Development of a Regional Coral Observation Method by a Fluorescence Imaging LIDAR Installed in a Towable Buoy
Abstract
:1. Introduction
2. Methods
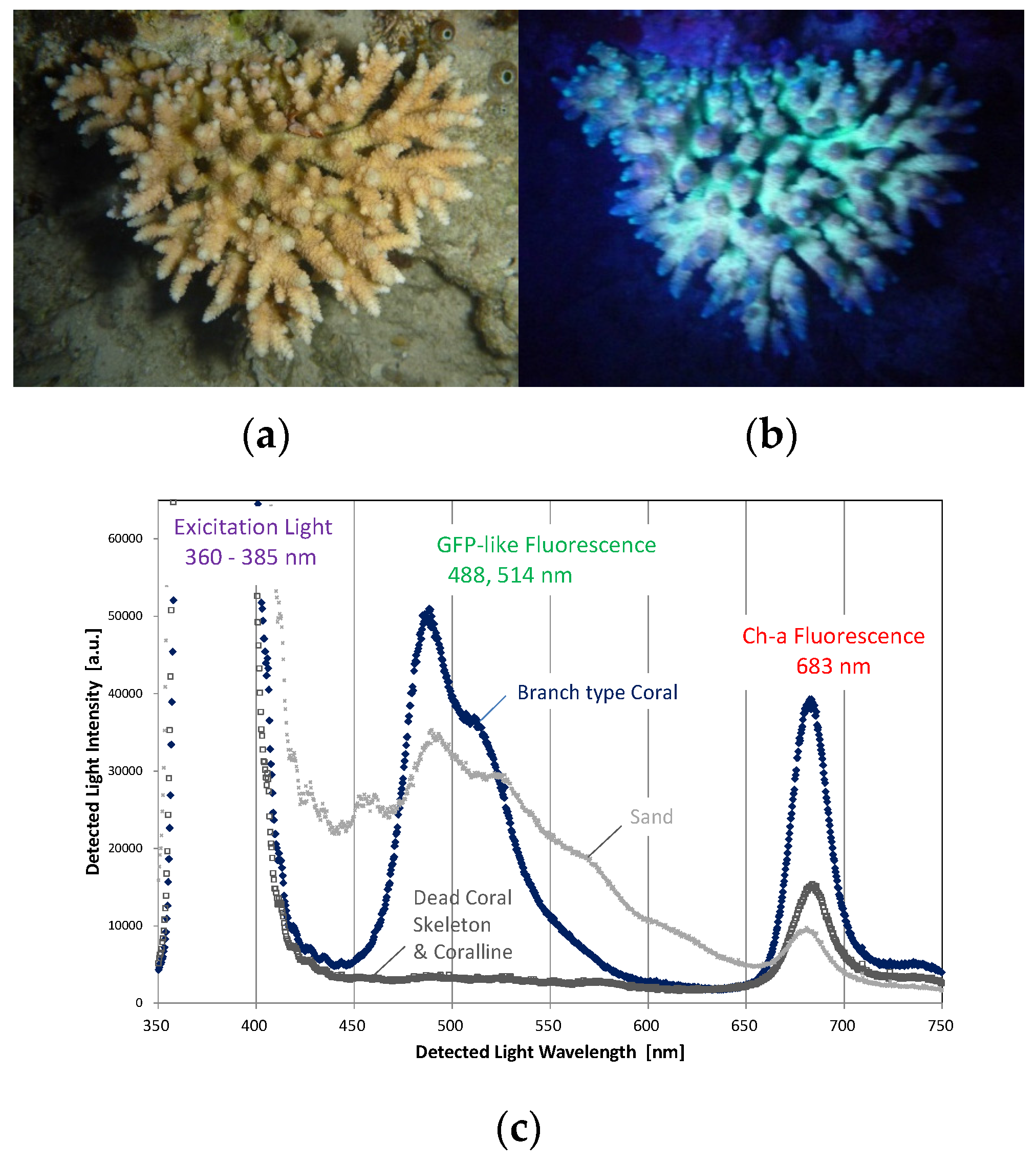
2.1. Fluorescence Properties of Corals
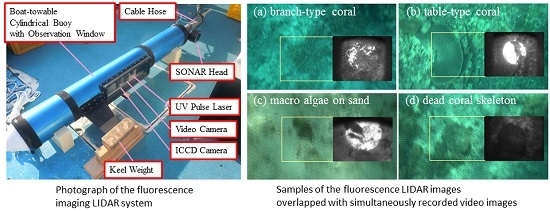
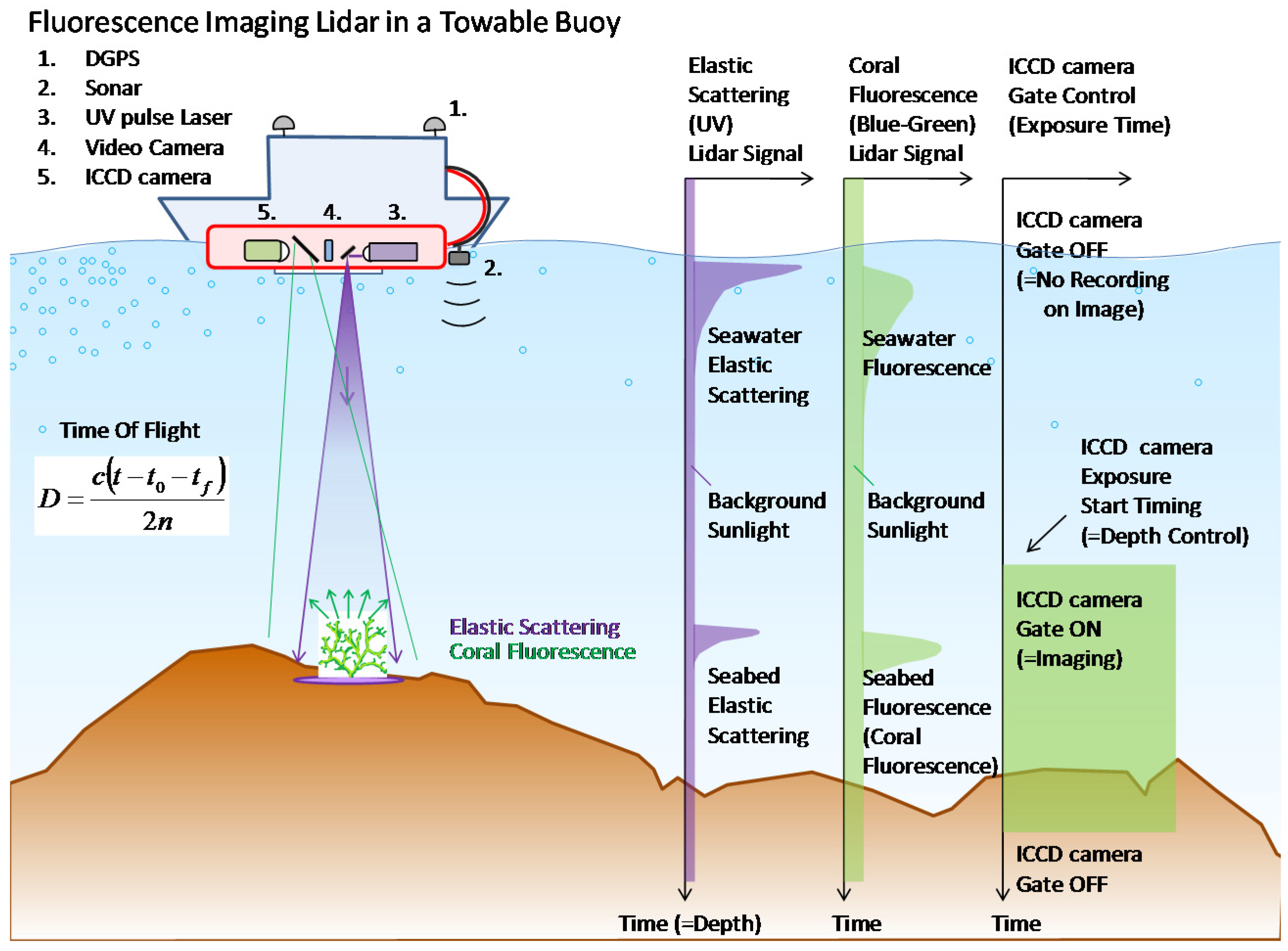
2.2. Fluorescence Imaging LIDAR System
