Comparative Analysis of Four Models to Estimate Chlorophyll-a Concentration in Case-2 Waters Using MODerate Resolution Imaging Spectroradiometer (MODIS) Imagery
Abstract
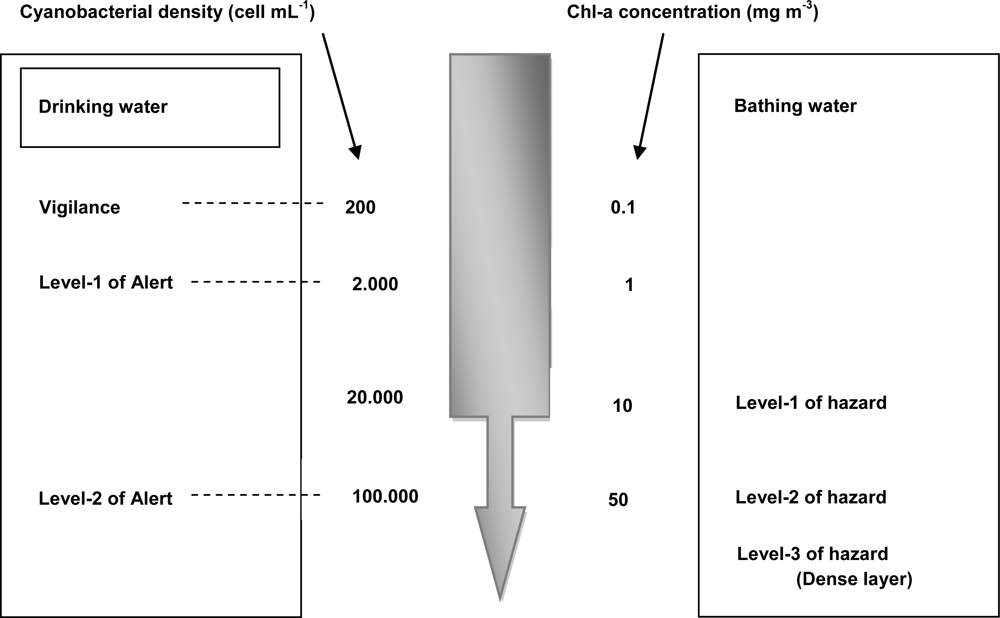
:1. Introduction
2. Material and Methods
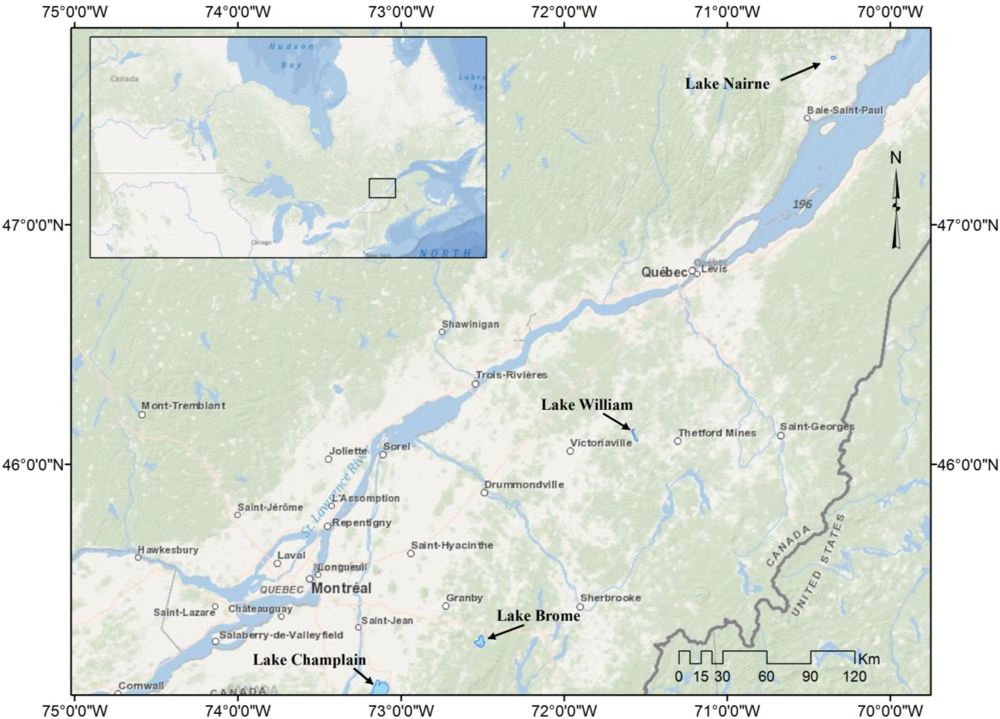
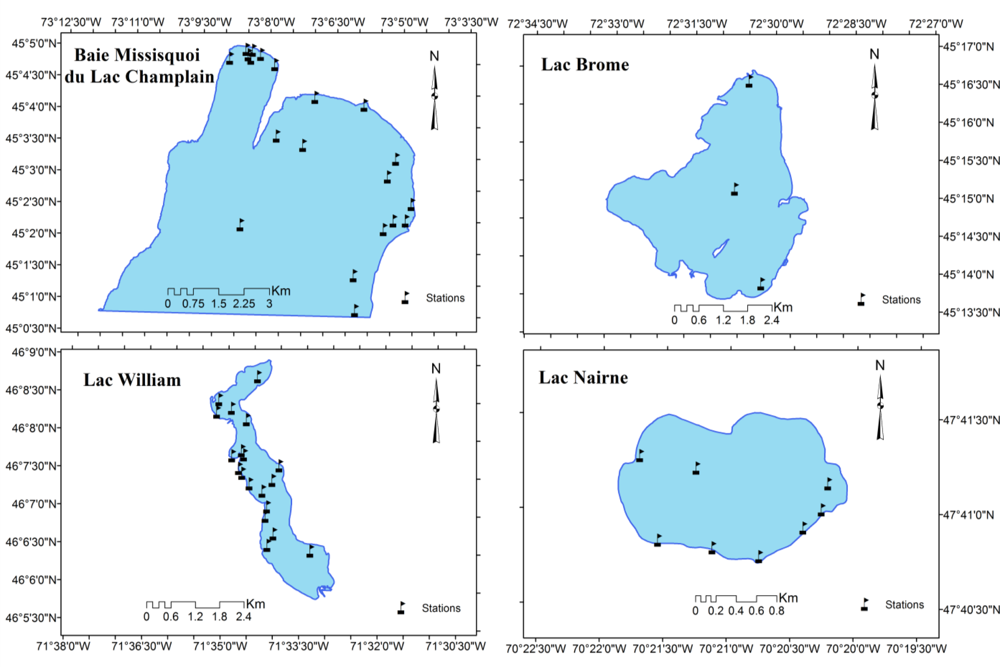
2.1. In situ Data
2.2. MODIS Data
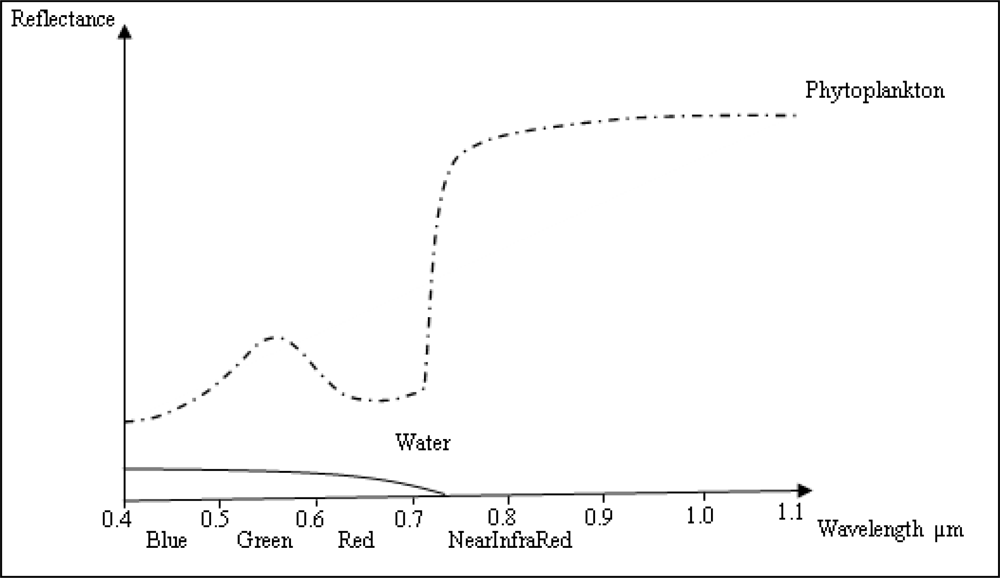
3. Algorithms Used to Estimated Chl-a Concentration
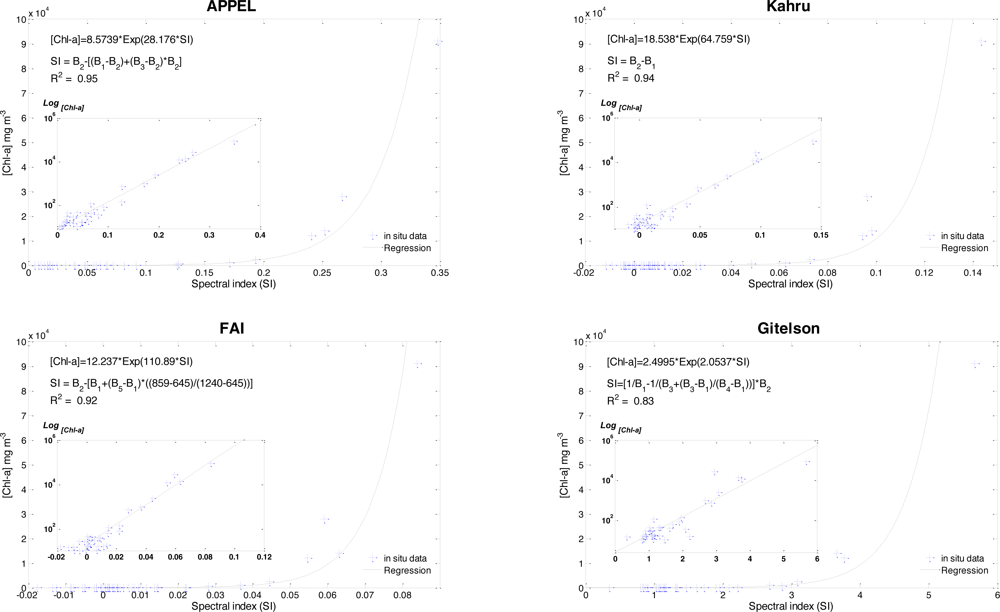
3.1. Kahru’s Model
3.2. Floating Algae Index (FAI)
3.3. Gitelson Model
