Climate Drivers of Pine Shoot Beetle Outbreak Dynamics in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Response Variable
2.2.1. Landsat Data
2.2.2. Field Data
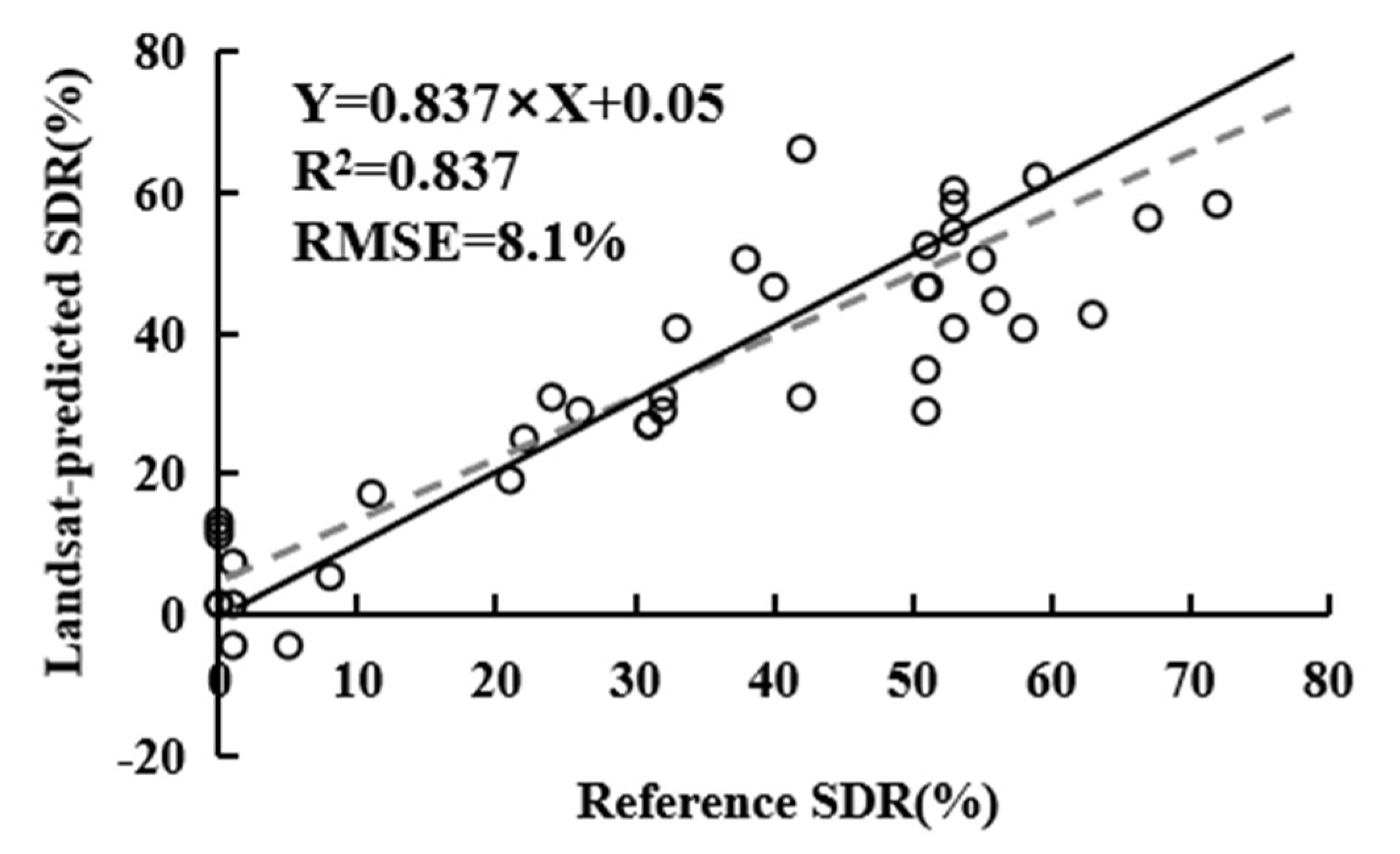
2.2.3. Statistical Modeling of Percent Shoot Damage Ratios
2.3. Explanatory Variables
2.4. Statistical Analysis
3. Results
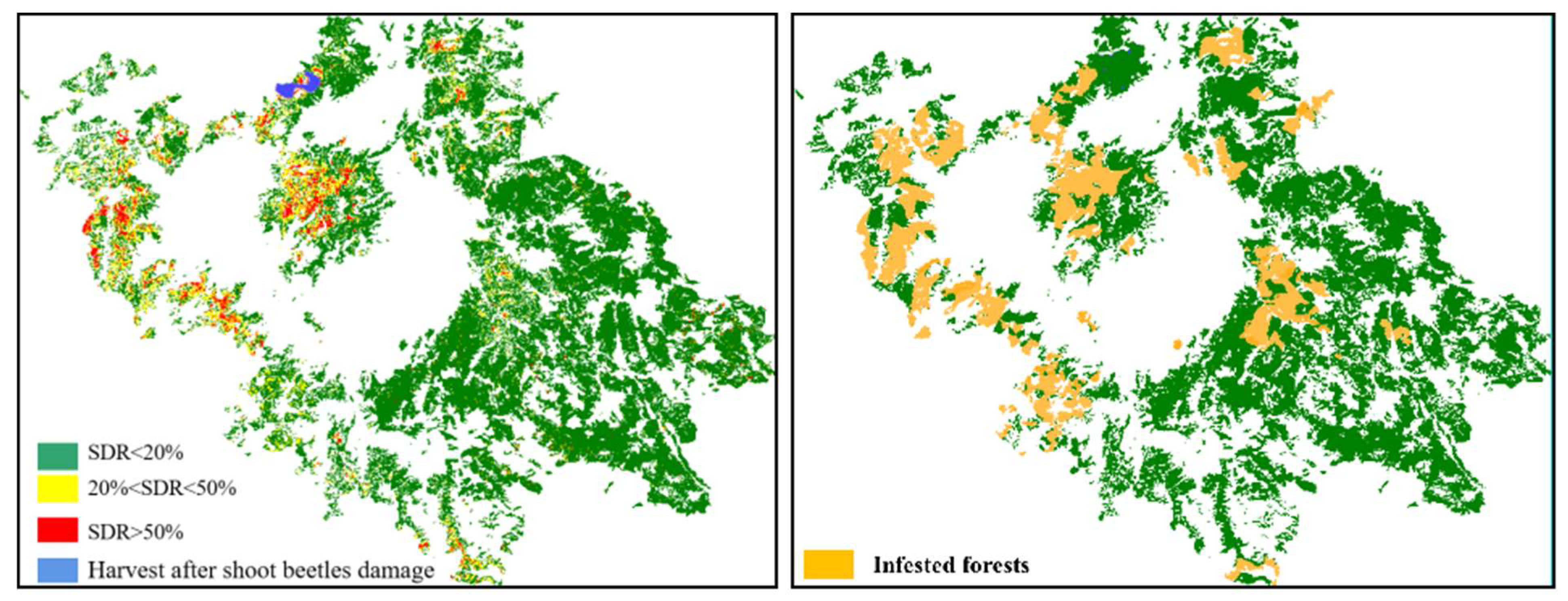
3.1. Changes in Beetle-Infested Total Forest Area from 2000 to 2017
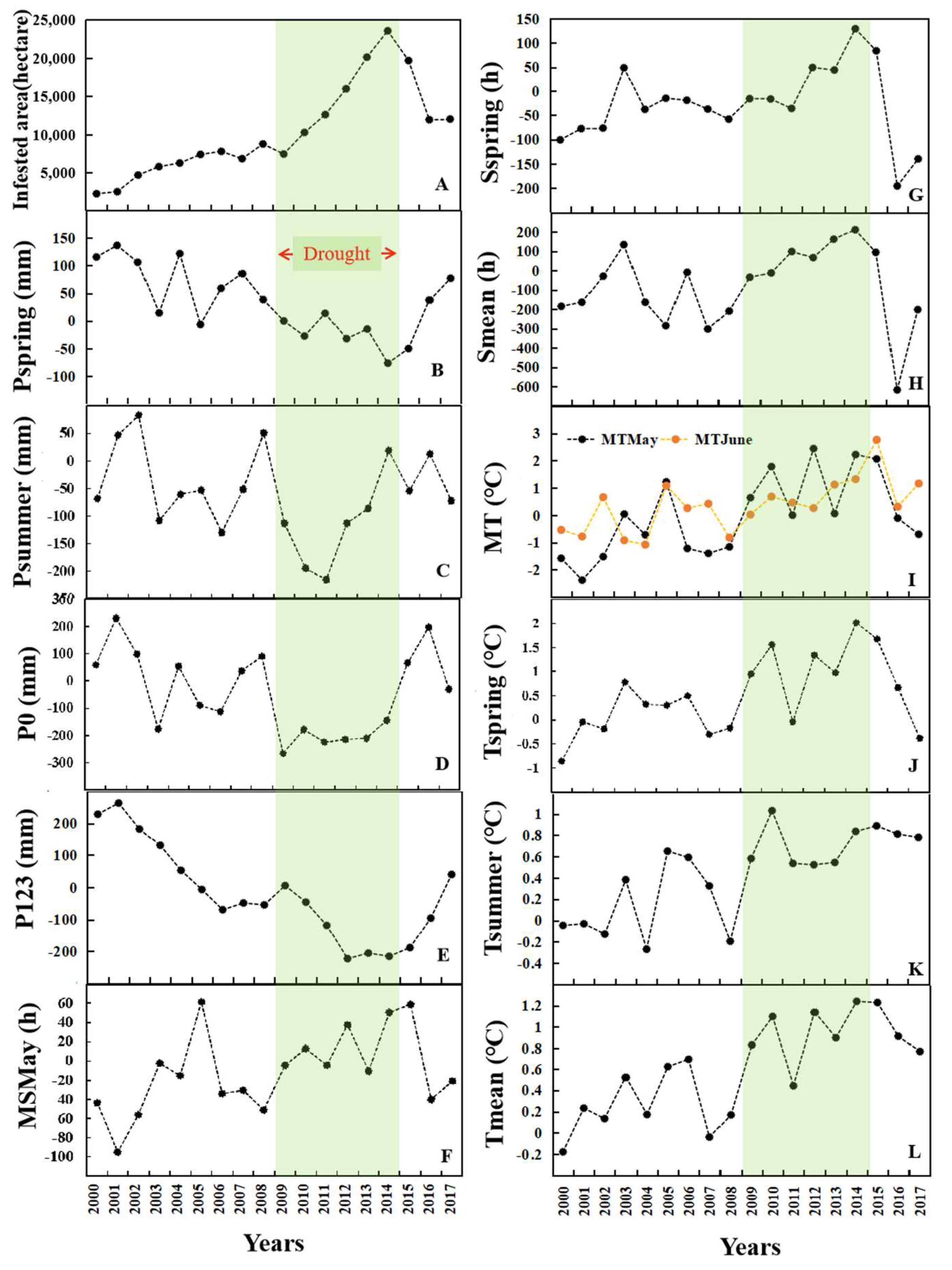
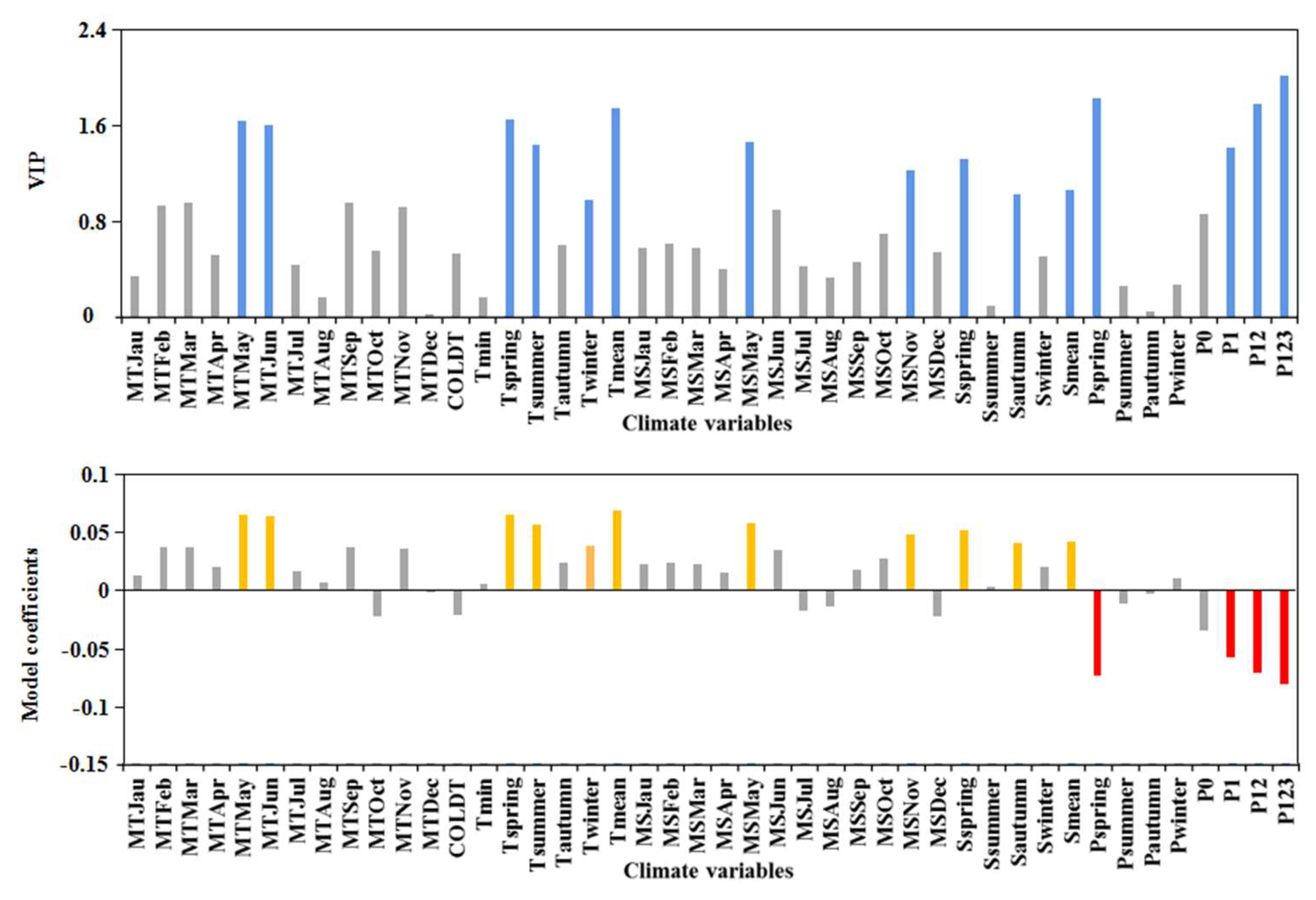
3.2. Responses of Pine Shoot Beetle Outbreaks to Climate Variation from 2000 to 2017
4. Discussion
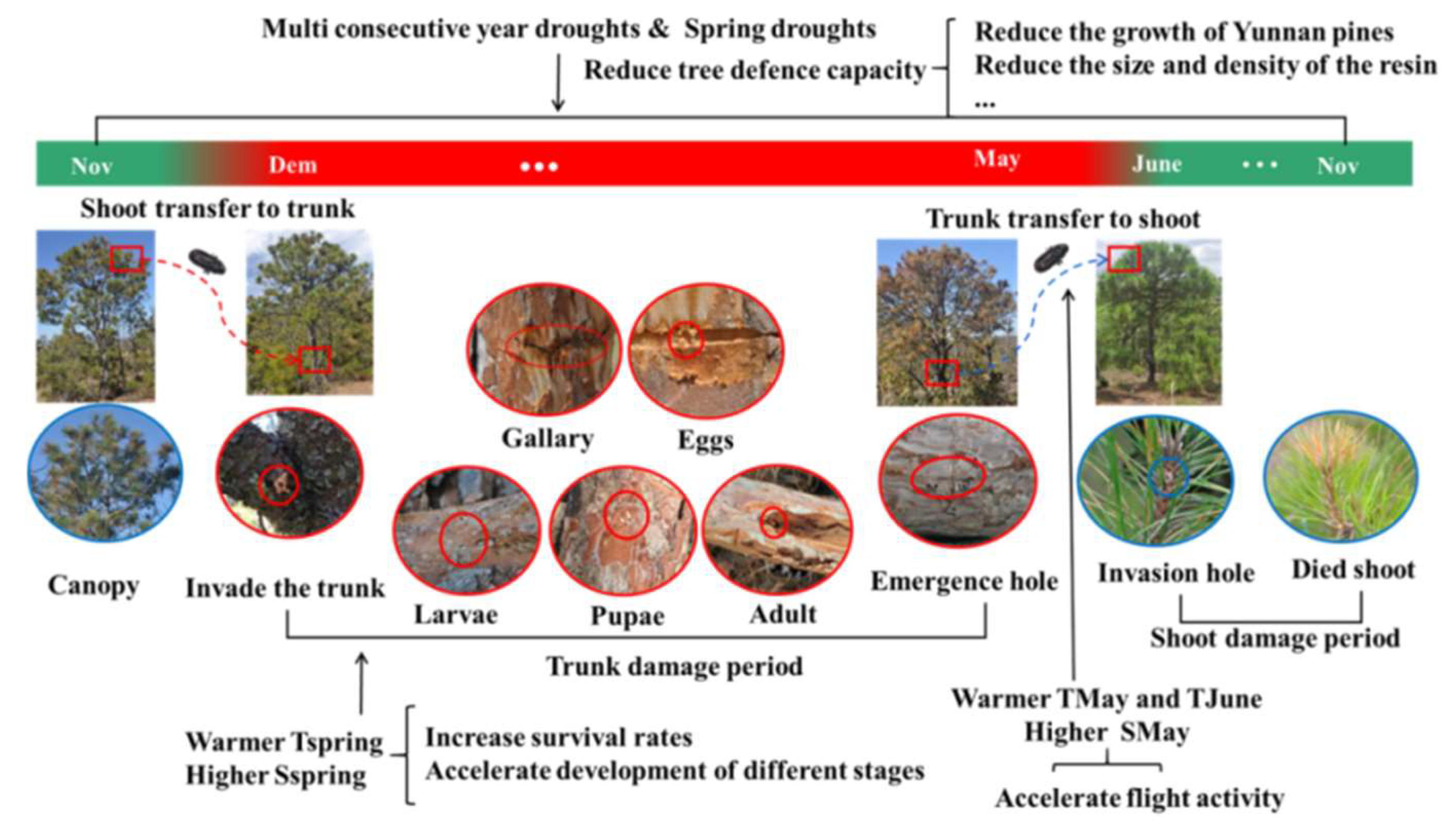
4.1. Influence of Drought on Pine Shoot Beetle Outbreaks
4.2. Influence of Warmer Temperature and Higher Solar Radiation Duration on Pine Shoot Beetle Outbreaks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, F.S.; Wan, G.H.; Pi, W.L. Studies on the geographical provenance of Pinus yunnanensis I. Seeding test. Acta Bot. Yunnanica 1987, 9, 427–435. [Google Scholar]
- Jin, Z.Z.; Peng, J. Pinus yunnanensis; Yunnan Science and Technology Publishing Press: Kunming, China, 2004; pp. 5–13. [Google Scholar]
