Abiotic Controls on Macroscale Variations of Humid Tropical Forest Height
Abstract
:1. Introduction
2. Materials and Methods
2.1. Remote Sensing Data
2.2. Climate and Soil Data
2.3. Developing of Gridded Data Layers
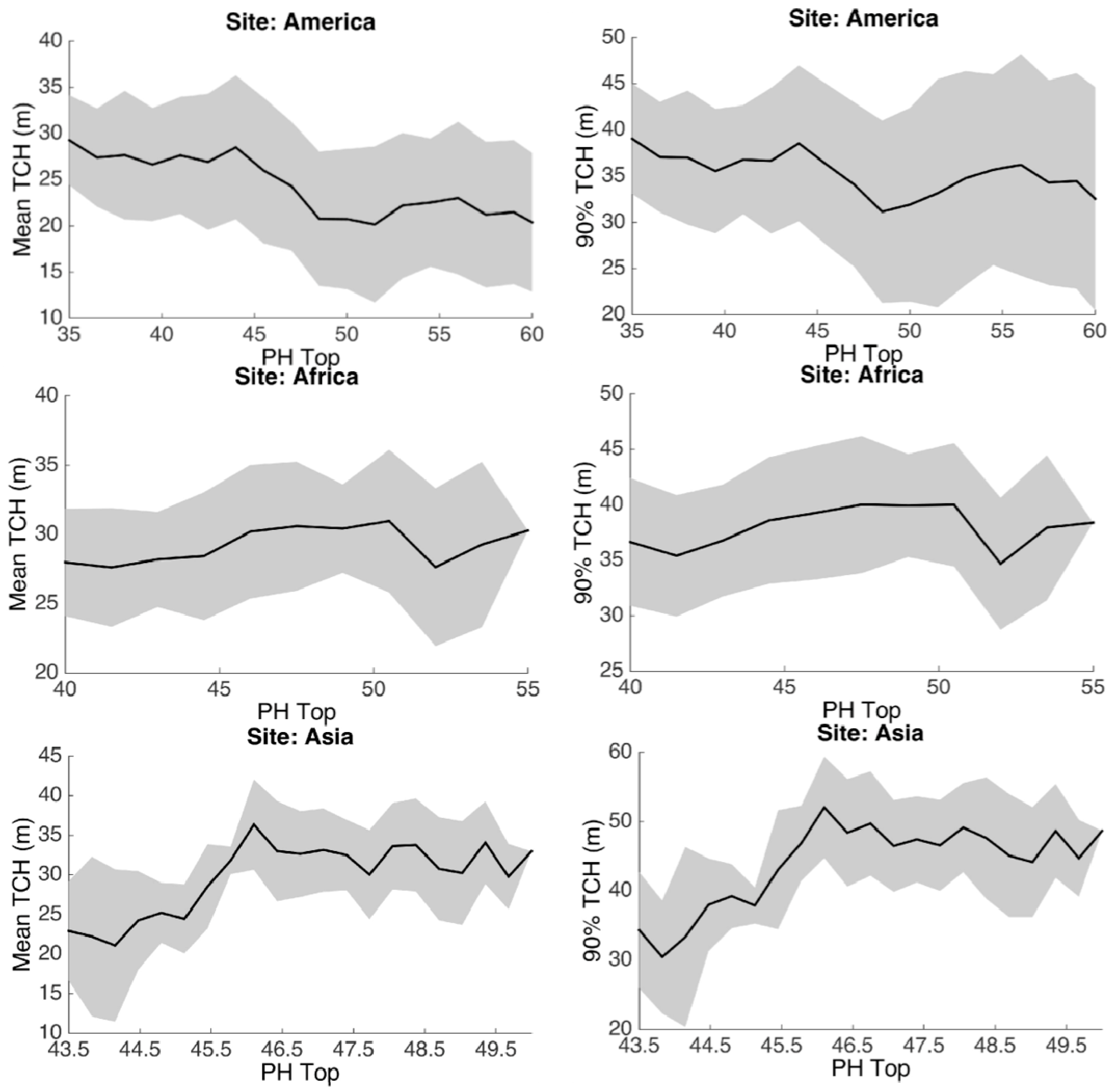
2.4. Spatial Analysis
3. Results
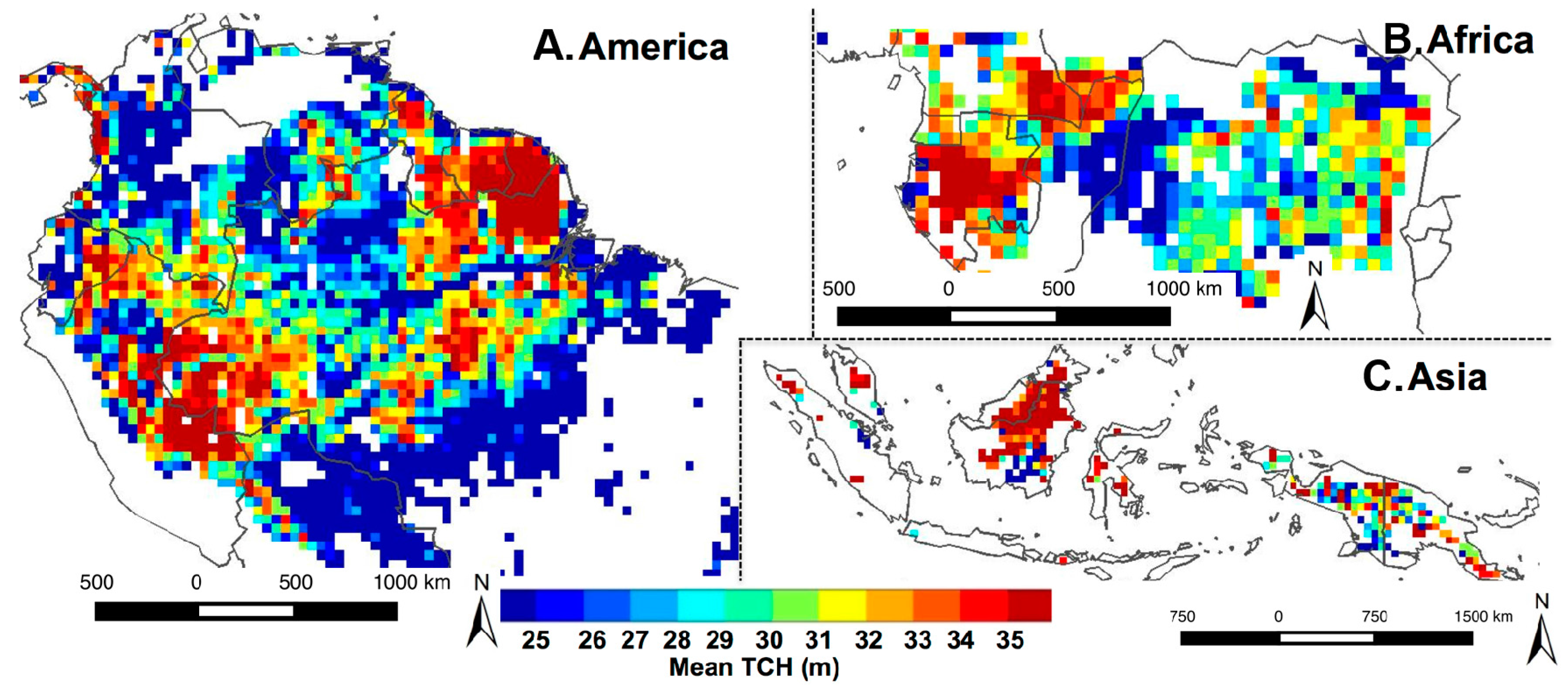
3.1. Spatial Patterns
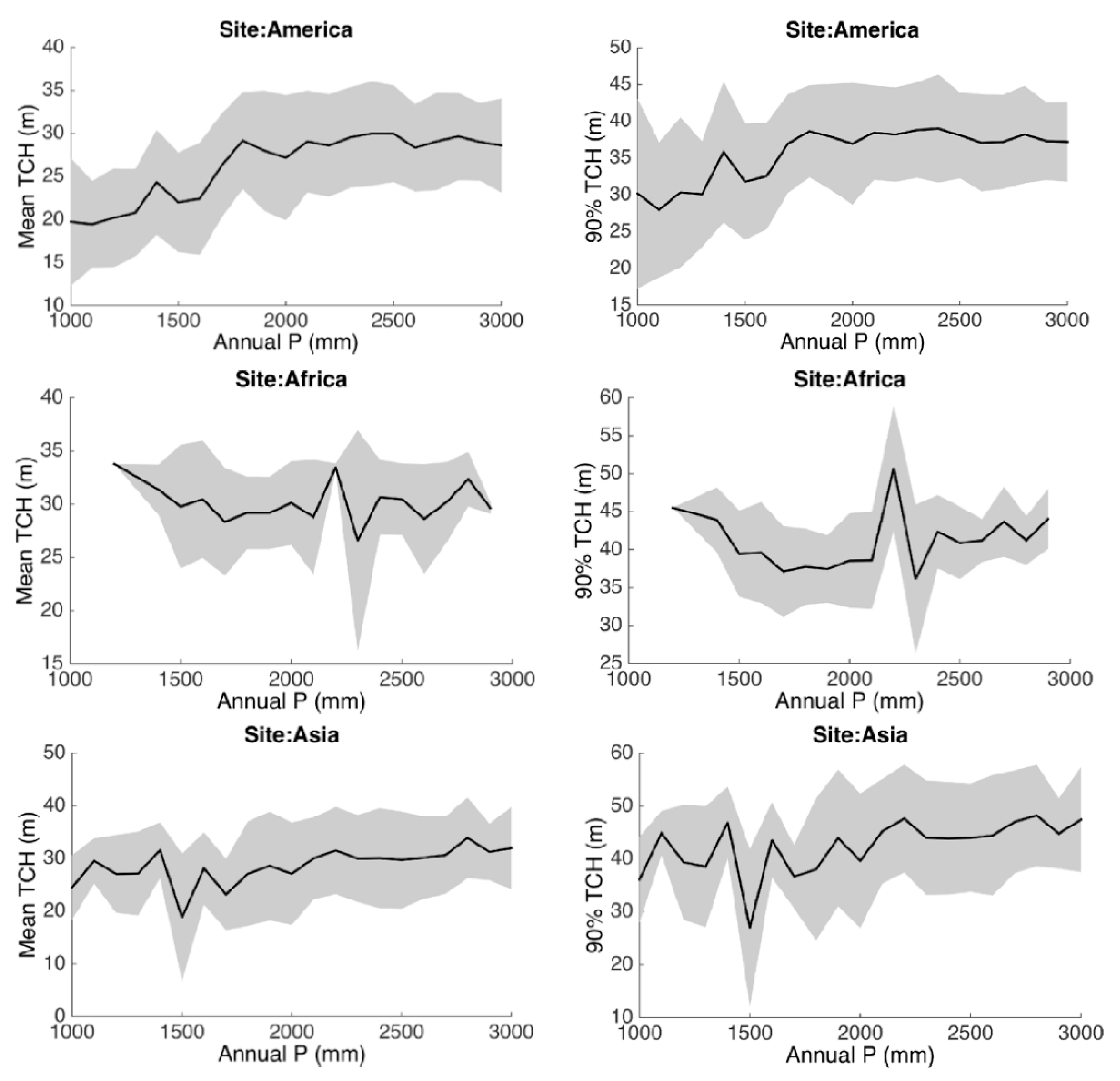
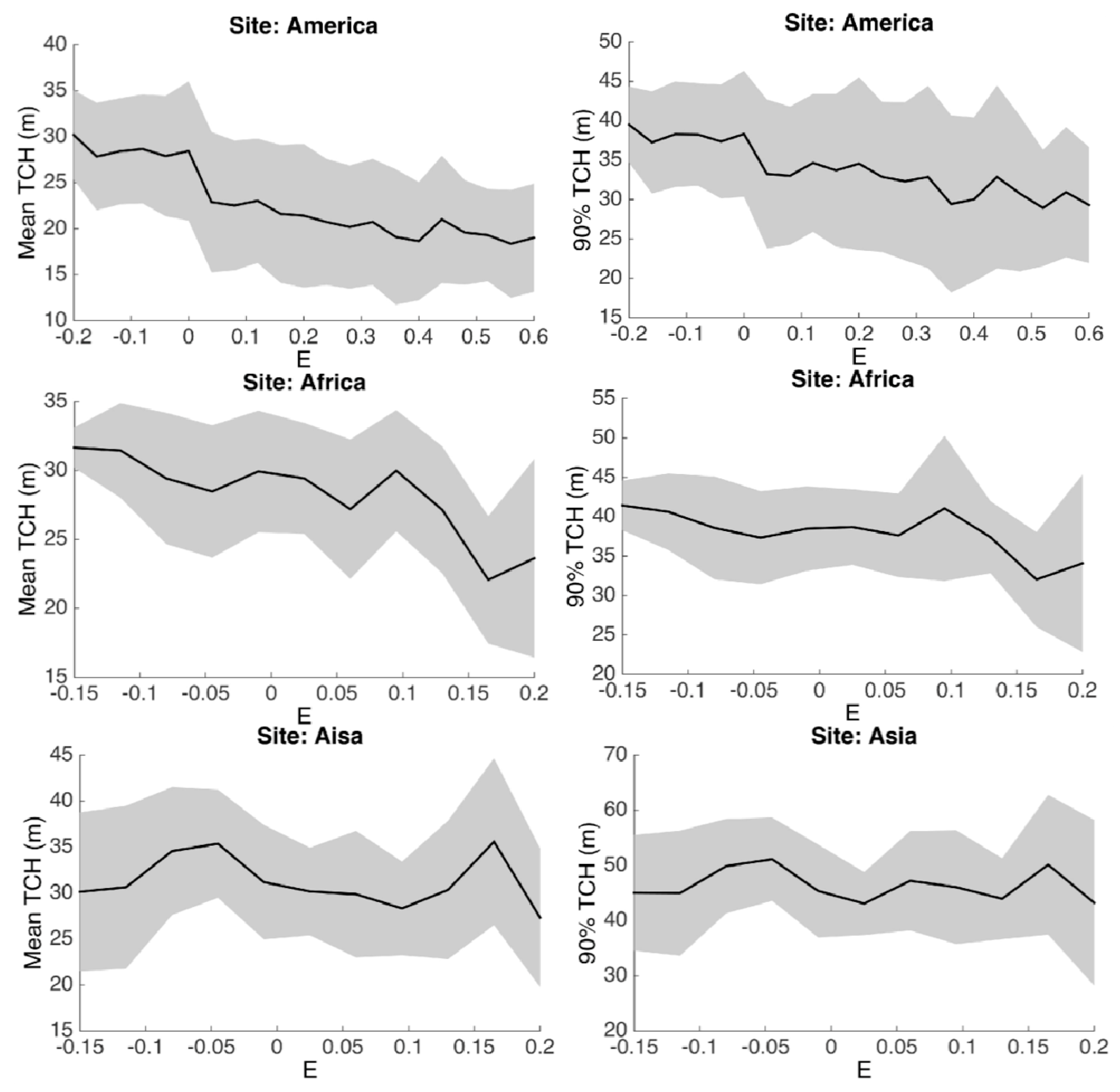
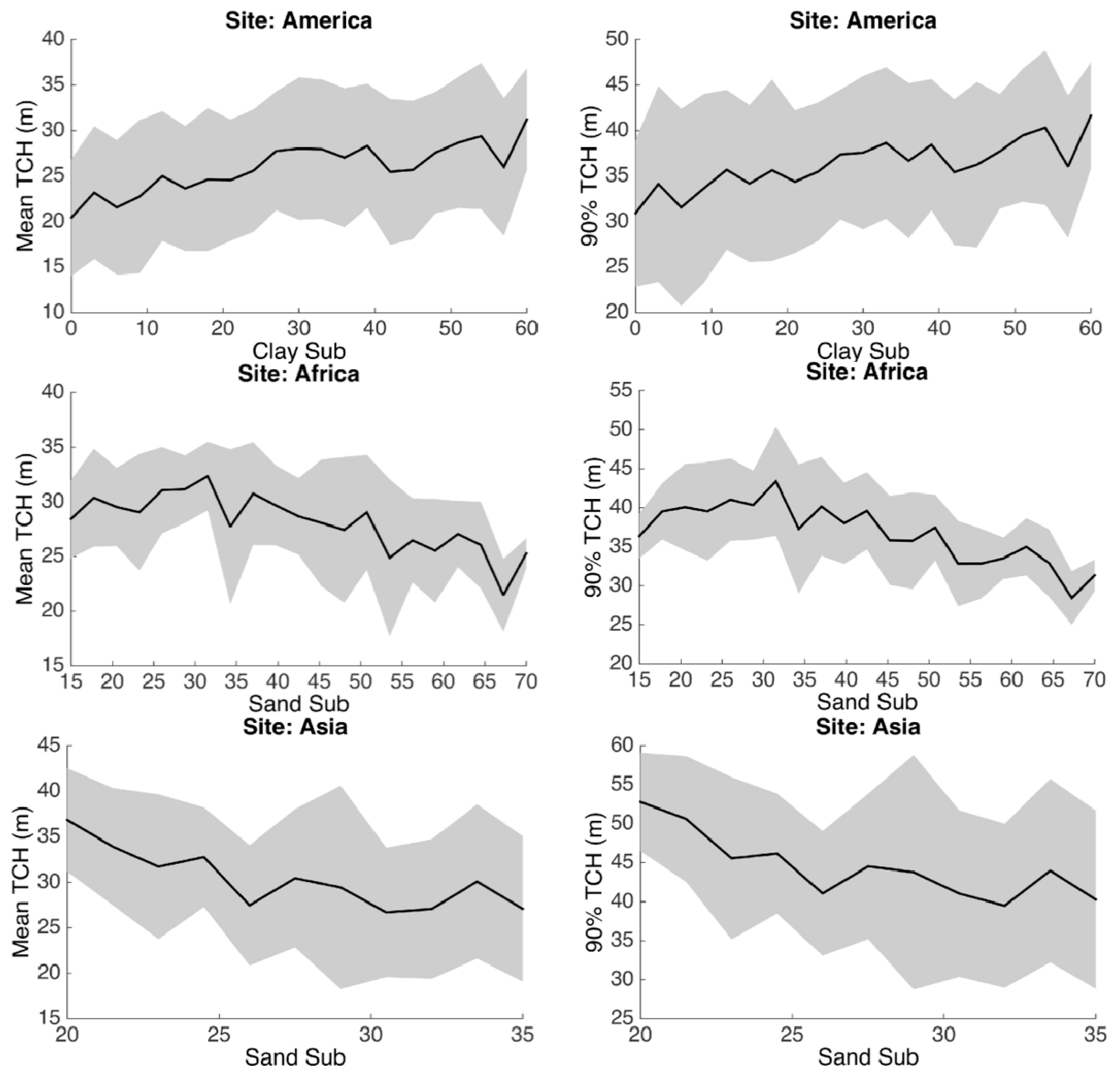
3.2. Statistical Analysis
3.3. Environmental Controls
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.R.; Phillips, O.L.; Malhi, Y.; Almeida, S.; Arroyo, L.; Di Fiore, A.; Erwin, T.; Killeen, T.J.; Laurance, S.G.; Laurance, W.F.; et al. Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob. Chang. Biol. 2004, 10, 545–562. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asner, G.P.; Mascaro, J.; Anderson, C.; Knapp, D.E.; Martin, R.E.; Kennedy-Bowdoin, T.; van Breugel, M.; Davies, S.; Hall, J.S.; Muller-Landau, H.C.; et al. High-fidelity national carbon mapping for resource management and REDD+. Carbon Balance Manag. 2013, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Clark, D.B.; Oberbauer, S.F. Field-quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climatic stress, 1997–2009. J. Geophys. Res. Biogeosci. 2013, 118, 783–794. [Google Scholar] [CrossRef]
- Espírito-Santo, F.D.B.; Gloor, M.; Keller, M.; Malhi, Y.; Saatchi, S.; Nelson, B.; Junior, R.C.O.; Pereira, C.; Lloyd, J.; Frolking, S.; et al. Size and frequency of natural forest disturbances and the Amazon forest carbon balance. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Doughty, C.E.; Goldsmith, G.R.; Metcalfe, D.B.; Girardin, C.A.J.; Marthews, T.R.; del Aguila-Pasquel, J.; Aragão, L.E.O.C.; Araujo-Murakami, A.; Brando, P.; et al. The linkages between photosynthesis, productivity, growth and biomass in lowland Amazonian forests. Glob. Chang. Biol. 2015, 21, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Marvin, D.C.; Asner, G.P.; Knapp, D.E.; Anderson, C.B.; Martin, R.E.; Sinca, F.; Tupayachi, R. Amazonian landscapes and the bias in field studies of forest structure and biomass. Proc. Natl. Acad. Sci. USA 2014, 111, E5224–E5232. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, S.; Mascaro, J.; Xu, L.; Keller, M.; Yang, Y.; Duffy, P.; Espírito-Santo, F.; Baccini, A.; Chambers, J.; Schimel, D. Seeing the forest beyond the trees. Glob. Ecol. Biogeogr. 2015, 24, 606–610. [Google Scholar] [CrossRef]