3.4. Novel Approach APPEL (APProach by ELimination)
4. Accuracy Assessment
5. Results and Discussion
5.1. Evaluation of Downscaling the MODIS Band Signal
5.2. Model Calibration
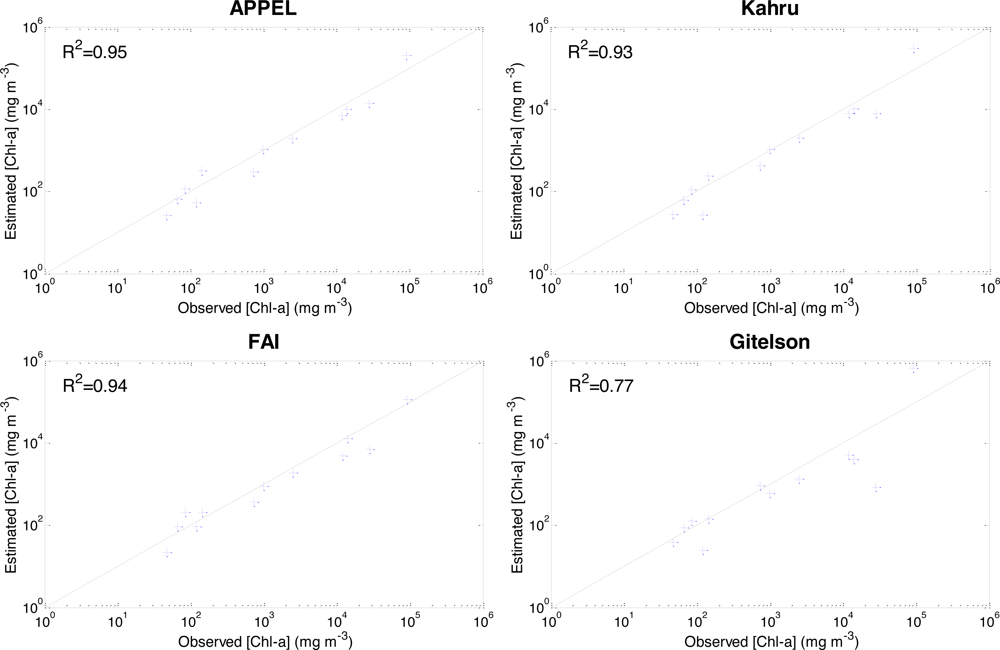
5.3. Model Validation
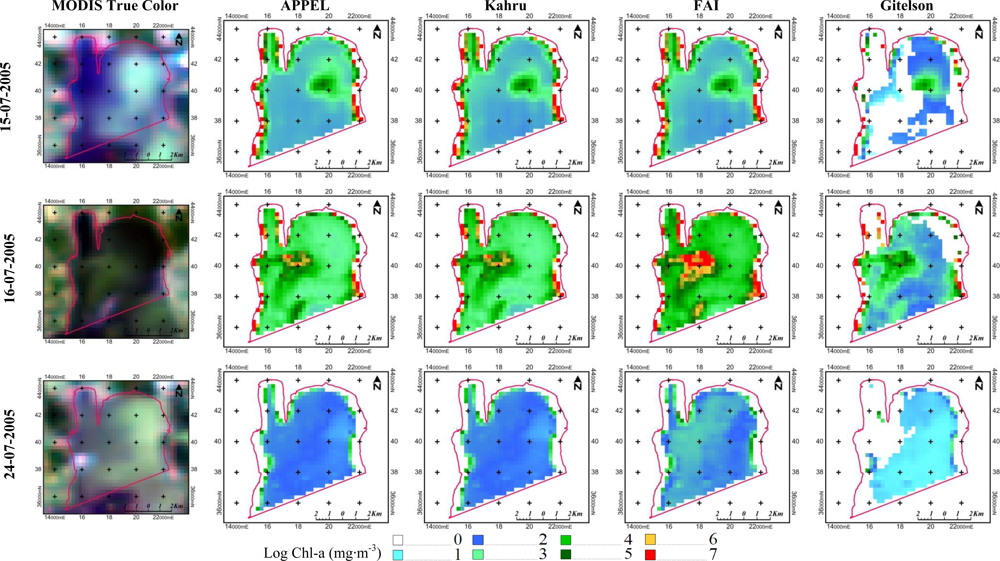
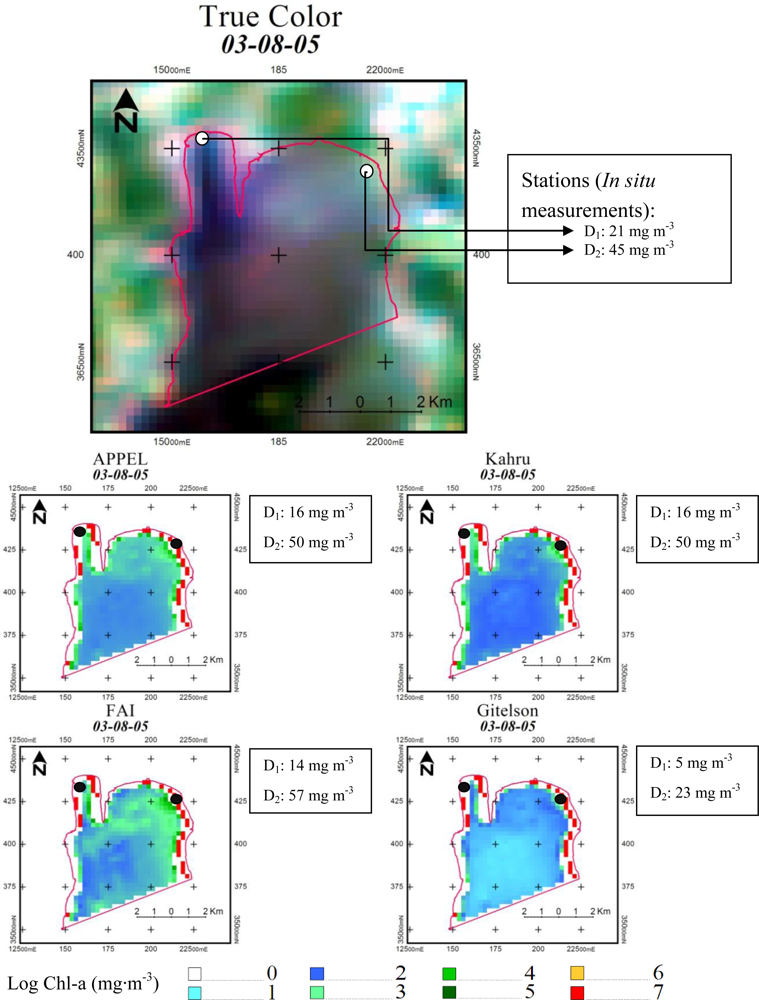
5.5. Qualitative Evaluation
6. Conclusions
Acknowledgments
References and Notes
- Gleick, P.H. The World’s Water; Island Press: Chicago, IL, USA, 2000; Volume 7, p. 315. [Google Scholar]
