Post-Exercise Appetite and Ad Libitum Energy Intake in Response to High-Intensity Interval Training versus Moderate- or Vigorous-Intensity Continuous Training among Physically Inactive Middle-Aged Adults
Abstract
:1. Introduction
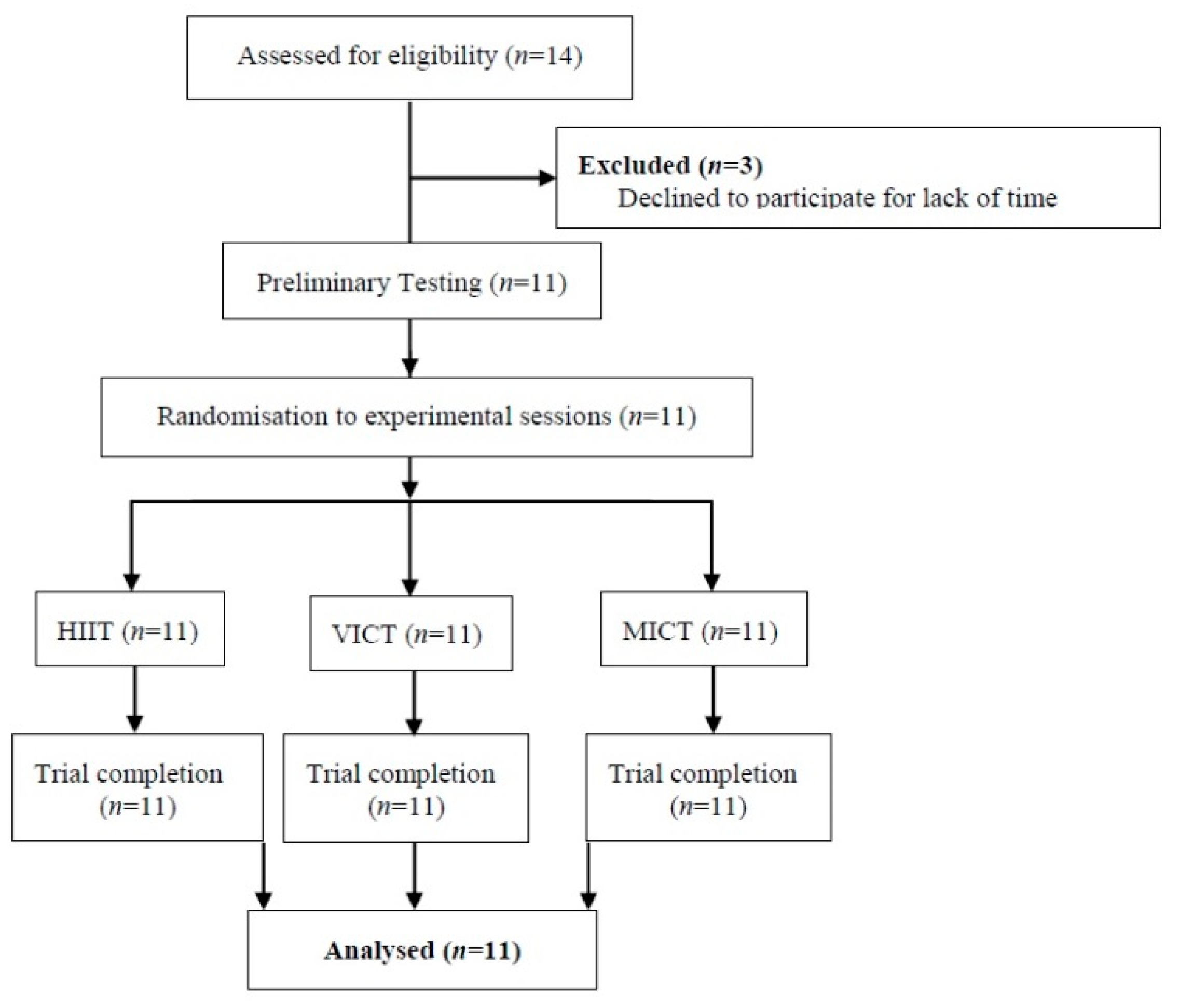
2. Materials and Methods
2.1. Participants
2.2. Preliminary Testing
2.3. Familiarisation Trial
2.4. Experimental Trials
