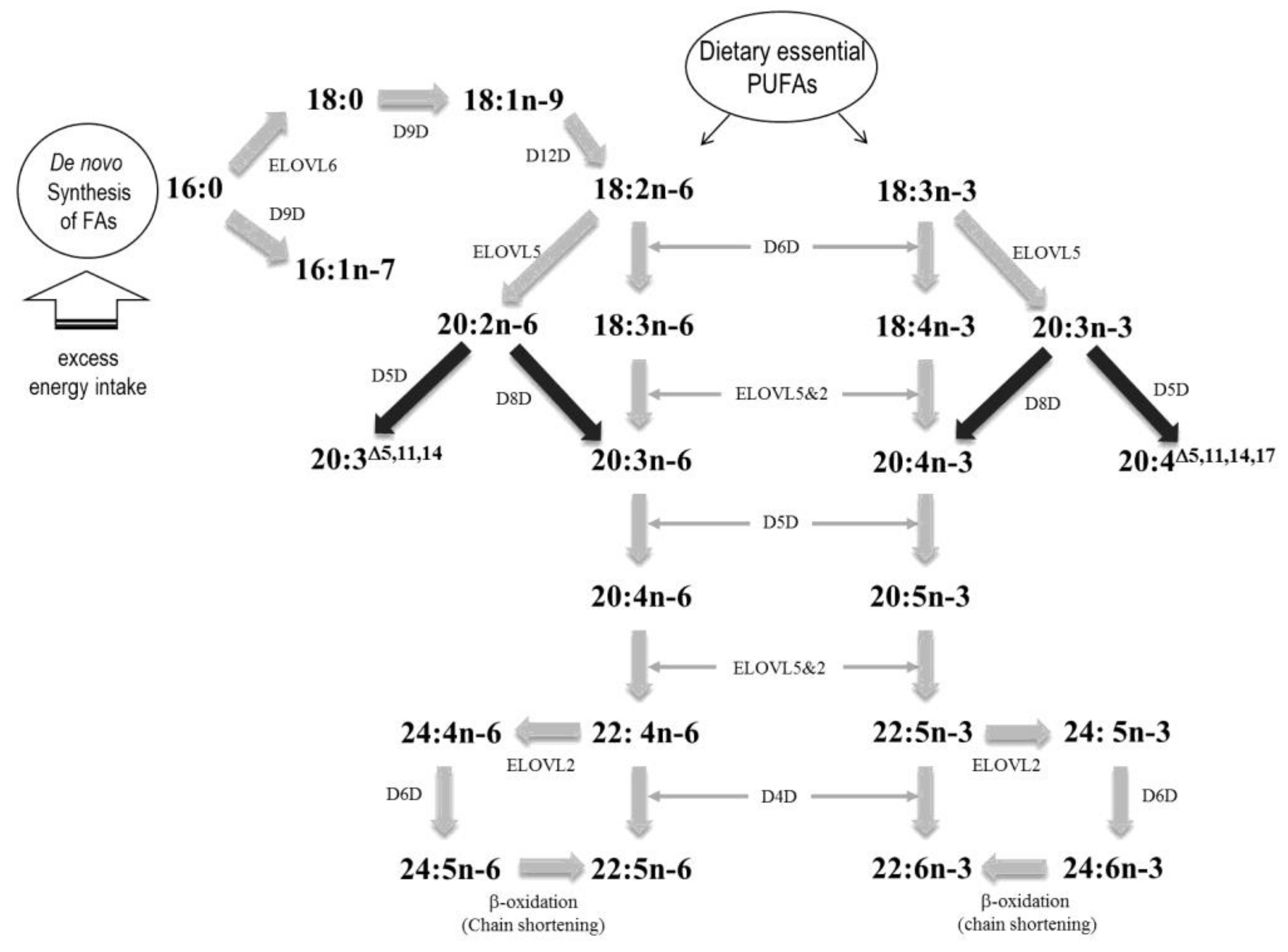
4. Blood or Tissue FAs as Predictors for the Risks of CVD and CKD
4.1. Impact of PUFAs on CVD Risk
4.2. Impact of SFAs, MUFAs, FA Desaturation/Elongation and TFA on CVD Risk
4.3. Impact of PUFAs on CKD Risk Prediction and Renal Progression
4.4. Impact of MUFAs on CKD Risk Prediction
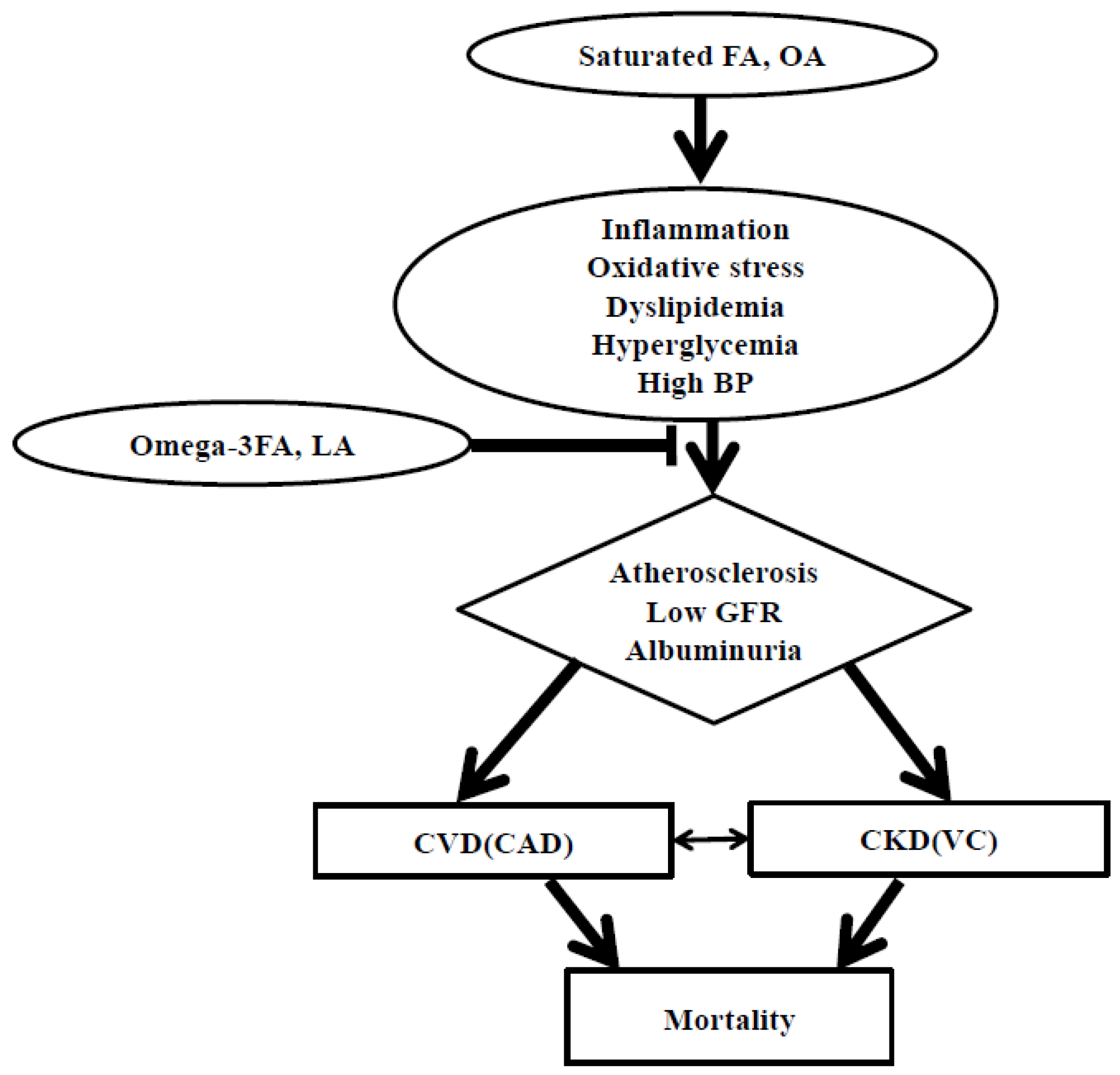
4.5. Impact of FAs on CVD Risk Prediction and Mortality in Patients with CKD
4.6. Impact of FAs on Vascular Calcification Prediction in Patients with CKD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fessler, M.B.; Rudel, L.L.; Brown, J.M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jump, D.B.; Tripathy, S.; Depner, C.M. Fatty acid-regulated transcription factors in the liver. Annu. Rev. Nutr. 2013, 33, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Folsom, A.R.; Shahar, E.; Eckfeldt, J.H. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am. J. Clin. Nutr. 1995, 62, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, R.M.; Galli, C.; Ferro-Luzzi, A.; Iacono, J.M. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: A study of normal subjects from Italy, Finland, and the USA. Am. J. Clin. Nutr. 1987, 45, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Kris-Etherton, P.M.; Harris, K.A. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr. Atheroscler. Rep. 2008, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Sundstrom, J.; Vessby, B.; Cederholm, T.; Riserus, U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: A population-based prospective study. Am. J. Clin. Nutr. 2008, 88, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Ohrvall, M.; Vessby, B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alvarenga, J.C.; Ebbesson, S.O.; Ebbesson, L.O.; Tejero, M.E.; Voruganti, V.S.; Comuzzie, A.G. Polyunsaturated fatty acids effect on serum triglycerides concentration in the presence of metabolic syndrome components. The Alaska-Siberia Project. Metabolism 2010, 59, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatri, M.; Moon, Y.P.; Scarmeas, N.; Gu, Y.; Gardener, H.; Cheung, K.; Wright, C.B.; Sacco, R.L.; Nickolas, T.L.; Elkind, M.S. The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin. J. Am. Soc. Nephrol. 2014, 9, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Rosner, B.A.; Sampson, L.A.; Hennekens, C.H. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 1993, 341, 581–585. [Google Scholar] [CrossRef]
- Grundy, S.M.; Becker, D.; Clark, L.T.; Cooper, R.S.; Denke, M.A.; Howard, J.; Hunninghake, D.B.; Illingworth, D.R.; Luepker, R.V.; McBride, P.; et al. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2889–2934. [Google Scholar] [CrossRef] [PubMed]
- The Korean Nutrition Society. Dietary Reference Intakes for Koreans; Ministry of Health and Welfare: Seoul, Korea, 2015. [Google Scholar]
- Kim, C.J.; Kim, J.; Kim, K.I.; Kim, D.; Kim, M.A.; Kim, S.H.; Kim, S.R.; Kim, Y.; Kim, Y.J.; Kim, E.M.; et al. 2015 Korean Guidelines for the Management of Dyslipidemia: Executive Summary (English Translation). Korean Circ. J. 2016, 46, 275–306. [Google Scholar]