- Frolking, S.; Palace, M.W.; Clark, D.B.; Chambers, J.Q.; Shugart, H.H.; Hurtt, G.C. Forest disturbance and recovery: A general review in the context of spaceborne remote sensing of impacts on aboveground biomass and canopy structure. J. Geophys. Res. Biogeosci. 2009, 114, G00E02. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Negron-Juarez, R.I.; Marra, D.M.; Vittorio, A.D.; Tews, J.; Roberts, D.; Ribeiro, G.H.P.M.; Trumbore, S.E.; Higuchi, N. The steady-state mosaic of disturbance and succession across an old-growth Central Amazon forest landscape. Proc. Natl. Acad. Sci. USA 2013, 110, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, S.; Asefi-Najafabady, S.; Malhi, Y.; Aragão, L.E.O.C.; Anderson, L.O.; Myneni, R.B.; Nemani, R. Persistent effects of a severe drought on Amazonian forest canopy. Proc. Natl. Acad. Sci. USA 2013, 110, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Melillo, J.M.; Kicklighter, D.W.; McGuire, A.D.; Helfrich, J.V.K.; Moore, B.; Vörösmarty, C.J. Effect of interannual climate variability on carbon storage in Amazonian ecosystems. Nature 1998, 396, 664–667. [Google Scholar] [CrossRef]
- Malhi, Y.; Roberts, J.T.; Betts, R.A.; Killeen, T.J.; Li, W.; Nobre, C.A. Climate change, deforestation, and the fate of the Amazon. Science 2008, 319, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.A.; Asner, G.P.; Perez, E.; Elespuru, N.; Tuomisto, H.; Ruokolainen, K.; Alonso, A. Use of Landsat and SRTM data to detect broad-scale biodiversity patterns in northwestern Amazonia. Remote Sens. 2012, 4, 2401–2418. [Google Scholar] [CrossRef]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef] [Green Version]
- Webb, C.; Cannon, C.; Davies, S. Ecological organization, biogeography, and the phylogenetic structure of tropical forest tree communities. In Tropical Forest Community Ecology; Carson, W., Schnitzer, S., Eds.; Blackwell: Malden, MA, USA, 2008; pp. 79–97. [Google Scholar]