- Dai, K.J.; He, F.; Shen, Y.X.; Zhou, W.J.; Li, Y.P.; Tang, L. Advances in the research on Pinus yunnanensis forest. J. Cent. South For. Univ. 2006, 26, 138–142. [Google Scholar]
- Ye, H. On the bionomy of Tomicus piniperda (L.) (Col., Scolytidae) in the Kunming region of China. J. Appl. Entomol. 1991, 112, 366–369. [Google Scholar]
- Duan, Y. Genetic Structuration and Host Tree Preference of T. piniperda Populations in Southwestern China, with Comparison to the French Population from Scots Pine. Ph.D. Thesis, Yunnan University, Kunming, China, 2003. [Google Scholar]
- Ji, M.; Dong, X.Q.; Liu, H.P.; Li, L.S.; Xu, H.; Yang, X.P.; Li, H.R.; Ze, S.Z. Preliminary study on remote sensing detection of yunnan pine forest damaged by Tomicus piniperda. J. West China For. Sci. 2007, 36, 87–90. [Google Scholar]
- Ye, H.; Li, L.S. The distribution of Tomicus piniperda (L.) population in the crown of Yunnan pine during the shoot feeding period. Acta Entomol. Sin. 1994, 37, 311–316. [Google Scholar]
- Ye, H. Studies on the biology of Tomicus piniperda (Col., Scolytidae) in the shoot-feeding period. Acta Entomol. Sin. 1996, 39, 58–62. [Google Scholar]