2.5. Post-Exercise Energy Intake
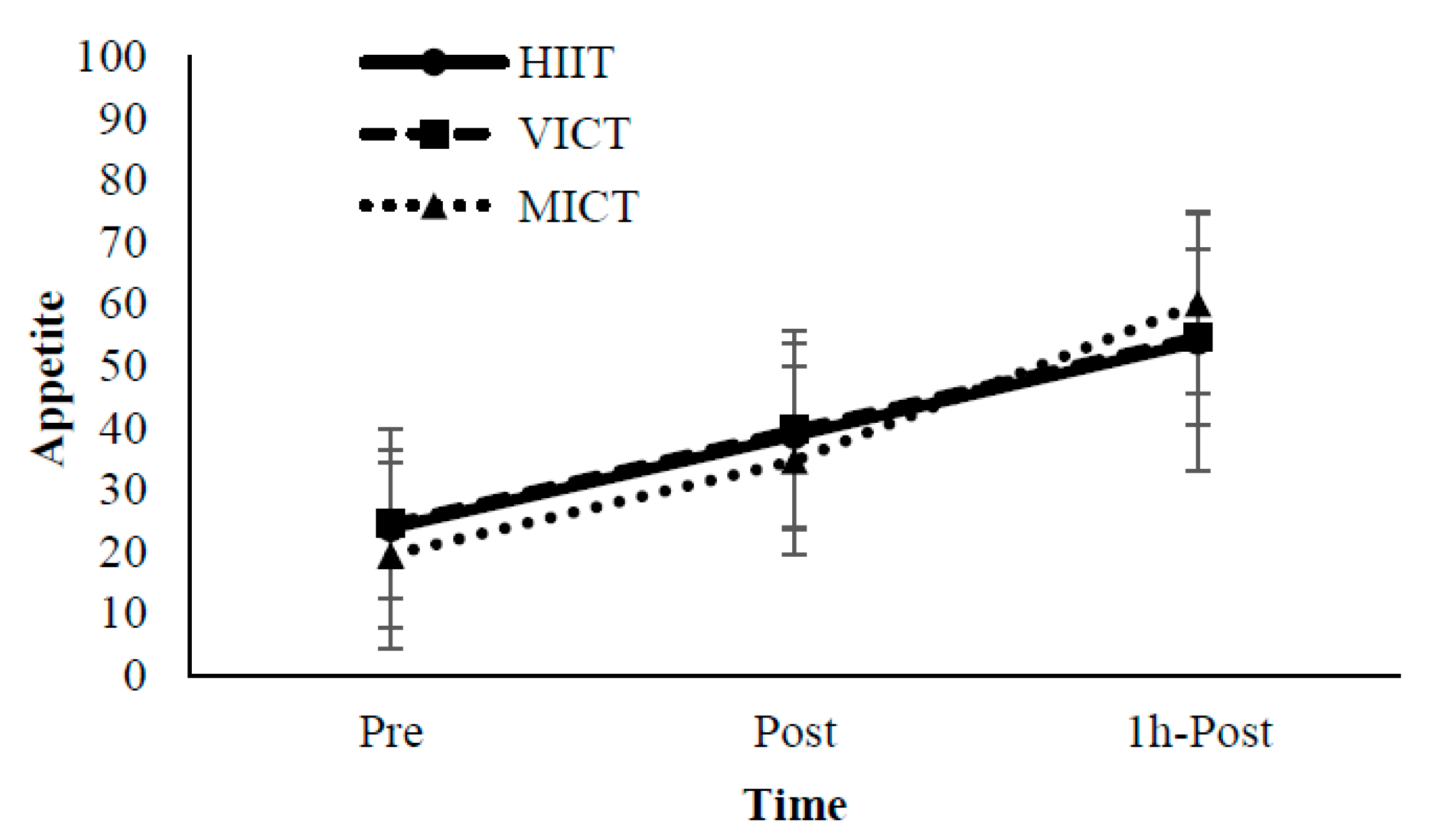
2.6. Perception of Appetite
- (1)
- How strong is your desire to eat? (‘very weak’ to ‘very strong’),
- (2)
- How hungry do you feel? (‘not hungry’ to ‘as hungry as I’ve ever felt’),
- (3)
- How full do you feel? (‘not full at all’ to ‘very full’), and
- (4)
- How much food do you think you could eat? (‘nothing at all’ to ‘a large amount’).