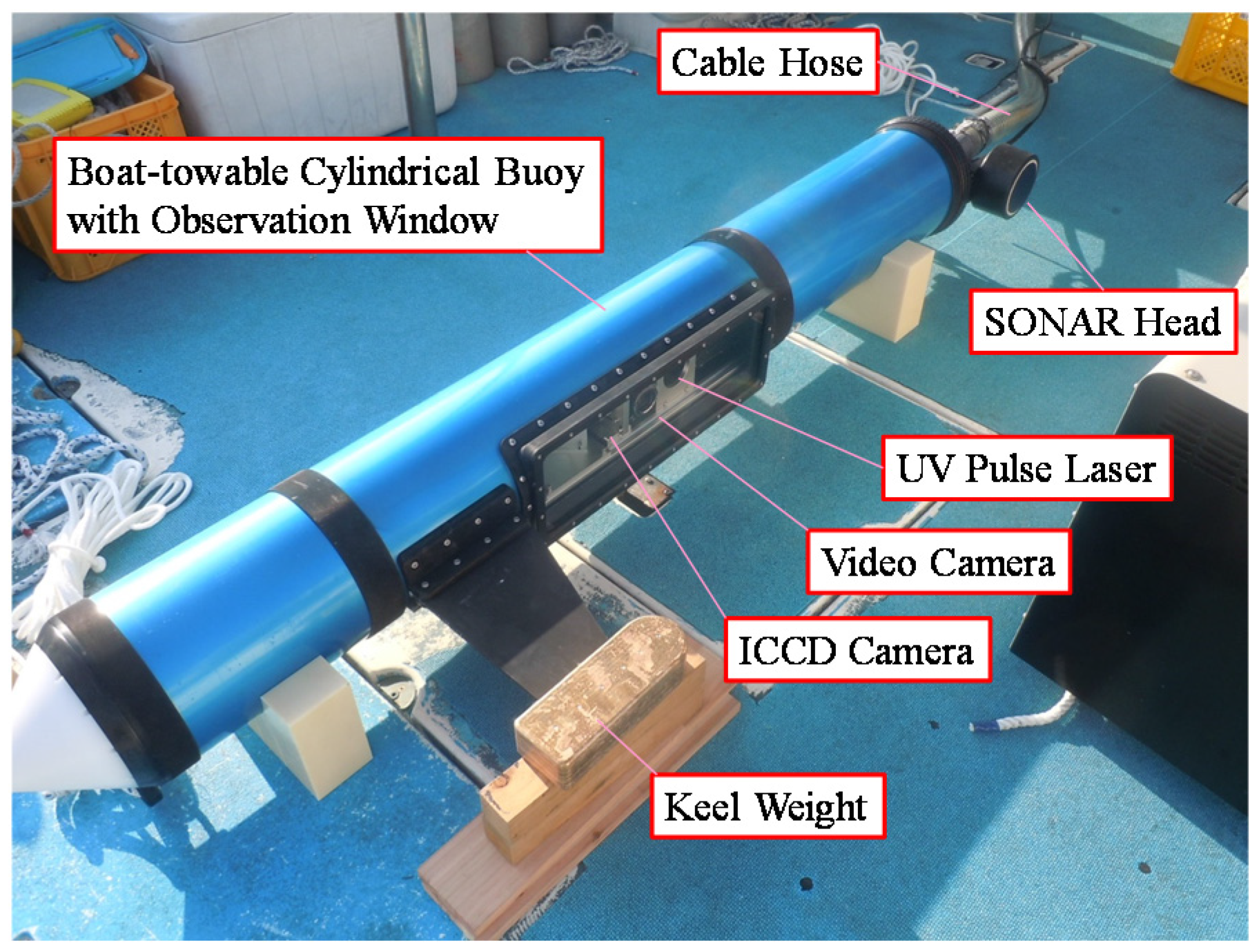
| UV—pulsed Laser (Quantel CFR400) | Type | neodymium-doped yttrium aluminium garnet (Nd:YAG) |
| Wavelength | 355 nm | |
| Energy | 90 mJ/pulse | |
| Pulse Width | 9 ns | |
| Repetition | 1–5 Hz (adjustable) | |
| Spread Angle | 45 to 350 mrad (adjustable) | |
| Gated ICCD Camera (Hamamatsu Photonics C10054-22) Collective Lens (Fujinon