- Volek, J.S.; Fernandez, M.L.; Feinman, R.D.; Phinney, S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008, 47, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Nunez, B.; Dijck-Brouwer, D.A.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Na, G.Y.; Yoon, S.R.; An, J.; Yeo, R.; Song, J.; Jo, M.; Han, S.; Kim, O.Y. The relationship between circulating neutrophil gelatinase associated lipocalin and early alteration of metabolic parameters is associated with dietary saturated fat intake in non-diabetic Korean women. Endocr. J. 2017, 64, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Hall, W.L.; Maniou, Z.; Lewis, F.; Seed, P.T.; Chowienczyk, P.J. Effect of low doses of long-chain n-3 PUFAs on endothelial function and arterial stiffness: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kwon, N.; Lee, M.H.; Ko, Y.G.; Lee, J.H.; Kim, O.Y. Association of serum phospholipid PUFAs with cardiometabolic risk: Beneficial effect of DHA on the suppression of vascular proliferation/inflammation. Clin. Biochem. 2014, 47, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.; Kok, F.J. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Zarate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Perez, J.A.; Rodriguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Theobald, H.E.; Goodall, A.H.; Sattar, N.; Talbot, D.C.; Chowienczyk, P.J.; Sanders, T.A. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J. Nutr. 2007, 137, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Nettleton, J.A.; Herrington, D.M.; Johnson, W.C.; Tsai, M.Y.; Siscovick, D. Relation of omega-3 fatty acid and dietary fish intake with brachial artery flow-mediated vasodilation in the Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2010, 92, 1204–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egert, S.; Rassoul, F.; Boesch-Saadatmandi, C.; Richter, V.; Rimbach, G.; Erbersdobler, H.F.; Somoza, V.; Wahrburg, U. Effects of controlled diets enriched with alpha-linolenic acid, eicosapentaenoic acid or docosahexaenoic acid on soluble adhesion molecules and endothelin-1 concentrations in healthy volunteers. Curr. Top. Nutraceutical Res. 2007, 5, 189–195. [Google Scholar]
- Gerda, K.; Pot, I.A.B.; Anke, E.; Ger, T.; Rijkers, E.K.; Anouk, G. No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: A randomized controlled trial in healthy, middle-aged individuals. Eur. J. Clin. Nutr. 2009, 63, 1353–1359. [Google Scholar]
- Schwingshackl, L.; Strasser, B. High-MUFA diets reduce fasting glucose in patients with type 2 diabetes. Ann. Nutr. Metab. 2012, 60, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Strasser, B.; Hoffmann, G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: A systematic review and meta-analysis. Ann. Nutr. Metab. 2011, 58, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Oomen, C.M.; Ocke, M.C.; Feskens, E.J.; van Erp-Baart, M.A.; Kok, F.J.; Kromhout, D. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: A prospective population-based study. Lancet 2001, 357, 746–751. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, C.; Bordiu, E.; Bagazgoitia, J.; Calle-Pascual, A.L. Polyunsaturated fatty acid consumption may play a role in the onset and regression of microalbuminuria in well-controlled type 1 and type 2 diabetic people: A 7-year, prospective, population-based, observational multicenter study. Diabetes Care 2004, 27, 1454–1457. [Google Scholar] [PubMed]