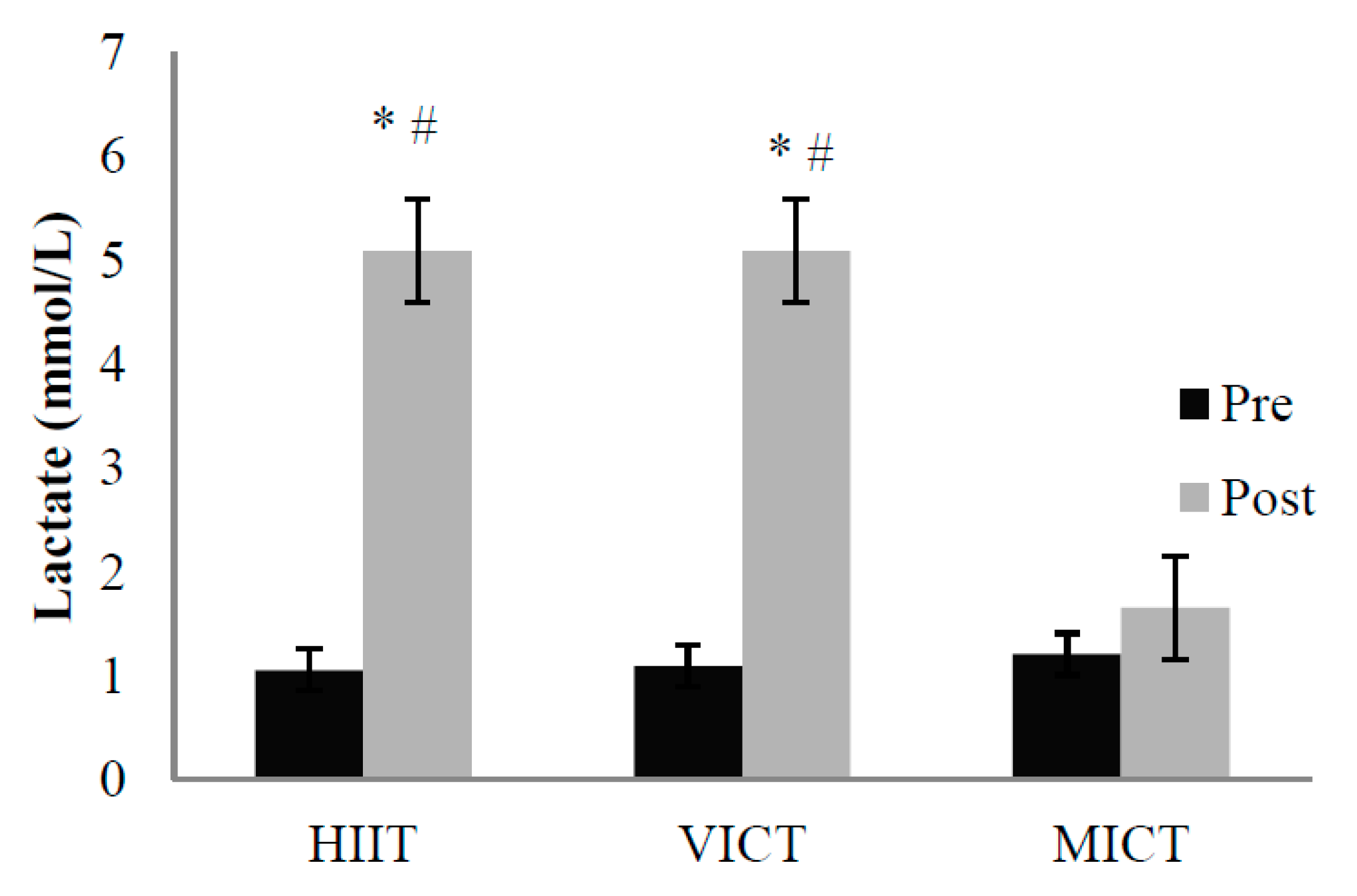
2.7. Blood Lactate
2.8. Dietary and Exercise Training Control
2.9. Statistical Analysis
3. Results
3.1. Post-Exercise Energy Intake
3.2. Perception of Appetite
3.3. Energy Expenditure and Blood Lactate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization Global Action Plan for the Prevention and Control of Ncds 2013–2020; WHO: Geneva, Switzerland, 2013; p. 55. [Google Scholar]
- Catenacci, V.A.; Wyatt, H.R. The role of physical activity in producing and maintaining weight loss. Nat. Clin. Pract. Endocrinol. Met. 2007, 3, 518–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, M.M.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 63, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Kayser, B.; Saris, W.H.M.; Pichard, C. Effects of physical activity on food intake. Clin. Nutr. 2005, 24, 885–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trost, S.G.; Owen, N.; Bauman, A.E.; Sallis, J.F.; Brown, W. Correlates of adults’ participation in physical activity: Review and update. Med. Sci. Sports Exerc. 2002, 34, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, M.W.; Jung, M.E.; Little, J.P. High-intensity interval training a review of physiological and psychological responses. ACSMS Health Fit. J. 2014, 18, 11–16. [Google Scholar]
- Thompson, W.R. Worldwide survey of fitness trends for 2018. ACSMS Health Fit. J. 2017, 21, 10–19. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Taylor, K.L.; Batterham, A.M.; Hopkins, W.G. Effects of low-volume high-intensity interval training (hit) on fitness in adults: A meta-analysis of controlled and non-controlled trials. Sports Med. 2014, 44, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Boutcher, S.H. High-intensity intermittent exercise and fat loss. J. Obes. 2011, 2011, 868305. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. Moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Zelt, J.G.E.; Castellani, L.N.; Little, J.P.; Jung, M.E.; Wright, D.C.; Tschakovsky, M.E.; Gurd, B.J. Changes in mechanisms proposed to mediate fat loss following an acute bout of high-intensity interval and endurance exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, S.J.; Ray, H.; Beale, L.; Hagger, M.S. Why sprint interval training is inappropriate for a largely sedentary population. Front. Psychol. 2014, 5, 1505. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- ACSM. Acsm’s Guidelines for Exercise Testing and Prescription, 10th ed; Wolters Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Martins, C.; Aschehoug, I.; Ludviksen, M.; Holst, J.; Finlayson, G.; Wisloff, U.; Morgan, L.; King, N.; Kulseng, B. High-intensity interval training, appetite, and reward value of food in the obese. Med. Sci. Sports Exerc. 2017, 49, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Karra, E.; Batterham, R.L.; Stensel, D.J. Appetite, energy intake, and pyy3-36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Appl. Physiol. Nutr. Metab.