- Ye, H.; Lv, J.; Lieutier, F. On the bionomics of Tomicus minor (Hartig) (Col., Scolytidae) in Yunnan Province. Acta Entomol. Sin. 2004, 47, 223–228. [Google Scholar]
- Li, L.S.; Wang, H.L.; Chai, X.S.; Wang, Y.X.; Shu, N.B.; Yang, D.S. Study on the biological characteristics of Tomicus piniperda and its damage. Yunnan For. Technol. 1997, 2, 4–10. [Google Scholar]
- Hui, Y.C.; Lieutier, F. Shoot aggregation by Tomicus piniperda L. (Coleoptera: Scolytidae). Southwest. China Ann. Sci. For. 1997, 54, 635–641. [Google Scholar]
- Langstrom, B.; Li, L.S.; Liu, H.P.; Cao, P.; Liu, H.R.; Hellqvist, C.; Lieutier, F. Shoot feeding ecology of Tomicus piniperda, and T. minor, (col. scolytidae) in southern China. J. Appl. Entomol. 2002, 126, 333–342. [Google Scholar] [CrossRef]
- Shen, S.W.; Luo, Y.Q.; Yu, L.F.; Lu, W.J.; Han, X.G.; Ren, L.L. Temporal and spatial niches of two sympatric Tomicus species pests of Pinus yunnanensis Faranch. Chin. J. Appl. Entomol. 2018, 55, 279–287. [Google Scholar]
- Lieutier, F.; Ye, H.; Yart, A. Shoot damage by Tomicus sp. (Coleoptera: Scolytidae) and effect on Pinus yunnanensis resistance to subsequent reproductive attacks in the stem. Agric. For. Entomol. 2003, 5, 227–233. [Google Scholar] [CrossRef]