- Kembel, S.W.; Hubbell, S.P. The phylogenetic structure of a Neotropical forest tree community. Ecology 2006, 87, S86–S99. [Google Scholar] [CrossRef]
- Iida, Y.; Poorter, L.; Sterck, F.; Kassim, A.R.; Potts, M.D.; Kubo, T.; Kohyama, T.S. Linking size-dependent growth and mortality with architectural traits across 145 co-occurring tropical tree species. Ecology 2014, 95, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Feldpausch, T.R.; Lloyd, J.; Lewis, S.L.; Brienen, R.J. W.; Gloor, M.; Monteagudo Mendoza, A.; Lopez-Gonzalez, G.; Banin, L.; Abu Salim, K.; Affum-Baffoe, K.; et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences 2012, 9, 3381–3403. [Google Scholar] [CrossRef] [Green Version]
- Banin, L.; Feldpausch, T.R.; Phillips, O.L.; Baker, T.R.; Lloyd, J.; Affum-Baffoe, K.; Arets, E.J.M.M.; Berry, N.J.; Bradford, M.; Brienen, R.J.W.; et al. What controls tropical forest architecture? Testing environmental, structural and floristic drivers. Glob. Ecol. Biogeogr. 2012, 21, 1179–1190. [Google Scholar] [CrossRef]
- DeWalt, S.J.; Chave, J. Structure and biomass of four lowland Neotropical Forests. Biotropica 2004, 36, 7–19. [Google Scholar] [CrossRef]
- Moser, B.; Fridley, J.D.; Askew, A.P.; Grime, J.P. Simulated migration in a long-term climate change experiment: invasions impeded by dispersal limitation, not biotic resistance. J. Ecol. 2011, 99, 1229–1236. [Google Scholar] [CrossRef]
- Slik, J.W.F.; Paoli, G.; McGuire, K.; Amaral, I.; Barroso, J.; Bastian, M.; Blanc, L.; Bongers, F.; Boundja, P.; Clark, C.; et al. Large trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Glob. Ecol. Biogeogr. 2013, 22, 1261–1271. [Google Scholar] [CrossRef]
- Kohyama, T.; Suzuki, E.; Partomihardjo, T.; Yamada, T.; Kubo, T. Tree species differentiation in growth, recruitment and allometry in relation to maximum height in a Bornean mixed dipterocarp forest. J. Ecol. 2003, 91, 797–806. [Google Scholar] [CrossRef]
- Lefsky, M.A. A global forest canopy height map from the Moderate Resolution Imaging Spectroradiometer and the Geoscience Laser Altimeter System. Geophys. Res. Lett. 2010, 37, L15401. [Google Scholar] [CrossRef]
