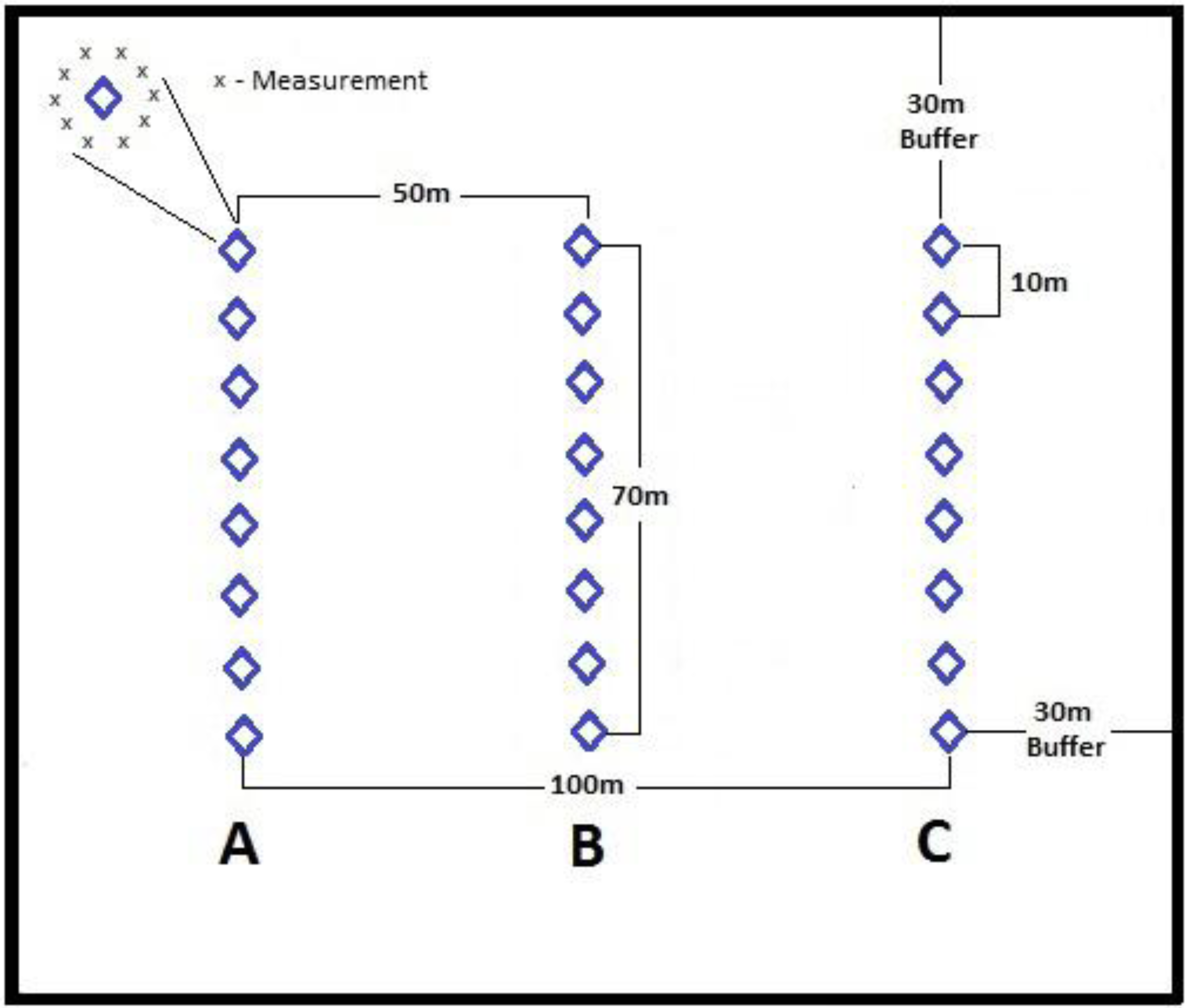
4.1.1. Interpretation of Spectral Reflectance for DAP
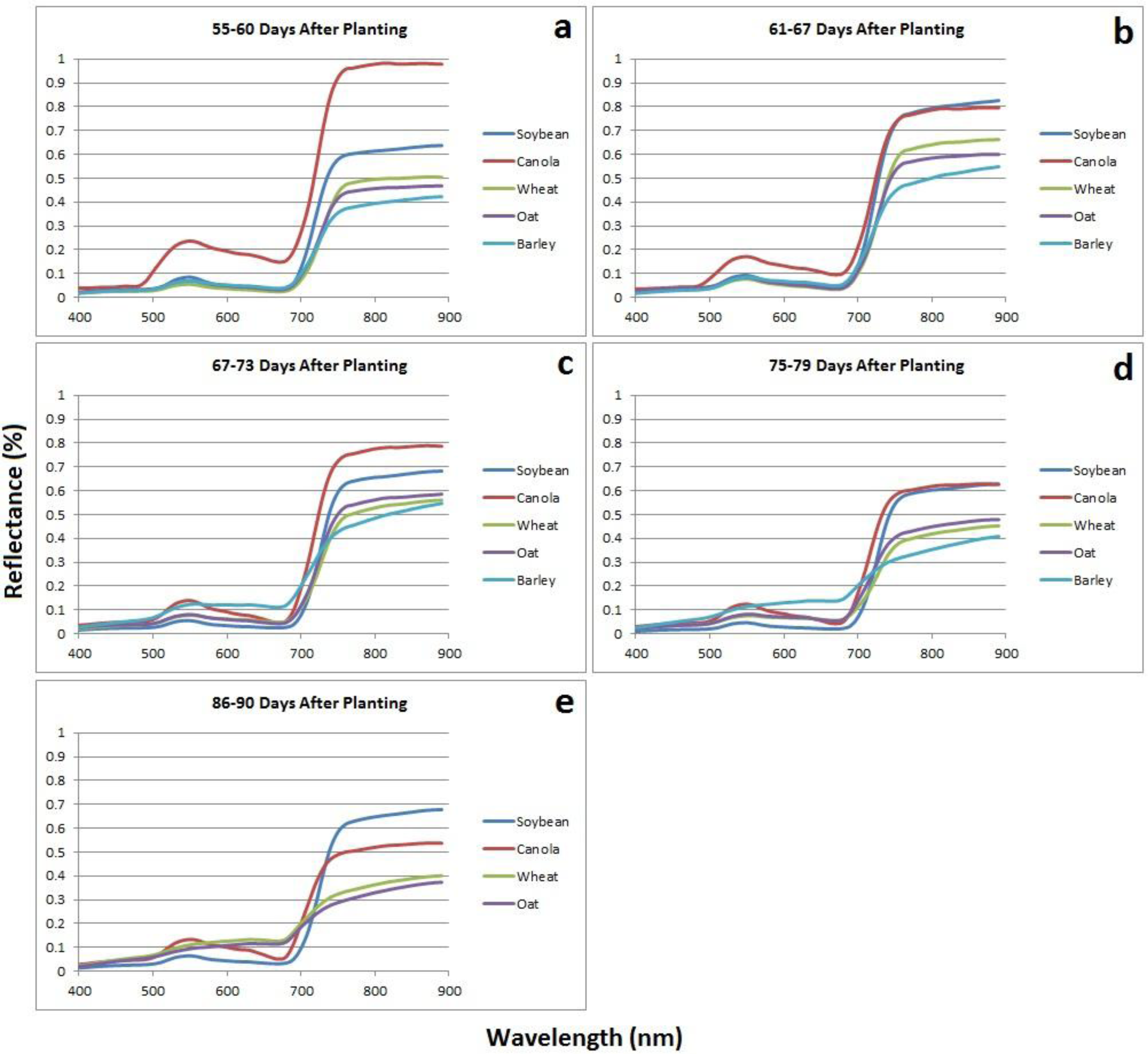
At 55–60 DAP (
Figure 5a) canola is very distinguishable from the other crops hyperspectrally with high reflectance in the green and NIR portions of the spectrum as a result of flowering. The reflectance in the visible spectrum is similar for soybean, wheat, oats, and barley, however, soybean has a slightly higher reflectance in the NIR indicating denser vegetation canopy. At 61–67 DAP (
Figure 5b), reflectance in the NIR for canola has dropped slightly, likely as a result of a change in plant structure due to the flower petals maturing and beginning to fall off. However, reflectance in the green and red portions of the spectrum for canola still remain slightly higher than for the other crops as there are still some yellow flowers on the plants. The yellow flowers of the canola crop cause increase reflectance in the green and red portions of the spectrum. Reflectance in the NIR for soybean has also reached its maximum of above 80%, which is likely a result of flowering and of the broad leaves reaching maturity before transferring their energy to pod production. At this stage in development, soybean has reached maturity and canopy coverage is dense allowing for very little light energy to pass through gaps in the canopy, ultimately resulting in strong reflectance back to the sensor. The spectral reflectance of the cereal crops (wheat, oats, and barley) are very similar with only minor variations in the NIR spectrum due to differences in the growth stage and the physical structure of the heads of each crop. Visually, 67–73 DAP (
Figure 5c) appears to be one of the best times for crop separability. However, a thorough examination of the signatures reveals that although soybean and canola have clearly distinguished themselves from each other and from the rest of the crops hyperspectrally, the spectral reflectance of the cereal crops is very similar with overlap occurring in the NIR, red-edge and visible portions of the spectrum. At 75–79 DAP (
Figure 5d) the cereal crops show good separation in the NIR, red-edge, and visible portions of the spectrum which are key areas for crop separability. The differences in spectral reflectance is attributed to the varying rates at which each crop begins to senesce and change from green to brown in colour. As the crops begin to senesce the structure of each crop changes dramatically and the canopy density quickly decreases.