- Bouchard, V.M. Floraisons de Cyanobactéries au lac Saint-Augustin: Dynamique à Court Terme et Stratification; Université Laval: Québec, QC, Canada, 2004. [Google Scholar]
- Laurion, I.; Rousseau, A.; Chokmani, K.; Drogui, P.; Bourget, S.; Warren, A.; Drevnick, P. Mémoire sur la Situation des lacs au Québec en regard des Cyanobactéries; INRS-Centre Eau Terre Environnement: Québec, QC, Canada, 2009; p. 25. [Google Scholar]
- Eija, R.; Rvi, R.O.; Seija, H.L.; Juha-Markku, L.N.; Mika, R. Effect of sampling frequency on detection of natural variability in phytoplankton: Unattended high-frequency measurements on board ferries in the baltic sea. J. Marine Sci 1998, 55, 8. [Google Scholar]
- Agence Française de Sécurité Sanitaire des Aliments, A. Rapport sur l’Évaluation des Risques liés à la Présence de Cyanobactéries et de leurs Toxines dans les eaux Destinées à l’Alimentation, à la Baignade et autres Activités Récréatives; AFSSET: Maisons-Alfort, France, 2006; p. 231. [Google Scholar]
- MDDEP. Les Lacs et Cours d’Eau Touchés par une Fleur d’Eau d’Algues Bleu-Vert; MDDEP: Québec, QC, Canada, 2009; Available online: http://www.mddep.gouv.qc.ca/eau/algues-bv/bilan/saison2009/bilan2009.pdf (accessed on 12 June 2012).