- Dos Santos, A.L.T.; Duarte, C.K.; Santos, M.; Zoldan, M.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J.; Lichtenstein, A.H.; Zelmanovitz, T. Low linolenic and linoleic acid consumption are associated with chronic kidney disease in patients with type 2 diabetes. PLoS ONE 2018, 13, e0195249. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Harris, D.C.; Flood, V.M.; Burlutsky, G.; Mitchell, P. Consumption of long-chain n-3 PUFA, alpha-linolenic acid and fish is associated with the prevalence of chronic kidney disease. Br. J. Nutr. 2011, 105, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hu, F.B.; Curhan, G.C. Associations of diet with albuminuria and kidney function decline. Clin. J. Am. Soc. Nephrol. 2010, 5, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Judd, S.; Le, A.; Ard, J.; Newsome, B.B.; Howard, G.; Warnock, D.G.; McClellan, W. Associations of dietary fat with albuminuria and kidney dysfunction. Am. J. Clin. Nutr. 2010, 92, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, M.D.; Dwyer, T. Microalbuminuria is positively associated with usual dietary saturated fat intake and negatively associated with usual dietary protein intake in people with insulin-dependent diabetes mellitus. Am. J. Clin. Nutr. 1998, 67, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kang, K.W.; Kim, J.G.; Lee, S.J. Concurrent renal dysfunction with ischemic heart disease is an important determinant for cardiac and cerebrovascular mortality in patients on chronic digoxin therapy for atrial fibrillation. Kidney Res. Clin. Pract. 2018, 37, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Gabbai, F.B.; Rahman, M.; Hu, B.; Appel, L.J.; Charleston, J.; Contreras, G.; Faulkner, M.L.; Hiremath, L.; Jamerson, K.A.; Lea, J.P.; et al. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M. Dyslipidemia and progression of kidney disease: Role of lipid-lowering drugs. Clin. Exp. Nephrol. 2014, 18, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Hemmelgarn, B.R.; Manns, B.J.; Lloyd, A.; James, M.T.; Klarenbach, S.; Quinn, R.R.; Wiebe, N.; Tonelli, M. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010, 303, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Heerspink, H.L.; Chalmers, J.; Woodward, M.; Jun, M.; Li, Q.; MacMahon, S.; Cooper, M.E.; Hamet, P.; Marre, M.; et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013, 83, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Steffen, L.M.; Palmas, W.; Burke, G.L.; Jacobs, D.R., Jr. Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2008, 87, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; An, W.S.; Park, Y. Erythrocyte n-3 polyunsaturated fatty acids and the risk of type 2 diabetes in Koreans: A case-control study. Ann. Nutr. Metab. 2013, 63, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Leeson, C.P.; Mann, A.; Kattenhorn, M.; Deanfield, J.E.; Lucas, A.; Muller, D.P. Relationship between circulating n-3 fatty acid concentrations and endothelial function in early adulthood. Eur. Heart. J. 2002, 23, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, O.Y.; Lim, H.H.; Lee, M.J.; Kim, J.Y.; Lee, J.H. Association of fatty acid composition in serum phospholipids with metabolic syndrome and arterial stiffness. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, O.Y.; Cho, Y.; Chung, J.H.; Jung, Y.S.; Hwang, G.S.; Shin, M.J. Plasma phospholipid fatty acid composition in ischemic stroke: Importance of docosahexaenoic acid in the risk for intracranial atherosclerotic stenosis. Atherosclerosis 2012, 225, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Sands, S.A.; Windsor, S.L.; Ali, H.A.; Stevens, T.L.; Magalski, A.; Porter, C.B.; Borkon, A.M. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: Correlation with erythrocytes and response to supplementation. Circulation 2004, 110, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.I.; Gurzell, E.A.; Davidson, E.A.; Harris, W.S. Red blood cell PUFAs reflect the phospholipid PUFA composition of major organs. Prostaglandins Leukot. Essent. Fatty Acids. 2016, 112, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Iggman, D.; Arnlov, J.; Cederholm, T.; Riserus, U. Association of Adipose Tissue Fatty Acids with Cardiovascular and All-Cause Mortality in Elderly Men. JAMA Cardiol. 2016, 1, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Tsuda, S.; Minatogawa, Y.; Iwahashi, H.; Kido, R.; Masuyama, Y. Decreased membrane fluidity of erythrocytes and cultured vascular smooth muscle cells in spontaneously hypertensive rats: An electron spin resonance study. Clin. Sci. 1988, 75, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Diep, Q.N.; Touyz, R.M.; Schiffrin, E.L. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension 2000, 36, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, E.A.; Banerjee, T.; Calder, P.C. The influence of different combinations of gamma-linolenic, stearidonic and eicosapentaenoic acids on the fatty acid composition of blood lipids and mononuclear cells in human volunteers. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Hiramatsu, M.; Hamaue, N.; Minami, M.; Hirafuji, M. Docosahexaenoic acid enhances cyclooxygenase-2 induction by facilitating p44/42, but not p38, mitogen-activated protein kinase activation in rat vascular smooth muscle cells. J. Pharmacol. Sci. 2005, 99, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr. Opin. Lipidol. 2003, 14, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Baek, S.H.; Kim, J.Y.; Lee, J.H.; Kim, O.Y. Serum phospholipid monounsaturated fatty acid composition and Delta-9-desaturase activity are associated with early alteration of fasting glycemic status. Nutr. Res. 2014, 34, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004, 43, 91–104. [Google Scholar] [CrossRef]
- Yao-Borengasser, A.; Rassouli, N.; Varma, V.; Bodles, A.M.; Rasouli, N.; Unal, R.; Phanavanh, B.; Ranganathan, G.; McGehee, R.E., Jr.; Kern, P.A. Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-gamma responsiveness. J. Clin. Endocrinol. Metab. 2008, 93, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Schaffer, J.E. Mechanisms of lipoapoptosis: Implications for human heart disease. Trends Cardiovasc. Med. 2002, 12, 134–138. [Google Scholar] [CrossRef]