The variable rates at which this occurs for each crop results in differences in spectral reflectance making crops more easily distinguishable. Barley has a shorter growing season and therefore has begun to senesce at a quicker rate, hence, the flattening of the spectral curve in relation to wheat and oats. The soybean and canola crops are still separable in the visible and red-edge portions of the spectrum. At 86–90 DAP (
Figure 5e) soybean and canola still remain very distinguishable from each other as canola has begun to senesce and turn brown, whereas, soybean still remains relatively green in colour. The spectral curves of wheat and oats have begun to flatten as the crops senesce and die. At this stage, crops are still relatively distinguishable hyperspectrally, however, barley has been harvested which indicates that 86–90 DAP or later, may not be the most ideal time for crop separability. Visually, either 67–73 DAP or 75–79 DAP seem to be the most suitable times for crop separability.
4.1.2. Identification of Significant Bands for Crop Separability for DAP
An initial discriminant analysis was run using all bands for all crops to determine which bands were significant for crop separability. Classification accuracies for the bands initially selected as significant were high, ranging from 95.8% to 100%. However, a number of these initial bands were from similar parts of the electromagnetic spectrum (EMS) and, therefore, contained similar information. To reduce redundant information, similar bands were removed and a second discriminant analysis was run on the newly created set of bands.
The newly selected bands were chosen based primarily on where they were located in the EMS and how significant each band was in discriminating crops. For example, at 55–60 DAP, a total of 9 bands were identified as significant, after the initial discriminant analysis, including one violet band (B405), two blue bands (B455, B485), one green band (B535), one yellow band (B595), two red-edge bands (B705, B715), and two NIR bands (B765, B885). The blue band in the visible portion of the spectrum is highly influenced by atmospheric conditions through scattering [
40] making it less desirable for crop discrimination; for this reason, bands B405, B455, and B485 were removed from further analysis. Band B535 was the only green band identified as significant and therefore was included for further analysis. The two red-edge bands identified as significant contained very similar information as they are very close to one another within the EMS. Discriminant analysis was run using each red-edge band and both generated similar classification results, however only one band was required for further analysis; for this reason, band B715 was chosen and band B705 was removed. Finally, both NIR bands were chosen for further analysis because band B765 is very close to the red-edge portion of the EMS while band B885 is well into the NIR spectrum; although both bands are located in the NIR spectrum, they were far enough apart to not contain very similar information. Bands located in transition zones from one part of the EMS to another (from blue to green, green to red, or red to NIR) are generally significant for crop discrimination especially the red-edge [
8,
31]. Heavy consideration was placed on identified red-edge bands as they are important for crop discrimination. In this way, the number of bands selected for further discriminant analysis was reduced from the initial 9 to a final of 4 for 55–60 DAP. The final sets of wavebands selected for crop discrimination are summarized in
Table 3 and the corresponding classification accuracies are summarized in
Table 4. Consequently, this method was used to determine smaller sets of bands for crop discrimination for all seven data acquisition dates throughout the growing season.
A total of 12 bands for Scenario 1 were deemed best suited for crop discrimination including B465, B525, B535, B545, B675, B695, B715, B725, B735, B765, B825, and B885. The significant bands vary by the number of DAP; however, there are some consistencies that should be noted. In general, almost every data acquisition date had a band in the green, red or red-edge and NIR portion of the EMS identified as significant. The most frequently occurring band was B535 in the green spectrum, which was identified as significant in four data acquisition dates. The second most frequently identified bands were B665 (red), B735 (red-edge), B765 (NIR), and B885 (NIR) each of which were identified twice.