C22-17A-M41) | ICCD Type | Image—intensifiedCCD with Gate function of usual OFF |
| Quantum Efficiency | 50% (Max) | |
| Image Gain | 5 × 106 (Max) | |
| ICCD Wavelength Range | 280–720 nm | |
| Max. sensitive wavelength | 530 nm | |
| CCD number of pixels | 768 × 494 | |
| Lens Diameter | 70 mm | |
| Lens Wavelength Range | 407 nm–Visible | |
| Field of View | 35 to 660 mrad (adjustable) | |
| Video Camera (Sony HDR-CX560V) | Type | 1/3 inch complementary metal–oxide–semiconductor (CMOS) |
| Field of View | 90 to 1050 mrad (adjustable) | |
| CMOS pixel size | 6.14 × 106 pixel (16:9) | |
| DGPS Sensor (Septentrio PolaRx2e@) | Type | Single frequency, 3 antenna |
| Position Accuracy | 1 m | |
| Attitude Accuracy | 5 mrad | |
| SONAR Depth Sounder (Tamaya Technics TDM-9000B) | Frequency | 200 kHz |
| Spread Angle | 200 mrad | |
| Observation Range | 1 to 100 m | |
| Depth Accuracy | 0.1 m | |
| Towable Buoy | Diameter | 200 mm |
| Length | 2 m | |
| Observation Window | Pyrex–reinforced glass |
3. Results
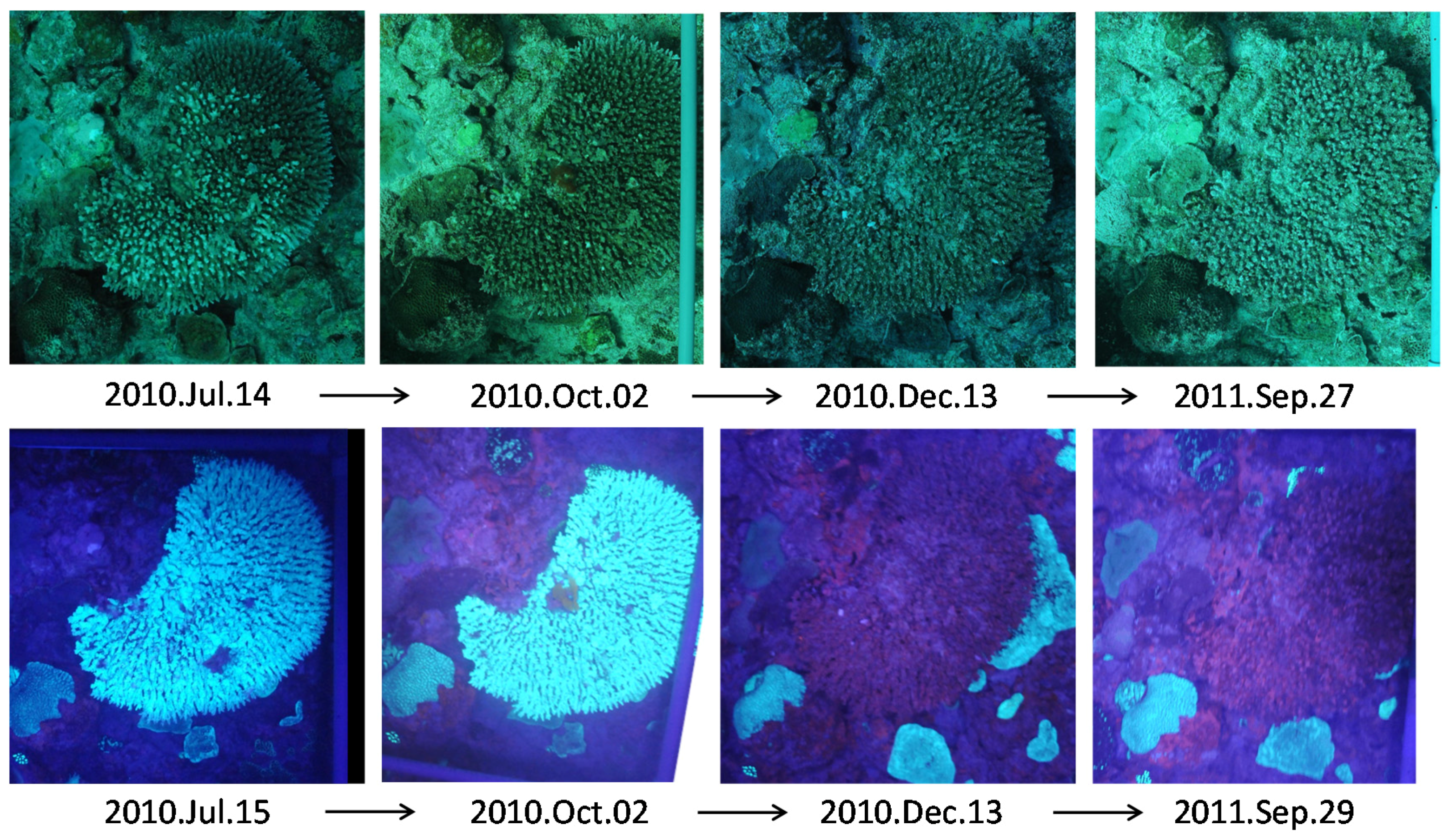
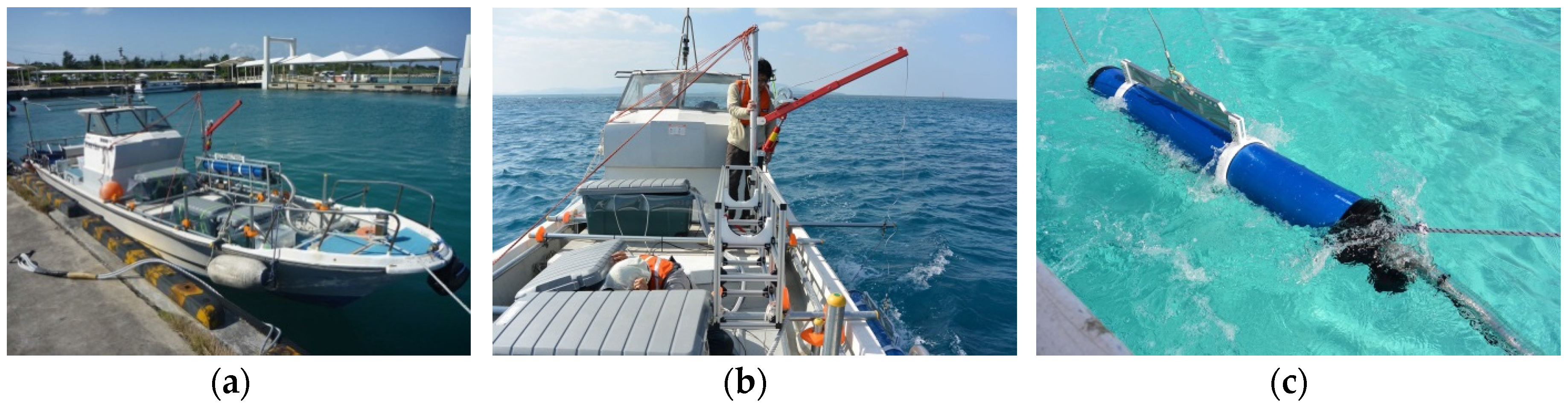