- Le Toan, T.; Quegan, S.; Davidson, M.W.J.; Balzter, H.; Paillou, P.; Papathanassiou, K.; Plummer, S.; Rocca, F.; Saatchi, S.; Shugart, H.; et al. The BIOMASS mission: Mapping global forest biomass to better understand the terrestrial carbon cycle. Remote Sens. Environ. 2011, 115, 2850–2860. [Google Scholar] [CrossRef]
- Saatchi, S.S.; Marlier, M.; Chazdon, R.L.; Clark, D.B.; Russell, A.E. Impact of spatial variability of tropical forest structure on radar estimation of aboveground biomass. Remote Sens. Environ. 2011, 115, 2836–2849. [Google Scholar] [CrossRef]
- Bontemps, S.; Defourny, P.; Van Bogaert, E.; Arino, O.; Kalogirou, V.; Ramos Perez, J. GlobCover 2009: Products Description and Validation Report; UCLouvain & ESA: Louvain-la-Neuve, Belgium, 2011. [Google Scholar]
- Abshire, J.B.; Sun, X.; Riris, H.; Sirota, J.M.; McGarry, J.F.; Palm, S.; Yi, D.; Liiva, P. Geoscience Laser Altimeter System (GLAS) on the ICESat Mission: On-orbit measurement performance. Geophys. Res. Lett. 2005, 32, L21S02. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Harding, D.J.; Keller, M.; Cohen, W.B.; Carabajal, C.C.; Espirito-Santo, F.D.B.; Hunter, M.O.; de Oliveira, R., Jr. Estimates of forest canopy height and aboveground biomass using ICESat. Geophys. Res. Lett. 2005, 32, L22S02. [Google Scholar] [CrossRef]
- Sun, G.; Ranson, K.J.; Kimes, D.S.; Blair, J.B.; Kovacs, K. Forest vertical structure from GLAS: An evaluation using LVIS and SRTM data. Remote Sens. Environ. 2008, 112, 107–117. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Keller, M.; Pang, Y.; De Camargo, P.B.; Hunter, M.O. Revised method for forest canopy height estimation from Geoscience Laser Altimeter System waveforms. J. Appl. Remote Sens. 2007, 1. [Google Scholar] [CrossRef]
- Lim, K.; Treitz, P.; Wulder, M.; St-Onge, B.; Flood, M. Lidar remote sensing of forest structure. Prog. Phys. Geogr. 2003, 27, 88–106. [Google Scholar] [CrossRef]
- Van Leeuwen, M.; Nieuwenhuis, M. Retrieval of forest structural parameters using Lidar remote sensing. Eur. J. For. Res. 2010, 129, 749–770. [Google Scholar] [CrossRef]