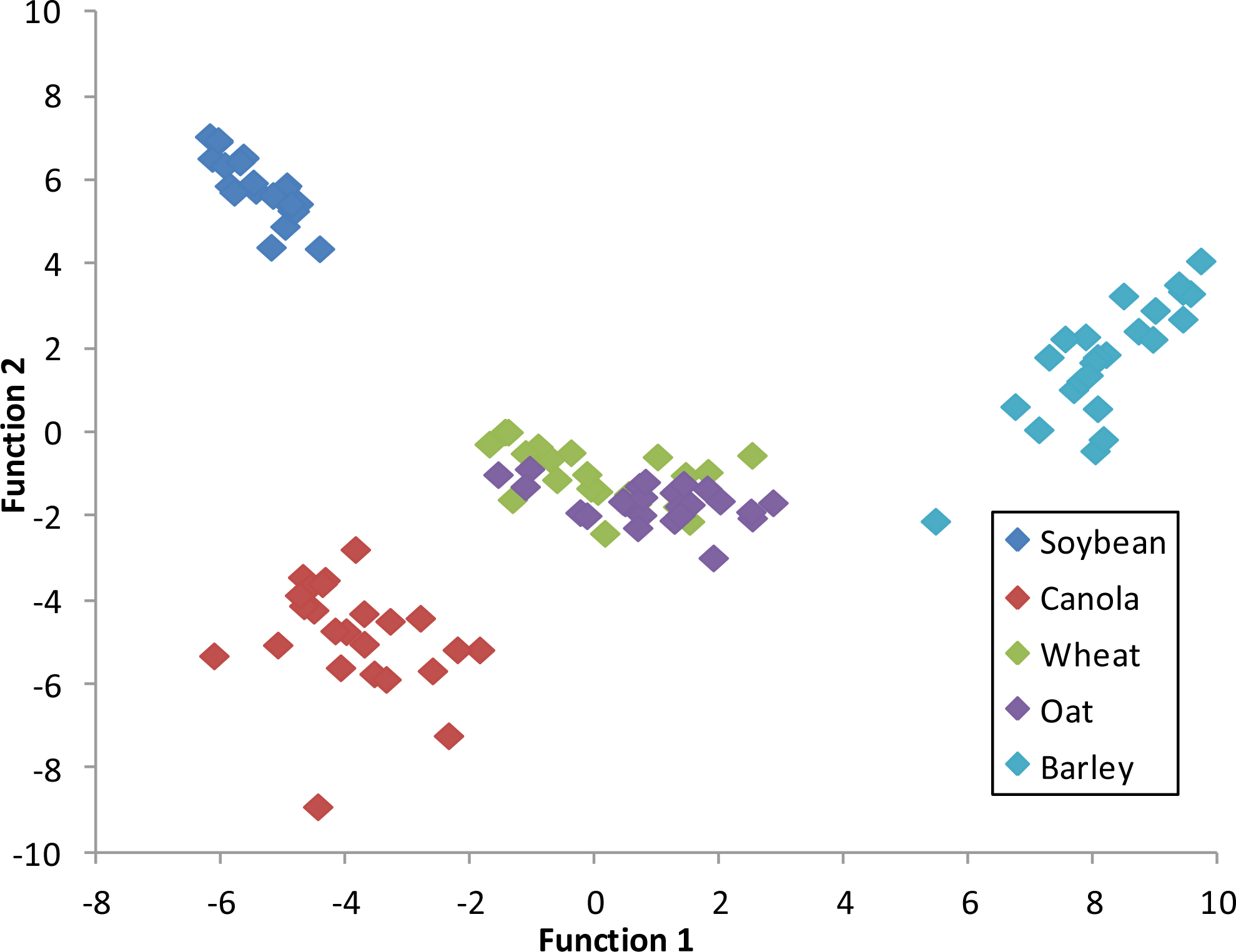
The results for Scenario 1 indicate that the optimal time for crop discrimination is approximately 75–79 DAP. At this point in the growing season soybean, canola, wheat, oats and barley were at BBCH stage 73, 76, 75, 83, and 83, respectively (
Table 5).