- Seppälä, R. Making Forests Fit for Climate Change: A Global View of Climate-Change Impacts on Forests and People and Options for Adaptation; Development Communication, Ministry for Foreign Affairs of Finland: Helsinki, Finland, 2009. [Google Scholar]
- Littell, J.S.; Oneil, E.E.; Mckenzie, D.; Hicke, J.A.; Lutz, J.A.; Norheim, R.A.; Elsner, M.M. Forest ecosystems, disturbance, and climatic change in washington state, USA. Clim. Chang. 2010, 102, 129–158. [Google Scholar] [CrossRef] [Green Version]
- Byron, J.C.; Charles, C.R.; Robert, M.H.; Michael, A.B. Tree regeneration and future stand development after bark beetle infestation and harvesting in Colorado lodgepole pine stands. For. Ecol. Manag. 2011, 261, 2168–2175. [Google Scholar]
- Stadelmann, G.; Bugmann, H.; Wermelinger, B.; Meier, F.; Bigler, C. A predictive framework to assess spatio-temporal variability of infestations by the European spruce bark beetle. Ecography 2013, 36, 1208–1217. [Google Scholar] [CrossRef]
- Bearup, L.A.; Maxwell, R.M.; Clow, D.W.; Mccray, J.E. Hydrological effects of forest transpiration loss in bark beetle-impacted watersheds. Nat. Clim. Chang. 2014, 4, 481–486. [Google Scholar] [CrossRef]