- Rabus, B.; Eineder, M.; Roth, A.; Bamler, R. The shuttle radar topography mission—A new class of digital elevation models acquired by spaceborne radar. ISPRS J. Photogramm. Remote Sens. 2003, 57, 241–262. [Google Scholar] [CrossRef]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The shuttle radar topography mission. Rev. Geophys. 2007, 45, RG2004. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Synes, N.W.; Osborne, P.E. Choice of predictor variables as a source of uncertainty in continental-scale species distribution modelling under climate change. Glob. Ecol. Biogeogr. 2011, 20, 904–914. [Google Scholar] [CrossRef]
- Nachtergaele, F.O.F.; Licona-Manzur, C. The Land Degradation Assessment in Drylands (LADA) Project: reflections on indicators for land degradation assessment. In The Future of Drylands; Lee, C., Schaaf, T., Eds.; Springer Netherlands: Tunis, Tunisia, 2008; pp. 327–348. [Google Scholar]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- FAO/IIASA/ISRIC/ISSCAS/JRC. Harmonized World Soil Database (Version 1.2); FAO: Rome, Italy, 2012. [Google Scholar]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Daniel Kissling, W.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Mauricio Bini, L.; Diniz-Filho, J.A.F.; Rangel, T.F.L.V.B.; Akre, T.S.B.; Albaladejo, R.G.; Albuquerque, F.S.; Aparicio, A.; Araújo, M.B.; Baselga, A.; Beck, J.; et al. Coefficient shifts in geographical ecology: An empirical evaluation of spatial and non-spatial regression. Ecography 2009, 32, 193–204. [Google Scholar] [CrossRef]
- Rangel, T.F.; Diniz-Filho, J.A.F.; Bini, L.M. SAM: A comprehensive application for spatial analysis in macroecology. Ecography 2010, 33, 46–50. [Google Scholar] [CrossRef]
- Matula, D.W.; Sokal, R.R. Properties of Gabriel graphs relevant to geographic variation research and the clustering of points in the plane. Geogr. Anal. 1980, 12, 205–222. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- O’brien, R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Laing, A.; Evans, J.L. Introduction to Tropical Meteorology; University Corporation Atmospheric Research: Boulder, CO, USA, 2011. [Google Scholar]
- Bloom, D.E.; Sachs, J.D.; Collier, P.; Udry, C. Geography, demography, and economic growth in Africa. Brook. Pap. Econ. Act. 1998, 1998, 207–295. [Google Scholar] [CrossRef]