It is at this point that the difference in hyperspectral reflectance of crops is maximized and classification results were most accurate. Soybean, canola, wheat, and oat were classified at 100% accuracy, while barley was classified at 95.8% accuracy; only one barley variable was misclassified as wheat. The cereal crops seem most difficult to separate hyperspectrally due to similarities in plant structure. When the cereal crops mature and the heads of the plants begin to fill with seeds, differences in spectral reflectance are maximized due to changes in plant structure. In the later growth stages, the heads of the barley crop begin to lodge and produce hairs that protrude, ultimately increasing canopy coverage and spectral reflectance. The heads of the wheat crops enlarge but lodge only slightly, therefore, gaps between the rows of wheat are still present, which could decrease the amount of reflectance returning back to the sensor. The structure of the oat crop differs from wheat and barley in that there is not one single head that contains all the seeds. In fact, the seeds are dispersed along the main stem and along branches off the main stem ultimately increasing canopy density, which could explain the increased reflectance in the NIR portion of the spectrum in the later growth stages.
The wavebands identified as significant for crop discrimination at 74–79 DAP included band B535 (green), B675 (red), B735 (red-edge), and B885 (NIR). This suggests that if all crops are planted near the same date, 75–79 DAP would be the optimal time in the Northeastern Ontario growing season to capture a hyperspectral satellite image in order to most accurately distinguish these five cash crops.
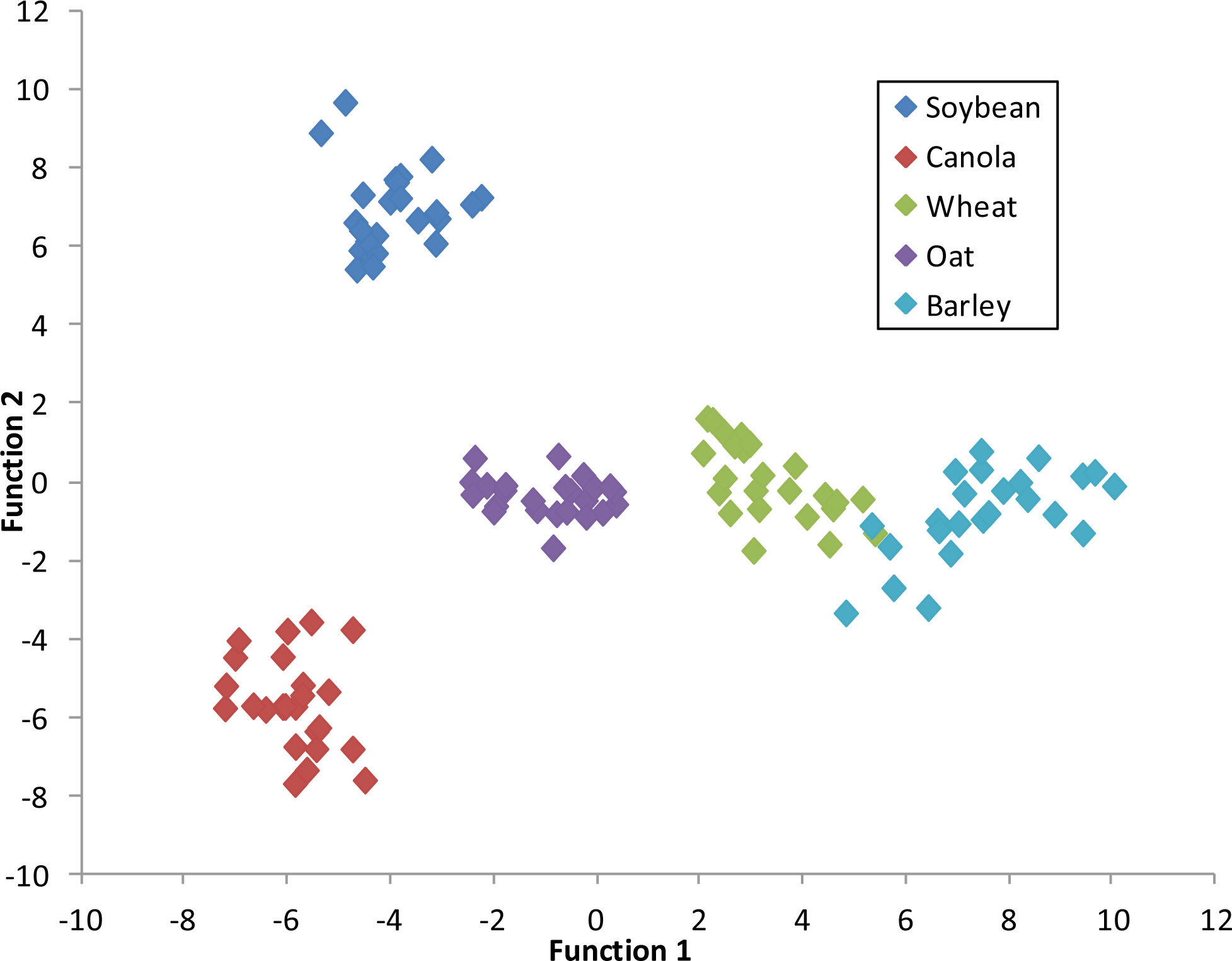
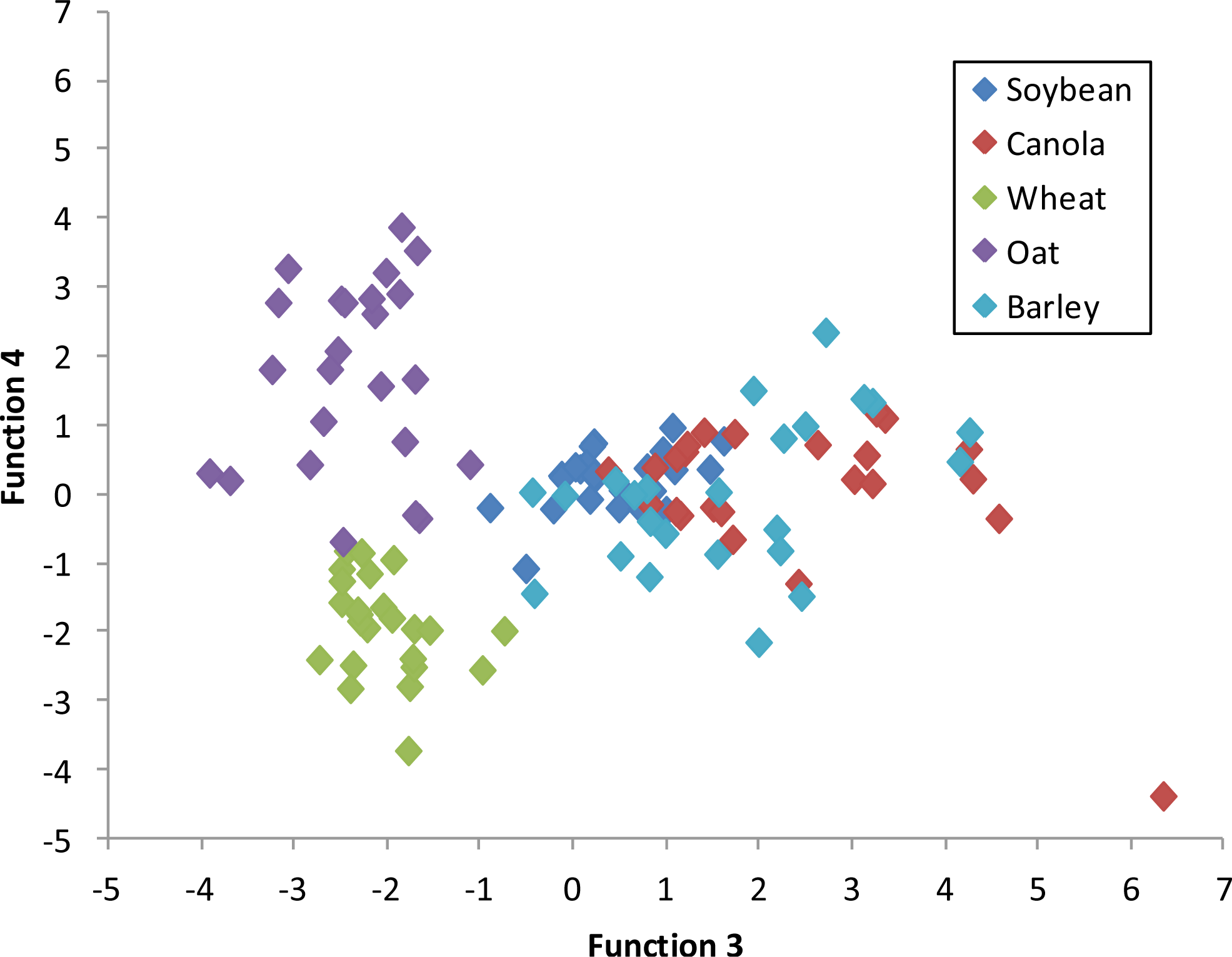
Figure 6 depicts a graph of the canonical discriminant function 1
versus function 2 at 75–79 DAP. Each class is clearly distinguishable from the other indicating a high degree of accuracy for crop classification based on the first two functions of the discriminant analysis.