- Coll, T.; Eyre, E.; Rodriguez-Calvo, R.; Palomer, X.; Sanchez, R.M.; Merlos, M.; Laguna, J.C.; Vazquez-Carrera, M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 2008, 283, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B. Dietary fat and insulin action in humans. Br. J. Nutr. 2000, 83, 91. [Google Scholar] [CrossRef]
- Ortinau, L.C.; Pickering, R.T.; Nickelson, K.J.; Stromsdorfer, K.L.; Naik, C.Y.; Haynes, R.A.; Bauman, D.E.; Rector, R.S.; Fritsche, K.L.; Perfield, J.W., 2nd. Sterculic Oil, a Natural SCD1 Inhibitor, Improves Glucose Tolerance in Obese ob/ob Mice. ISRN Endocrinol. 2012, 2012, 947323. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B.; Mozaffarian, D.; Sotoodehnia, N.; Rea, T.D.; Kuller, L.H.; Tracy, R.P.; Siscovick, D.S. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: The cardiovascular health study. Circulation 2006, 114, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B.; Raghunathan, T.E.; Pearce, R.M.; Weinmann, S.; Knopp, R.H.; Copass, M.K.; Cobb, L.A.; Siscovick, D.S. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation 2002, 105, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Miller, E.R., 3rd; Ruggiero, C.; Cherubini, A.; Guralnik, J.M.; Ferrucci, L. Plasma polyunsaturated fatty acids and the decline of renal function. Clin. Chem. 2008, 54, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., 3rd; Juraschek, S.P.; Anderson, C.A.; Guallar, E.; Henoch-Ryugo, K.; Charleston, J.; Turban, S.; Bennett, M.R.; Appel, L.J. The effects of n-3 long-chain polyunsaturated fatty acid supplementation on biomarkers of kidney injury in adults with diabetes: Results of the GO-FISH trial. Diabetes Care 2013, 36, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Eide, I.A.; Jenssen, T.; Hartmann, A.; Diep, L.M.; Dahle, D.O.; Reisaeter, A.V.; Bjerve, K.S.; Christensen, J.H.; Schmidt, E.B.; Svensson, M. The association between marine n-3 polyunsaturated fatty acid levels and survival after renal transplantation. Clin. J. Am. Soc. Nephrol. 2015, 10, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.S.; Waikar, S.S.; Ferguson, M.A.; Collings, F.B.; Sunderland, K.; Gioules, C.; Bradwin, G.; Matsouaka, R.; Betensky, R.A.; Curhan, G.C.; et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 2008, 1, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Bonventre, J.V. Biomarkers for the diagnosis of acute kidney injury. Curr. Opin. Nephrol. Hypertens 2007, 16, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Eide, I.A.; Dorje, C.; Svensson, M.; Jenssen, T.; Hammarstrom, C.; Scott, H.; Bjerve, K.S.; Christensen, J.H.; Schmidt, E.B.; Hartmann, A.; et al. Development of Kidney Transplant Fibrosis Is Inversely Associated with Plasma Marine Fatty Acid Level. J. Ren. Nutr. 2018, 28, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Elajami, T.K.; Alfaddagh, A.; Lakshminarayan, D.; Soliman, M.; Chandnani, M.; Welty, F.K. Eicosapentaenoic and Docosahexaenoic Acids Attenuate Progression of Albuminuria in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Chung, S.H.; Park, Y.; Park, M.K.; Son, Y.K.; Kim, S.E.; An, W.S. Effect of Omega-3 Fatty Acid on the Fatty Acid Content of the Erythrocyte Membrane and Proteinuria in Patients with Diabetic Nephropathy. Int. J. Endocrinol. 2015, 2015, 208121. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Carrero, J.J.; Heimburger, O.; Barany, P.; Stenvinkel, P. Plasma fatty acids in chronic kidney disease: Nervonic acid predicts mortality. J. Ren. Nutr. 2012, 22, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Block, R.; Kakinami, L.; Liebman, S.; Shearer, G.C.; Kramer, H.; Tsai, M. Cis-vaccenic acid and the Framingham risk score predict chronic kidney disease: The multi-ethnic study of atherosclerosis (MESA). Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.E.; Maddux, F.W.; Tolkoff-Rubin, N.; Karumanchi, S.A.; Thadhani, R.; Hakim, R.M. Early outcomes among those initiating chronic dialysis in the United States. Clin. J. Am. Soc. Nephrol. 2011, 6, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Terashima, Y.; Itomura, M.; Sawazaki, S.; Inagaki, H.; Kuroda, M.; Tomita, S.; Hirata, H.; Inadera, H.; Hamazaki, T. Docosahexaenoic acid is an independent predictor of all-cause mortality in hemodialysis patients. Am. J. Nephrol. 2011, 33, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Saha, C.; Watkins, B.A. Feasibility study of erythrocyte long-chain omega-3 polyunsaturated fatty acid content and mortality risk in hemodialysis patients. J. Ren. Nutr. 2008, 18, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Block, R.C.; Harris, W.S.; Reid, K.J.; Spertus, J.A. Omega-6 and trans fatty acids in blood cell membranes: A risk factor for acute coronary syndromes? Am. Heart J. 2008, 156, 1117–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paganelli, F.; Maixent, J.M.; Duran, M.J.; Parhizgar, R.; Pieroni, G.; Sennoune, S. Altered erythrocyte n-3 fatty acids in Mediterranean patients with coronary artery disease. Int. J. Cardiol. 2001, 78, 27–32. [Google Scholar] [CrossRef]
- An, W.S.; Kim, S.E.; Kim, K.H.; Lee, S.; Park, Y.; Kim, H.J.; Vaziri, N.D. Comparison of fatty acid contents of erythrocyte membrane in hemodialysis and peritoneal dialysis patients. J. Ren. Nutr. 2009, 19, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.K.; Lee, S.M.; Kim, S.E.; Kim, K.H.; Lee, S.Y.; Bae, H.R.; Han, J.Y.; Park, Y.; An, W.S. Association between vascular calcification scores on plain radiographs and fatty acid contents of erythrocyte membrane in hemodialysis patients. J. Ren. Nutr. 2012, 22, 58–66. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Lee, S.M.; Son, Y.K.; Kim, S.E.; Kim, K.H.; Han, J.Y.; Bae, H.R.; Rha, S.H.; Park, Y. Omega-3 fatty acid supplementation increases 1,25-dihydroxyvitamin D and fetuin-A levels in dialysis patients. Nutr. Res. 2012, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Lee, S.M.; Son, Y.K.; Kim, S.E.; Kim, K.H.; Han, J.Y.; Bae, H.R.; Park, Y. Effect of omega-3 fatty acids on the modification of erythrocyte membrane fatty acid content including oleic acid in peritoneal dialysis patients. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Karpati, I.; Paragh, G.; Buris, L.; Kakuk, G. Relative abundance of some free fatty acids in plasma of uremic patients: Relationship between fatty acids, lipid parameters, and diseases. Nephron 1997, 77, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Yu, Z.