- Carder, K.L.; Chen, F.R.; Lee, Z.P.; Hawes, S.K.; Kamykowski, D. Semianalytic moderate-resolution imaging spectrometer algorithms for chlorophyll a and absorption with bio-optical domains based on nitrate-depletion temperatures. J. Geophys. Res 1999, 104, 5403–5421. [Google Scholar]
- Morel, A.; Prieur, L. Analysis of variations in ocean color. Limnol. Oceanogr 1977, 22, 709–722. [Google Scholar]
- Baruah, P.J.; Tumura, M.; Oki, K.; Nishimura, H. Neural Network Modeling of Lake Surface Chlorophyll and Sediment Content from Landsat TM Imagery. Proceedings of 22nd Asian Conference on Remote Sensing, Singapore, 5–9 November 2001; p. 6.
- Ahn, Y.H.; Shanmugam, P. Detecting the red tide algal blooms from satellite ocean colour observations in optically complex Nourtheast-Asia coastal waters. Remote Sens. Environ 2006, 103, 419–437. [Google Scholar]
- Becker, R.H.; Sultan, M.I.; Boyer, G.L.; Twiss, M.R.; Konopko, E. Mapping cyanobacterial blooms in the great lakes using modis. J. Great Lakes Res 2009, 35, 447–453. [Google Scholar]
- Gitelson, A.A.; Schalles, J.F.; Hladik, C.M. Remote chlorophyll-a retrieval in turbid, productive estuaries: Chesapeake bay case study. Remote Sens. Environ 2007, 109, 464–472. [Google Scholar]
- Hu, C. A novel ocean color index to detect floating algae in the global oceans. Remote Sens. Environ 2009, 113, 2118–2129. [Google Scholar]
- Tarrant, P.; Neuer, S. Monitoring algal blooms in a southwestern U.S. Reservoir system. Eos Trans. AGU 2009, 90, 38. [Google Scholar]
- Darecki, M.; Stramski, D. An evaluation of modis and seawifs bio-optical algorithms in the baltic sea. Remote Sens. Environ 2004, 89, 326–350. [Google Scholar]
- Mishra, S.; Mishra, D.; Schluchter, W. A novel algorithm for predicting phycocyanin concentrations in cyanobacteria: A proximal hyperspectral remote sensing approach. Remote Sens 2009, 1, 758–775. [Google Scholar]
- Heim, B.; Oberhaensli, H.; Fietz, S.; Kaufmann, H. Variation in Lake Baikal’s phytoplankton distribution and fluvial input assessed by seawifs satellite data. Glob. Planet. Change 2005, 46, 9–27. [Google Scholar]
- Semovski, S.V.; Mogilev, N.Y.; Sherstyankin, P.P. Lake Baikal ice: Analysis of AVHRR imagery and simulation of under-ice phytoplankton bloom. J. Marine Syst 2000, 27, 117–130. [Google Scholar]
- Hu, C.; Chen, Z.; Clayton, T.D.; Swarzenski, P.; Brock, J.C.; Muller-Karger, F.E. Assessment of estuarine water-quality indicators using MODIS medium-resolution bands: Initial results from tampa bay. Remote Sens. Environ 2004, 93, 423–441. [Google Scholar]
- Matthews, M.W.; Bernard, S.; Winter, K. Remote sensing of cyanobacteria-dominant algal blooms and water quality parameters in Zeekoevlei, A small hypertrophic lake, using MERIS. Remote Sens. Environ 2010, 114, 2070–2087. [Google Scholar]
- Wheeler, S.M.; Morrissey, L.A.; Levine, S.N.; Livingston, G.P.; Vincent, W.F. Mapping cyanobacterial blooms in Lake Champlain’s missisquoi bay using Quickbird and MERIS satellite data. J. Great Lakes Res 2012, 38(Supplement 1), 68–75. [Google Scholar]
- Dall'Olmo, G.; Gitelson, A.A.; Rundquist, D.C.; Leavitt, B.; Barrow, T.; Holz, J.C. Assessing the potential of seawifs and modis for estimating chlorophyll concentration in turbid productive waters using red and near-infrared bands. Remote Sens. Environ 2005, 96, 176–187. [Google Scholar]