- Malhi, Y.; Saatchi, S.; Girardin, C.; AragãO, L.E.O.C. The production, storage, and flow of Carbon in Amazonian forests. In Amazonia and Global Change; Keller, M., Bustamante, M., Gash, J., Dias, P.S., Eds.; American Geophysical Union: Washington, DC, USA, 2009; pp. 355–372. [Google Scholar]
- Cox, P.M.; Pearson, D.; Booth, B.B.; Friedlingstein, P.; Huntingford, C.; Jones, C.D.; Luke, C.M. Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature 2013, 494, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Huntingford, C.; Zelazowski, P.; Galbraith, D.; Mercado, L.M.; Sitch, S.; Fisher, R.; Lomas, M.; Walker, A.P.; Jones, C.D.; Booth, B.B.B.; et al. Simulated resilience of tropical rainforests to CO2-induced climate change. Nat. Geosci. 2013, 6, 268–273. [Google Scholar] [CrossRef]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, B.M.J.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Enquist, B.J.; West, G.B.; Brown, J.H. Extensions and evaluations of a general quantitative theory of forest structure and dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 7046–7051. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Ruhiyat, D. Soils of Tropical Forest Ecosystems: Characteristics, Ecology and Management; Springer: Berlin, Germany, 1998. [Google Scholar]
- Richter, D.; Babbar, L.I. Soil Diversity in the Tropics; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Slik, J.W.F.; Aiba, S.-I.; Brearley, F.Q.; Cannon, C.H.; Forshed, O.; Kitayama, K.; Nagamasu, H.; Nilus, R.; Payne, J.; Paoli, G.; et al. Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo’s tropical forests. Glob. Ecol. Biogeogr. 2010, 19, 50–60. [Google Scholar] [CrossRef]
- He, L.; Chen, J.M.; Zhang, S.; Gomez, G.; Pan, Y.; McCullough, K.; Birdsey, R.; Masek, J.G. Normalized algorithm for mapping and dating forest disturbances and regrowth for the United States. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 236–245. [Google Scholar] [CrossRef]


| Soil Property | Description | Unit |
|---|---|---|
| CEC_T/CEC_S | Topsoil /Subsoil CEC in the soil | Cmol kg‒1 |
| SLIT_T/SLIT_S | Topsoil/Subsoil Silt Fraction | % |
| OC_T/OC_S | Topsoil Organic Carbon | % weight |
| CLAY_T/CLAY_S | Topsoil/Subsoil Clay Fraction | % |