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B. Fatty acids and other risk factors for sudden cardiac death in patients starting hemodialysis. Am. J. Nephrol. 2013, 38, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Yu, Z.; Tabbey, R.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B.A. Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 2013, 83, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, E.; Svensson, M.; Strandhave, C.; Schmidt, E.B.; Jorgensen, K.A.; Christensen, J.H. Marine n-3 fatty acids, atrial fibrillation and QT interval in haemodialysis patients. Br. J. Nutr. 2012, 107, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Terashima, Y.; Itomura, M.; Sawazaki, S.; Inagaki, H.; Kuroda, M.; Tomita, S.; Hirata, H.; Hamazaki, T. The relationship between n-3 long-chain polyunsaturated fatty acids and pulse wave velocity in diabetic and non-diabetic patients under long-term hemodialysis. A. horizontal study. Clin. Nephrol. 2009, 71, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Stenvinkel, P.; Qureshi, A.R.; Riserus, U.; Cederholm, T.; Barany, P.; Heimburger, O.; Lindholm, B.; Carrero, J.J. Essential polyunsaturated fatty acids, inflammation and mortality in dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 3615–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Liu, J.; Zhu, L.; Tan, F.; Qin, Y.; Huang, H.; Yu, Y. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis. 2018, 17, 185. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Xu, H.; Huang, X.; Arnlov, J.; Qureshi, A.R.; Cederholm, T.; Sjogren, P.; Lindholm, B.; Riserus, U.; Carrero, J.J. Nonesterified fatty acids and cardiovascular mortality in elderly men with CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Eide, I.A.; Dahle, D.O.; Svensson, M.; Hartmann, A.; Asberg, A.; Bjerve, K.S.; Christensen, J.H.; Schmidt, E.B.; Lauritsen, M.E.; Lund, K.; et al. Plasma levels of marine n-3 fatty acids and cardiovascular risk markers in renal transplant recipients. Eur. J. Clin. Nutr. 2016, 70, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kwon, Y.E.; Park, K.S.; Park, J.T.; Han, S.H.; Kang, S.W.; Kim, H.J.; Yoo, T.H. Changes in geriatric nutritional risk index and risk of major adverse cardiac and cerebrovascular events in incident peritoneal dialysis patients. Kidney Res. Clin. Pract. 2017, 36, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salusky, I.B.; Goodman, W.G. Cardiovascular calcification in end-stage renal disease. Nephrol. Dial. Transplant 2002, 17, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Hsu, J.J.; Lu, J.; Ting, T.C.; Miyazaki, M.; Demer, L.L.; Tintut, Y. Role of cellular cholesterol metabolism in vascular cell calcification. J. Biol. Chem. 2011, 286, 33701–33706. [Google Scholar] [CrossRef] [PubMed]
- Crouthamel, M.H.; Lau, W.L.; Leaf, E.M.; Chavkin, N.W.; Wallingford, M.C.; Peterson, D.F.; Li, X.; Liu, Y.; Chin, M.T.; Levi, M.; et al. Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: Redundant roles for PiT-1 and PiT-2. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quinones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Miyazaki-Anzai, S.; Keenan, A.L.; Okamura, K.; Kendrick, J.; Chonchol, M.; Offermanns, S.; Ntambi, J.M.; Kuro, O.M.; Miyazaki, M. Saturated phosphatidic acids mediate saturated fatty acid-induced vascular calcification and lipotoxicity. J. Clin. Investig. 2015, 125, 4544–4558. [Google Scholar] [CrossRef] [PubMed]