-Physiol. Appl. Nutr. Metab. 2013, 38, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.H.; Wong, S.H.S.; Chen, S.H.; Poon, T.C. Carbohydrate electrolyte solutions enhance endurance capacity in active females. Nutrients 2015, 7, 3739–3750. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.; Kilpatrick, M.W.; Salomon, K.; Jung, M.E.; Little, J.P. Affective and enjoyment responses to high-intensity interval training in overweight-to-obese and insufficiently active adults. J. Sport Exerc. Psy. 2015, 37, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, M.W.; Martinez, N.; Little, J.P.; Jung, M.E.; Jones, A.M.; Price, N.W.; Lende, D.H. Impact of high-intensity interval duration on perceived exertion. Med. Sci. Sports Exerc. 2015, 47, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Ishikawa, C.; Suzuki, M.; Harano, Y.; Aoyama, A.; Fujimoto, A.; Onisi, K. Energy expenditure rate calculated from daily exercise and indirect calorimetry. Adv. Diabetes Mellit. Southeast Asia 1997, 1141, 265–268. [Google Scholar]
- Thum, J.S.; Parsons, G.; Whittle, T.; Astorino, T.A. High-intensity interval training elicits higher enjoyment than moderate intensity continuous exercise. PLoS ONE 2017, 12, e0166299. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Bourne, J.E.; Little, J.P. Where does hit fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS ONE 2014, 9, e114541. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.L.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E539–E552. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keating, S.E.; Johnson, N.A.; Mielke, G.I.; Coombes, J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes. Rev. 2017, 18, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Scheurink, A.J.W.; Ammar, A.A.; Benthem, B.; van Dijk, G.; Sodersten, P.A.T. Exercise and the regulation of energy intake. Int. J. Obes. 1999, 23, S1. [Google Scholar] [CrossRef]
- Crisp, N.A.; Fournier, P.A.; Licari, M.K.; Braham, R.; Guelfi, K.J. Adding sprints to continuous exercise at the intensity that maximises fat oxidation: Implications for acute energy balance and enjoyment. Metabolism 2012, 61, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Stensvold, D.; Finlayson, G.; Holst, J.; Wisloff, U.; Kulseng, B.; Morgan, L.; King, N.A. Effect of moderate- and high-intensity acute exercise on appetite in obese individuals. Med. Sci. Sports Exerc. 2015, 47, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.P.; Smith, L.R.; Chrismas, B.C.; Taylor, L.; Stensel, D.J.; Deighton, K.; Douglas, J.A.; Kerr, C.J. Appetite and gut hormone responses to moderate-intensity continuous exercise versus high-intensity interval exercise, in normoxic and hypoxic conditions. Appetite 2015, 89, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, V.A.F.; Souza, D.C.; Santos, V.O.A.; Medeiros, I.F.; Browne, R.A.V.; Nascimento, P.R.P.; Marinho, C.S.R.; Serquiz, A.C.; Costa, E.C.; Fayh, A.P.T. Acute