- Gitelson, A.A.; Dall'Olmo, G.; Moses, W.; Rundquist, D.C.; Barrow, T.; Fisher, T.R.; Gurlin, D.; Holz, J. A simple semi-analytical model for remote estimation of chlorophyll-a in turbid waters: Validation. Remote Sens. Environ 2008, 112, 3582–3593. [Google Scholar]
- Gitelson, A.A.; Kondratyev, K.Y. Optical models of mesotrophic and eutrophic water bodies. Int. J. Remote Sens 1991, 12, 373–385. [Google Scholar]
- Gons, H.J.; Rijkeboer, M.; Ruddick, K.G. A chlorophyll-retrieval algorithm for satellite imagery (medium resolution imaging spectrometer) of inland and coastal waters. J. Plankton Res 2002, 24, 947–951. [Google Scholar]
- Kahru, M.; Mitchell, B.G.; Diaz, A.; Miura, M. Modis detects a devastating algal bloom in Paracas Bay, Peru. EOS 2004, 85, 465–472. [Google Scholar]
- Trishchenko, A.P.; Luo, Y.; Khlopenkov, K.V. A method for downscaling MODIS land channels to 250 m spatial resolution using adaptive regression and normalization. Proc. SPIE 2006, 6366, 36607–36607. [Google Scholar]
- Kallio, K.; Attila, J.; Härmä, P.; Koponen, S.; Pulliainen, J.; Hyytiäinen, U.-M.; Pyhälahti, T. Landsat ETM+ images in the estimation of seasonal lake water quality in boreal river basins. Environ. Manag 2008, 42, 511–522. [Google Scholar]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Estimation of chlorophyll-a concentration in Case II waters using modis and meris data—Successes and challenges. Environ. Res. Lett 2009, 4, 045005. [Google Scholar]
- Siegel, D.A.; Wang, M.; Maritorena, S.; Robinson, W. Atmospheric correction of satellite ocean color imagery: The black pixel assumption. Appl. Opt 2000, 39, 3582–3591. [Google Scholar]
- Ferrier, G. Evaluation of Apparent Surface Reflectance Estimation Methodologies; Taylor & Francis: Abingdon, UK, 1995; Volume 16. [Google Scholar]
- Popp, T. Correcting atmospheric masking to retrieve the spectral albedo of land surfaces from satellite measurements. Int. J. Remote Sens 1995, 16, 3483–3508. [Google Scholar]
- Vermote, E.F.; Tanre, D.; Deuze, J.L.; Herman, M.; Morcette, J.J. Second simulation of the satellite signal in the solar spectrum, 6s: An overview. IEEE Trans. Geosci. Remote Sens 1997, 35, 675–686. [Google Scholar]
- Proud, S.R.; Rasmussen, M.O.; Fensholt, R.; Sandholt, I.; Shisanya, C.; Mutero, W.; Mbow, C.; Anyamba, A. Improving the smac atmospheric correction code by analysis of meteosat second generation ndvi and surface reflectance data. Remote Sens. Environ 2010, 114, 1687–1698. [Google Scholar]
- Smith, G.M.; Milton, E.J. The use of the empirical line method to calibrate remotely sensed data to reflectance. Int. J. Remote Sens 1999, 20, 2653–2662. [Google Scholar]
- Chavez, P.S. Image-Based Atmospheric Corrections: Revisited and Improved; American Society for Photogrammetry and Remote Sensing: Bethesda, MD, USA, 1996; Volume 62. [Google Scholar]
- Trishchenko, A.P.; Luo, Y.; Khlopenkov, K.V.; Park, W.M. Multi-Spectral Clear-Sky Composites of MODIS/Terra Land Channels (B1–B7) over Canada at 250 m Spatial Resolution and 10-Day Intervals since March, 2000: Top of the Atmosphere (TOA) Data; GeoGratis Client Services: Sherbrooke, QC, Canada, 2010. [Google Scholar]
- Giardino, C.; Bresciani, M.; Pilkaityte, R.; Bartoli, M.; Razinkovas, A. In situ measurements and satellite remote sensing of Case 2 waters: First results from the curonian lagoon. Oceanologia 2010, 52, 197–210. [Google Scholar]