| PH_T/PH_S | Topsoil/Subsoil PH (H2O) | Unitless |
| SAND_T/SAND_S | Topsoil/Subsoil Sand Fraction | % |
| M Diurnal Range | Mean of monthly (max temp-min temp) | °C × 10 |
| Isothermality | Mean Diurnal Range/Temp Annual Range | Unitless |
| T Annual Range | Max temp of warmest Month-Min temp of coldest month | °C × 10 |
| M T wettest Q | Mean Temperature of Wettest Quarter | °C × 10 |
| Max T warmest m | Min Temperature of Warmest Month | °C × 10 |
| Min T coldest m | Min Temperature of Coldest Month | °C × 10 |
| Annual M T | Annual Mean Temperature | °C × 10 |
| T seasonality | Temperature Seasonality (Coefficient of Variation) | Unitless |
| M T driest Q | Mean Temperature of Driest Quarter | °C × 10 |
| M T warmest Q | Mean Temperature of Warmest Quarter | °C × 10 |
| P seasonality | Precipitation Seasonality (Coefficient of Variation) | Unitless |
| P driest Q | Precipitation of Driest Quarter | mm |
| P warmest Q | Precipitation of Warmest Quarter | mm |
| P coldest Q | Precipitation of Coldest Quarter | mm |
| Annual P | Annual Precipitation | mm |
| P wettest Q | Precipitation of Wettest Quarter | mm |
| E | Bioclimatic stress variable (Chave et al., 2014) | Unitless |
| SRTM | Mean ground elevation from SRTM | m |
| SRTM SD | Standard deviation of ground elevation from SRTM | m |
| LCF | Linear combination of spatial filters retrieved from SEVM | m |
| America | Africa | Asia | |||
|---|---|---|---|---|---|
| Variable | Coeff. | Variable | Coeff. | Variable | Coeff. |
| CEC_T | −0.004 | CEC_T | −0.312 *** | CEC_S | −0.267 *** |
| SILT_S | −0.063 * | SILT_T | −0.007 | CLAY_T | 0.167 *** |
| OC_T | −0.168 *** | OC_T | 0.231 *** | PH_T | 0.185 *** |
| OC_S | −0.117 *** | CLAY_S | 0.079 * | SAND_S | −0.15 *** |
| CALY_S | 0.226 *** | PH_T | 0.155 *** | E | −0.062 |
| PH_T | −0.109 *** | E | −0.31 *** | M Diural Range | 0.07 |
| SAND_T | 0.126 ** | T Seasonality | 0.237 *** | Max T warmest m | −0.045 |
| SAND_S | −0.015 | Max T warmest m | 0.042 | P seasonality | −0.303 *** |
| E | −0.195 *** | T Annual Range | −0.108 * | P wettest Q | 0.071 |
| Isothermality | −0.068 | P seasonality | −0.245 *** | P warmest Q | 0.176 *** |