- Ting, T.C.; Miyazaki-Anzai, S.; Masuda, M.; Levi, M.; Demer, L.L.; Tintut, Y.; Miyazaki, M. Increased lipogenesis and stearate accelerate vascular calcification in calcifying vascular cells. J. Biol. Chem. 2011, 286, 23938–23949. [Google Scholar] [CrossRef] [PubMed]
- Kanai, S.; Uto, K.; Honda, K.; Hagiwara, N.; Oda, H. Eicosapentaenoic acid reduces warfarin-induced arterial calcification in rats. Atherosclerosis 2011, 215, 43–51. [Google Scholar] [CrossRef] [PubMed]
| CVD Risk φ | CKD | ||
|---|---|---|---|
| Non-DM | DM | ||
| PUFA | BP ↓, TG ↓ [23,24] | - | Albuminuria ↓ [33,34] GFR decline ↓ [33,34] |
| n-6 PUFA φφ | - | - | Albuminuria ↓ [33,34] GFR decline ↓ [33,34] |
| n-3 PUFA | CVD mortality ↓ [7,21,22,23] | - | - |
| ALA | - | GFR decline ↑ [35] | Albuminuria ↓ [33,34] GFR decline ↓ [33,34] |
| DHA | diastolic BP ↓ [25] | - | - |
| MUFA | FBG ↓, HbA1c ↓ [29,30] | GFR decline ↑ [36] | Albuminuria ↓ [33,34] GFR decline ↓ [33,34] |
| SFA | TC ↑, LDL ↑, LDLR ↓ [17] NGAL ↑ [20] | Albuminuria ↑ [37] GFR decline ↑ [36,37] | Albuminuria ↑ [33,38] |
| TFA | CVD ↑ [12,31] LDL ↑, HDL ↓ [32] | GFR decline ↑ [37] | - |
| CVD | CKD | |||||||
|---|---|---|---|---|---|---|---|---|
| DM or DL or MetS | AS | ICAS or CAD | Mortality | Proteinuria or GFR Decline | VC | CVD | Mortality | |
| PUFA | ↓ [34,72,73] | ↓ [91] | ||||||
| n-6 | ||||||||
| LA | ↓ [6,48] φ | ↓ [49] | ↓ [55] | ↓ [34] | ↓ [96] | |||
| trans-LA | ↑ [70] | ↑ [70,71] | ||||||
| DGLA | ↑ [6,48] | |||||||
| n-3 | ↓ [46] | ↓ [74] | ↓ [92,93] | |||||
| ALA | ↓ [34] | |||||||
| DHA | ↓ [46] | ↓ [48] φ | ↓ [49] | ↓ [95] φ | ↓ [94] | ↓ [83,84,92,93] | ||
| EPA | ↓ [46] | ↓ [111] | ||||||
| n-3 index | ↓ [46] | ↓ [50,53] | ||||||
| MUFA | ↑ [46,63] | ↑ [88] | ↑ [91] | |||||
| OA | ↑ [46,63] | ↑ [53] | ↑ [88] | |||||
| trans-OA | ↓ [70] | |||||||
| Palmitoleic acid | ↑ [46,63] | |||||||
| SFA | ↑ [18,63] | ↑ [110] | ↑ [91] | ↑ [92,93] | ||||
| Δ-6-desaturase | ↑ [46,63] | |||||||
| Δ-9-desaturase | ↑ [46,63] | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, O.Y.; Lee, S.M.; An, W.S. Impact of Blood or Erythrocyte Membrane Fatty Acids for Disease Risk Prediction: Focusing on Cardiovascular Disease and Chronic Kidney Disease. Nutrients 2018, 10, 1454. https://doi.org/10.3390/nu10101454
Kim OY, Lee SM, An WS. Impact of Blood or Erythrocyte Membrane Fatty Acids for Disease Risk Prediction: Focusing on Cardiovascular Disease and Chronic Kidney Disease. Nutrients. 2018; 10(10):1454. https://doi.org/10.3390/nu10101454
Chicago/Turabian StyleKim, Oh Yoen, Su Mi Lee, and Won Suk An. 2018. "Impact of Blood or Erythrocyte Membrane Fatty Acids for Disease Risk Prediction: Focusing on Cardiovascular Disease and Chronic Kidney Disease" Nutrients 10, no. 10: 1454. https://doi.org/10.3390/nu10101454
APA StyleKim, O. Y., Lee, S. M., & An, W. S. (2018). Impact of Blood or Erythrocyte Membrane Fatty Acids for Disease Risk Prediction: Focusing on Cardiovascular Disease and Chronic Kidney Disease. Nutrients, 10(10), 1454. https://doi.org/10.3390/nu10101454