- González Vilas, L.; Spyrakos, E.; Torres Palenzuela, J.M. Neural network estimation of chlorophyll a from meris full resolution data for the coastal waters of galician rias (NW Spain). Remote Sens. Environ 2011, 115, 524–535. [Google Scholar]
- Gordon, H.R.; Wang, M. Retrieval of water-leaving radiance and aerosol optical thickness over the oceans with seawifs: A preliminary algorithm. Appl. Opt 1994, 33, 443–452. [Google Scholar]
- Hu, C.; Muller-Karger, F.E.; Andrefouet, S.; Carder, K.L. Atmospheric correction and cross-calibration of Landsat-7/ETM+ imagery over aquatic environments: A multiplatform approach using SeaWifs/MODIS. Remote Sens. Environ 2001, 78, 99–107. [Google Scholar]
- Markham, B.L.; Barker, J.L.; Kaita, E.; Seiferth, J.; Morfitt, R. On-orbit performance of the Landsat-7 ETM+ radiometric calibrators. Int. J. Remote Sens 2003, 24, 265–285. [Google Scholar]
- Kahru, M.; Mitchell, B.G. Spectral reflectance and absorption of massive red tide off southern California. J. Geophys. Res 1998, 103, 21601–21609. [Google Scholar]
- Miller, P.I.; Shutler, J.D.; Moore, G.F.; Groom, S.B. SeaWifs discrimination of harmful algal bloom evolution. Int. J. Remote Sens 2006, 27, 2287–2301. [Google Scholar]
- Aiken, J.; Fishwick, J.R.; Lavender, S.J.; Barlow, R.; Moore, G.F.; Sessions, H.; Bernard, S.; Ras, J.; Hardman-Mountford, N.J. Validation of MERIS reflectance and chlorophyll during the bencal cruise october 2002: Preliminary validation of new demonstration products for phytoplankton functional types and photosynthetic parameters. Int. J. Remote Sens 2007, 30, 497–516. [Google Scholar]
- Kuster, T. Passive optical remote sensing of cyanobacteria and other intense phytoplancton blooms in coastal and inland waters. Int. J. Remote Sens 2009, 30, 4401–4425. [Google Scholar]
- Stumpf, R.P.; Arnone, R.A.; Gould, R.W.; Martinolich, V.; Tester, P.A.; Steward, R.G.; Subramaniam, A.; Culver, M.E.; Pennock, J.R. Seawifs Ocean Colour Data for US Southeast Coastal Waters. Proceeding of 6th Internaional Conference on Remote Sensing for Marine and Coastal Environment, Charleston, SC, USA, 1–3 May 2000; pp. 25–27.
- Kuster, T.; Metsamaa, L.; Vahtmae, E.; Strombeck, N. On suitablility of MODIS 250-m resolution band data for quntitative mapping cyanobacterial blooms. Proc. Estonian Acad. Sci. Biol. Ecol 2006, 55, 318–529. [Google Scholar]
- Reinart, A.; Kuster, T. Comparison of different satellite sensors in detecting cyanobacterial bloom events in the Baltic Sea. Remote Sens. Environ 2006, 102, 74–85. [Google Scholar]
- Hu, C.; He, M. Origin and offshore extent of floating algae in olympic sailing area. Eos Trans. AGU 2008, 89, 302. [Google Scholar]
- Hu, C.; Lee, Z.; Ma, R.; Yu, K.; Li, D.; Shang, S. Moderate resolution imaging spectroradiometer (MODIS) observations of cyanobacteria blooms in Taihu Lake, China. J. Geophys. Res 2010, 115, 20. [Google Scholar]
- Gordon, H.R.; Brown, O.B.; Evans, R.H.; Brown, J.W.; Smith, R.C.; Baker, K.S.; Clark, D.K. A semianalytic radiance model of ocean color. J. Geophys. Res 1988, 93, 10909. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol 2003, 160, 271–282. [Google Scholar]
- Gitelson, A.A.; Dall’Olmo, G. Effect of bio-optical parameter variability on the remote estimation of chlorophyll-a concentration in turbid productive waters: Experimental results. Appl. Opt 2005, 44, 412–422. [Google Scholar]