effects of high-intensity interval and moderate-intensity continuous exercise on glp-1, appetite and energy intake in obese men: A crossover trial. Nutrients 2018, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Biddle, S.J.H.; Batterham, A.M. High-intensity interval exercise training for public health: A big hit or shall we hit it on the head? Int. J. Behav. Nutr. Phys. 2015, 12, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, C.K.L.; Chari, M.; Wang, P.Y.T.; Lam, T.K.T. Central lactate metabolism regulates food intake. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E491–E496. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.M.; Jauch-Chara, K.; Born, J.; Schultes, B.; Hallschmid, M. Lactate infusion during euglycemia but not during hypoglycemia reduces subsequent food intake. Exp. Clin. Endocrinol. Diabetes 2007, 115, P01_142. [Google Scholar] [CrossRef]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Alajmi, N.; Deighton, K.; King, J.A.; Reischak-Oliveira, A.; Wasse, L.K.; Jones, J.; Batterham, R.L.; Stensel, D.J. Appetite and energy intake responses to acute energy deficits in females versus males. Med. Sci. Sports Exerc. 2016, 48, 412–420. [Google Scholar] [CrossRef] [PubMed]

| Variable | (n = 11) |
|---|---|
| Age (years) | 45.7 ± 7.4 |
| Height (cm) | 168.4 ± 6.8 |
| Weight (kg) | 66.6 ± 8.3 |
| Body fat (%) | 20.1 ± 3.5 |
| BMI (kg m−2) | 23.5 ± 2.1 |
| VO2max (mL min−1 kg−1) | 38.6 ± 5.4 |
| HIIT | VICT | MICT | |
|---|---|---|---|
| Energy Intake (kcal) | 645 ± 262.9 | 623.1 ± 249.0 | 614.7 ± 271.2 |
| Carbohydrates (g) | 102.6 ± 41.8 | 96.9 ± 40.2 | 92.8 ± 40.3 |
| Protein (g) | 24.8 ± 9.9 | 26.3 ± 12.8 | 23.0 ± 10.2 |
| Fat (g) | 17.5 ± 6.8 | 17.2 ± 7.6 | 17.2 ± 8.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poon, E.T.-C.; Sun, F.-H.; Chung, A.P.-W.; Wong, S.H.-S. Post-Exercise Appetite and Ad Libitum Energy Intake in Response to High-Intensity Interval Training versus Moderate- or Vigorous-Intensity Continuous Training among Physically Inactive Middle-Aged Adults. Nutrients 2018, 10, 1408. https://doi.org/10.3390/nu10101408
Poon ET-C, Sun F-H, Chung AP-W, Wong SH-S. Post-Exercise Appetite and Ad Libitum Energy Intake in Response to High-Intensity Interval Training versus Moderate- or Vigorous-Intensity Continuous Training among Physically Inactive Middle-Aged Adults. Nutrients. 2018; 10(10):1408. https://doi.org/10.3390/nu10101408
Chicago/Turabian StylePoon, Eric Tsz-Chun, Feng-Hua Sun, Anthony Pui-Wan Chung, and Stephen Heung-Sang Wong. 2018. "Post-Exercise Appetite and Ad Libitum Energy Intake in Response to High-Intensity Interval Training versus Moderate- or Vigorous-Intensity Continuous Training among Physically Inactive Middle-Aged Adults" Nutrients 10, no. 10: 1408. https://doi.org/10.3390/nu10101408