3.1. Long Line Observation

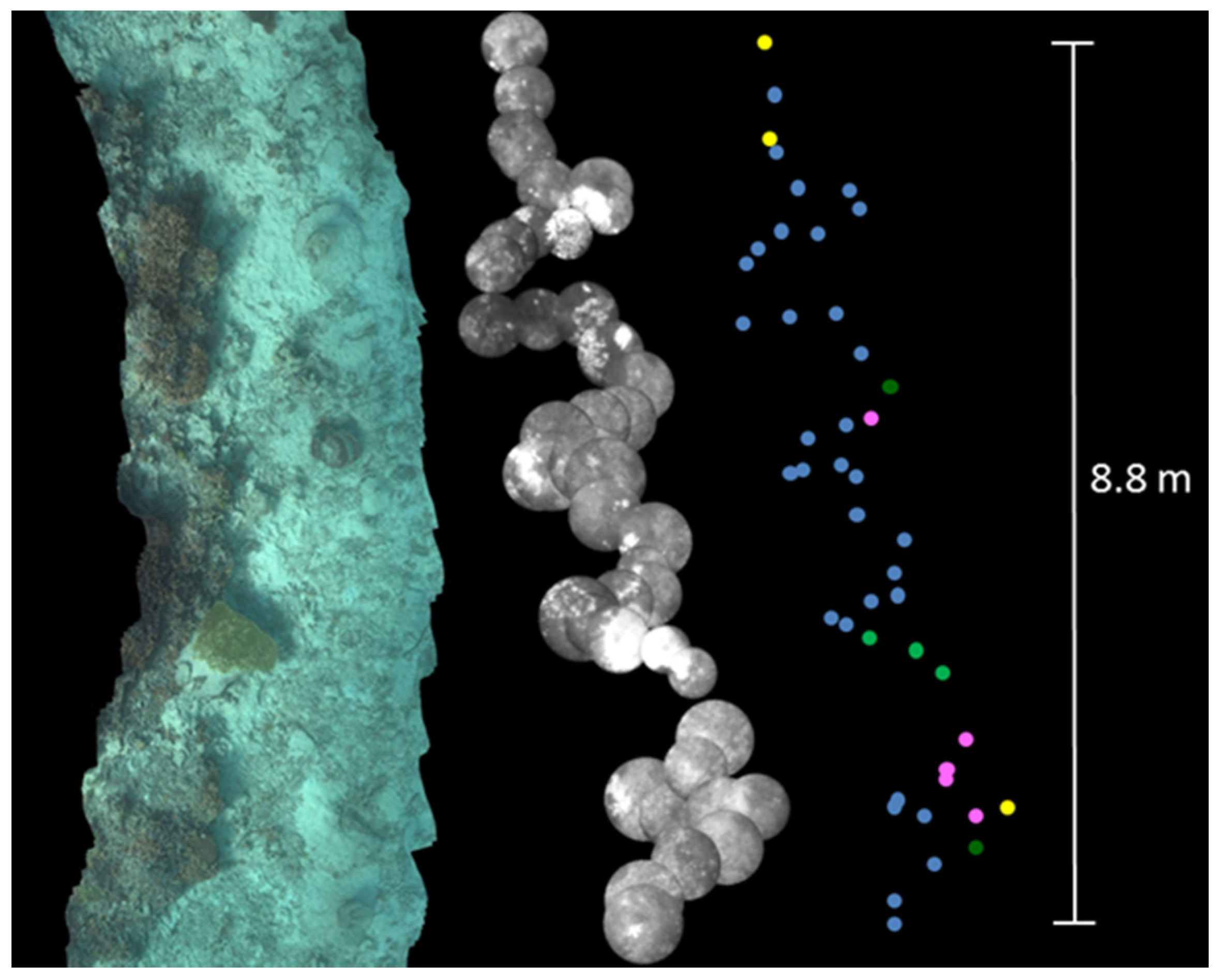
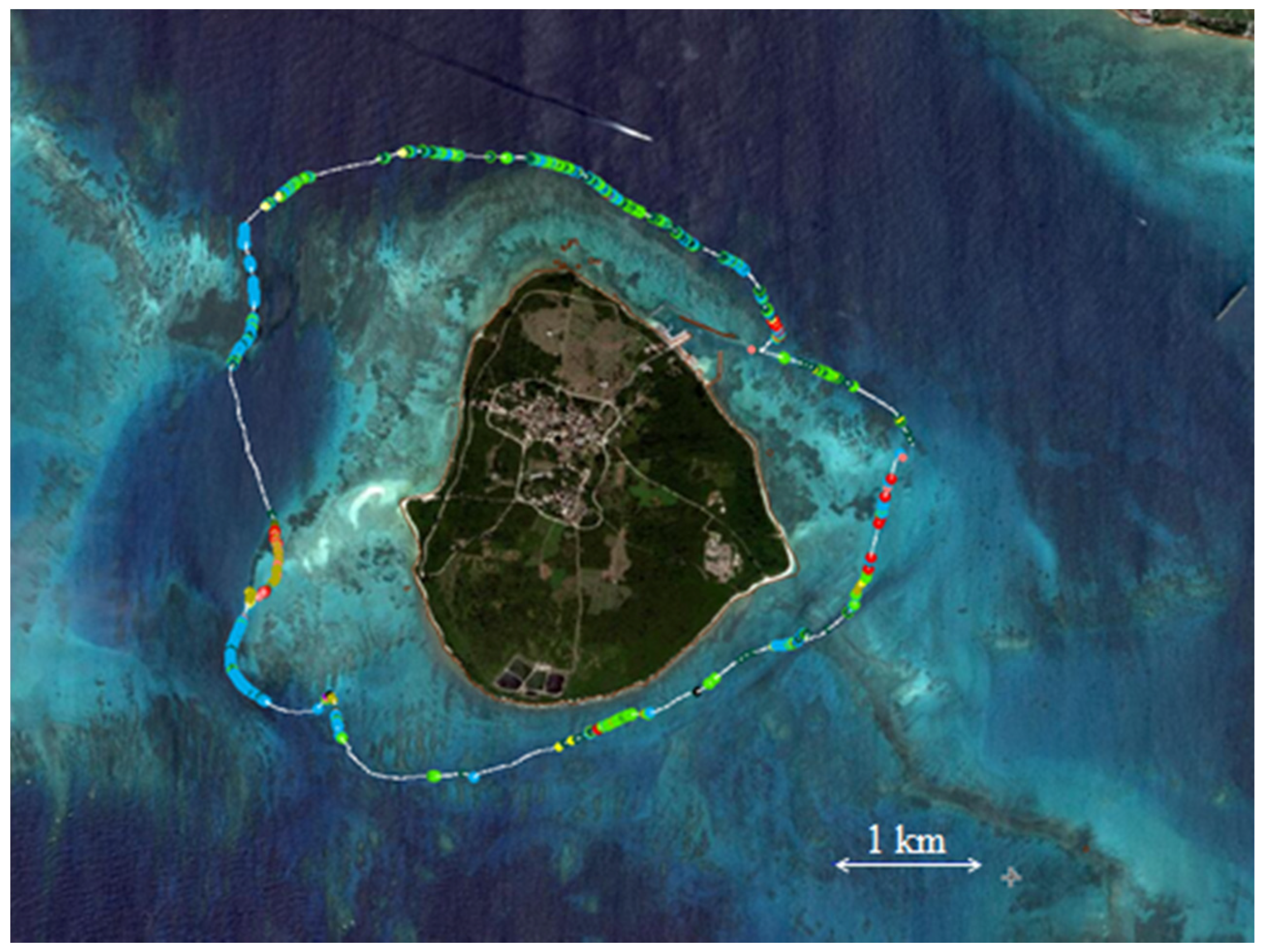
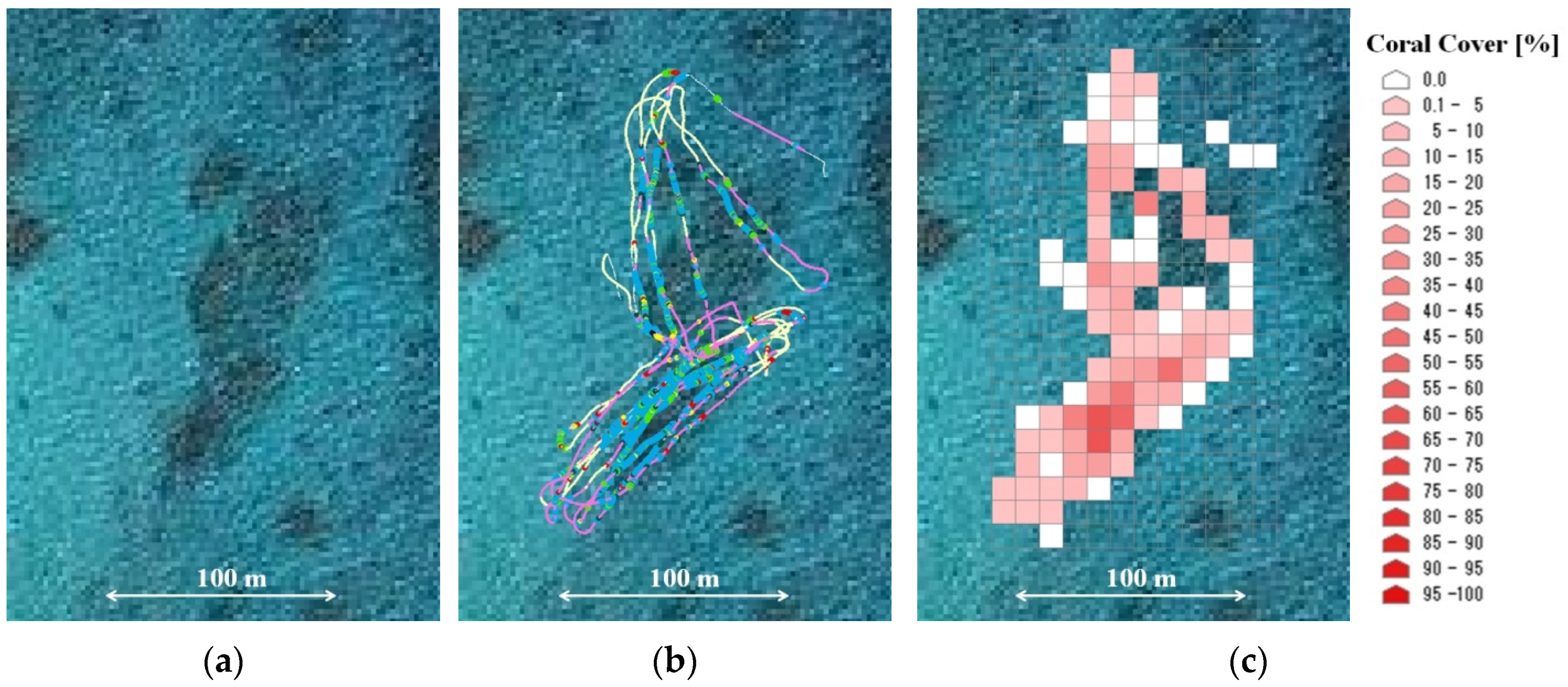
3.2. Coral Cover Observations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, C.; Hoegh-Guldberg, O.; Naumann, M.S.; Colombo-Pallotta, M.F.; Ateweberhan, M.; Fitt, W.K.; Iglesias-Prieto, R.; Palmer, C.; Bythell, J.C.; Ortiz, J.-C.; et al. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 2011, 62, 205–215. [Google Scholar] [CrossRef]
- Status of Coral Reefs of the World: 2008, Global Coral Reef Monitoring Network Report 2008. Available online: http://www.icriforum.org/sites/default/files/GCRMN_Status_Coral_Reefs_2008.pdf (accessed on 14 September 2015).