- Babin, M.; Stramski, D. Light absorption by aquatic particles in the near-infrared spectral region. Limnol. Oceanogr 2002, 47, 911–915. [Google Scholar]
- Gons, H.J. Optical teledetection of chlorophyll a in turbid inland waters. Environ. Sci. Technol 1999, 33, 1127–1132. [Google Scholar]
- Liu, W.; Liu, Y.L.; Chris, M.; Wu, G. Monitoring variation of water turbidity and related environmental factors in Poyang Lake National Nature Reserve, China. Proc. SPIE 2007, 6754, 67541H. [Google Scholar]
- Luciani, X.; Pierre, C.; Frédéric, T.; David, B.; André, B.; Stéphane, M.; Claude, J. Analyse numérique des spectres de 3d issus de mélanges non linéaires. Sud Toulon Var. 2007. [Google Scholar]
- Krause, P.; Boyle, D.P.; Bäse, F. Comparison of different efficiency criteria for hydrological model assessment. Adv. Geosci 2005, 5, 89–97. [Google Scholar]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management; WHO. E & FN Spon: London, UK, 1999; p. 416. [Google Scholar]
- Watson, S.B.; Mc-Cauley, E.; Downing, J.A. Patterns in Phytoplankton Taxonomic Composition across Temperate Lakes of Differing Nutrient Status; American Society of Limnology and Oceanography: Waco, TX, USA, 1997; p. 42. [Google Scholar]





| Primary Use | Band | Bandwidth (nm) | Spatial Resolution (m) |
|---|---|---|---|
| Land/Cloud/Aerosols Boundaries | 1 | 620–670 | 250 |
| 2 | 841–876 | 250 | |
| Land/Cloud/Aerosols properties | 3 | 459–479 | 500 |
| 4 | 545–565 | 500 | |
| 5 | 1230–1250 | 500 | |
| 6 | 1628–1652 | 500 | |
| 7 | 2105–2155 | 500 | |
| Indexes for Model Evaluation | APPEL | Kahru | FAI | Gitelson |
|---|---|---|---|---|
| R2 | 0.93 | 0.91 | 0.89 | 0.79 |
| Nr | 0.97 | 0.95 | 0.91 | 0.34 |
| RMESr (%) | 69 | 83 | 116 | 307 |
| BIASr (%) | −17 | −23 | −32 | −87 |
| Indexes for Model Evaluation | APPEL | Kahru | FAI | Gitelson |
|---|---|---|---|---|
| R2 | 0.95 | 0.93 | 0.94 | 0.77 |
| Nr | 0.927 | 0.88 | 0.934 | 0.30 |
| RMESr (%) | 61 | 80 | 58 | 189 |
| BIASr (%) | 1 | −2 | 4 | −28 |
| Indexes for Model Evaluation | APPEL | Kahru | FAI | Gitelson |
|---|---|---|---|---|
| R2 | 0.30 | 0.18 | 0.15 | 0.11 |
| Nr | 0.62 | 0.49 | 0.34 | −7.87 |
| RMSEr (%) | 71 | 82 | 132 | 341 |
| BIASr (%) | –23 | −30 | −47 | −110 |
Share and Cite
El-Alem, A.; Chokmani, K.; Laurion, I.; El-Adlouni, S.E. Comparative Analysis of Four Models to Estimate Chlorophyll-a Concentration in Case-2 Waters Using MODerate Resolution Imaging Spectroradiometer (MODIS) Imagery. Remote Sens. 2012, 4, 2373-2400. https://doi.org/10.3390/rs4082373
El-Alem A, Chokmani K, Laurion I, El-Adlouni SE. Comparative Analysis of Four Models to Estimate Chlorophyll-a Concentration in Case-2 Waters Using MODerate Resolution Imaging Spectroradiometer (MODIS) Imagery. Remote Sensing. 2012; 4(8):2373-2400. https://doi.org/10.3390/rs4082373
Chicago/Turabian StyleEl-Alem, Anas, Karem Chokmani, Isabelle Laurion, and Sallah E. El-Adlouni. 2012. "Comparative Analysis of Four Models to Estimate Chlorophyll-a Concentration in Case-2 Waters Using MODerate Resolution Imaging Spectroradiometer (MODIS) Imagery" Remote Sensing 4, no. 8: 2373-2400. https://doi.org/10.3390/rs4082373
APA StyleEl-Alem, A., Chokmani, K., Laurion, I., & El-Adlouni, S. E. (2012). Comparative Analysis of Four Models to Estimate Chlorophyll-a Concentration in Case-2 Waters Using MODerate Resolution Imaging Spectroradiometer (MODIS) Imagery. Remote Sensing, 4(8), 2373-2400. https://doi.org/10.3390/rs4082373