| T Annual Range | −0.35 *** | P warmest Q | −0.013 | STRM | −0.02 |
| M T warmest Q | −0.035 | P coldest Q | −0.197 *** | STRM SD | 0.072 |
| Annual P | 0.025 | STRM | −0.148 * | LCF | 0.504 *** |
| P seasonality | −0.151 *** | STRM_SD | 0.365 *** | ||
| P warmest Q | 0.131 *** | LCF | 0.718 *** | ||
| P coldest Q | −0.279 *** | ||||
| STRM | −0.002 | ||||
| STRM SD | 0.03 | ||||
| LCF | 0.591 *** | ||||
| America | Africa | Asia | |||
|---|---|---|---|---|---|
| Variable | Coeff. | Variable | Coeff. | Variable | Coeff. |
| CEC_S | 0.056 * | CEC_T | −0.086 | CEC_S | −0.179 ** |
| OC_T | −0.185 *** | SILT_S | 0.091 * | OC_T | −0.098 |
| OC_S | −0.129 *** | OC_S | −0.036 | CLAY_T | 0.128 ** |
| CLAY_S | 0.175 *** | CLAY_S | 0.122 ** | PH_T | 0.126 *** |
| PH_T | −0.118 *** | PH_T | 0.013 | SAND_T | −0.088 ** |
| SAND_T | 0.156 *** | SAND_S | −0.122 *** | E | −0.378 *** |
| E | −0.168 *** | E | −0.321 *** | M Diural Range | −0.186 *** |
| Max T warmest m | −0.068 | Max T warmest m | 0.057 | Min T_Coldest m | −0.584 *** |
| M T driest Q | −0.031 | T Annual Range | 0.004 | P seasonality | −0.283 *** |
| P driest M | 0.16 *** | P westtest M | −0.071 | P warmest Q | 0.156 *** |
| P wettest Q | −0.009 | P driest Q | −0.245 *** | STRM | −0.006 |
| STRM | −0.031 | P warmest Q | 0.202 *** | STRM SD | 0.093 |
| STRM SD | 0.219 *** | STRM | −0.051 | LCF | 0.519 *** |
| LCF | 0.572 *** | STRM SD | 0.473 *** | ||
| LCF | 0.531 *** | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Saatchi, S.S.; Xu, L.; Yu, Y.; Lefsky, M.A.; White, L.; Knyazikhin, Y.; Myneni, R.B. Abiotic Controls on Macroscale Variations of Humid Tropical Forest Height. Remote Sens. 2016, 8, 494. https://doi.org/10.3390/rs8060494
Yang Y, Saatchi SS, Xu L, Yu Y, Lefsky MA, White L, Knyazikhin Y, Myneni RB. Abiotic Controls on Macroscale Variations of Humid Tropical Forest Height. Remote Sensing. 2016; 8(6):494. https://doi.org/10.3390/rs8060494
Chicago/Turabian StyleYang, Yan, Sassan S. Saatchi, Liang Xu, Yifan Yu, Michael A. Lefsky, Lee White, Yuri Knyazikhin, and Ranga B. Myneni. 2016. "Abiotic Controls on Macroscale Variations of Humid Tropical Forest Height" Remote Sensing 8, no. 6: 494. https://doi.org/10.3390/rs8060494