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Hedley, J.D.; Mumby, P.J. Biological and remote sensing perspectives of pigmentation in coral reef organisms. Adv. Mar. Biol. 2002, 43, 277–317. [Google Scholar] [PubMed]
- Treibitz, T.; Neal, B.P.; Kline, D.I.; Beijbom, O.; Roberts, P.L.; Mitchell, B.G.; Kriegman, D. Wide field-of-view fluorescence imaging of coral reefs. Sci. Rep. 2015, 5, 7694. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Sato, T.; Morita, S. Basic coral distribution data for long term monitoring at Sekisei Lagoon. In Proceedings of the OCEANS 2000 MTS/IEEE Conference and Exhibition, Providence, RI, USA, 11–14 September 2000; pp. 1383–1387.
- Alieva, N.O.; Konzen, K.A.; Field, S.F.; Meleshkevitch, E.A.; Hunt, M.E.; Beltran-Ramirez, V.; Miller, D.J.; Wiedenmann, J.; Salih, A.; Matz, M.V. Diversity and evolution of coral fluorescent proteins. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.; Trevor-Jones, A. Fluorescence census techniques for the early detection of coral recruits. Coral Reefs 2006, 25, 73–76. [Google Scholar]
- Zawada, D.G.; Mazel, C.H. Fluorescence-based classification of caribbean coral reef organisms and substrates. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Mazel, C.H.; Cronin, T.W.; Caldwell, R.L.; Marshall, N.J. Fluorescent enhancement of signaling in a mantis shrimp. Science 2004, 303, 51. [Google Scholar] [CrossRef] [PubMed]
- Research on Cooperative Coral Monitoring for Environmental Impact Assessment of Ocean-Warming and Acidification. Available online: http://www.env.go.jp/earth/kenkyuhi/report/pdf/11_2_3.pdf (accessed on 30 December 2015).
- Sasano, M.; Matsumoto, A.; Imasato, M.; Yamano, H.; Oguma, H. Coral observation by fluorescence imaging LIDAR on a glass-bottom boat. J. Remote Sens. Soc. Jpn. 2013, 33, 377–389. (In Japanese) [Google Scholar]
- Gilmore, A.M.; Larkum, A.W.D.; Salih, A.; Itoh, S.; Shibata, Y.; Bena, C.; Yamasaki, H.; Papina, M.; van Woesik, R. Simultaneous time resolution of the emission spectra of fluorescent proteins and zooxanthellar chlorophyll in reef-building corals. Photochem. Photobiol. 2003, 77, 515–523. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasano, M.; Imasato, M.; Yamano, H.; Oguma, H. Development of a Regional Coral Observation Method by a Fluorescence Imaging LIDAR Installed in a Towable Buoy. Remote Sens. 2016, 8, 48. https://doi.org/10.3390/rs8010048
Sasano M, Imasato M, Yamano H, Oguma H. Development of a Regional Coral Observation Method by a Fluorescence Imaging LIDAR Installed in a Towable Buoy. Remote Sensing. 2016; 8(1):48. https://doi.org/10.3390/rs8010048
Chicago/Turabian StyleSasano, Masahiko, Motonobu Imasato, Hiroya Yamano, and Hiroyuki Oguma. 2016. "Development of a Regional Coral Observation Method by a Fluorescence Imaging LIDAR Installed in a Towable Buoy" Remote Sensing 8, no. 1: 48. https://doi.org/10.3390/rs8010048