- Kamal, J.K.G.; Chelsea, N.M.; Paula, J.F.; John, M.F. Bark beetle outbreaks alter biotic components of forested ecosystems. Bark Beetle Manag. Ecol. Clim. Chang. 2022, 227–259. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- Rouault, G.; Candau, J.-N.; Lieutier, F.; Nageleisen, L.-M.; Martin, J.-C.; Warzee, N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann. For. Sci. 2006, 63, 613–624. [Google Scholar] [CrossRef]
- Bentz, B.J.; Jacques, R.; Fettig, C.J.; Matthew, H.E.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the Western United States and Canada: Direct and indirect effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Logan, J.A.; Macfarlane, W.W.; Willcox, L. Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the Greater Yellowstone ecosystem. Ecol. Appl. 2010, 20, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.J.; Hood, S.; Lichstein, J.W.; Macalady, K.; McDowell, N.G.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Hicke, J.A.; Meddens, A.H.H.; Kolden, C.A. Recent tree mortality in the western United States from bark beetles and forest fires. For. Sci. 2016, 62, 141–153. [Google Scholar] [CrossRef]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Weed, A.S. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For. Ecol. Manag. 2016, 380, 321–334. [Google Scholar] [CrossRef]
- Davidson, J. The relationship between temperature and rate of development of insects at constant temperatures. J. Anim. Ecol. 1944, 13, 26–38. [Google Scholar] [CrossRef]
- Bentz, B.J.; Logan, J.A.; Amman, G.D. Temperature-dependent development of the mountain pine-beetle (Coleoptera, Scolytidae) and simulation of its phenology. Can. Entomol. 1991, 123, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, N.; Raworth, D. Insects and temperature—A general theory. Can. Entomol. 1996, 128, 1–13. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Charnov, E.L.; West, G.B.; Savage, V.M.; Brown, J.H. Effects of size and temperature on developmental time. Nature 2002, 417, 70–73. [Google Scholar] [CrossRef]
- Chown, S.L.; Nicolson, S.W. Insect Physiological Ecology. In Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Altermatt, F. Climatic warming increases voltinism in european butterflies and moths. Proc. R. Soc. B: Biol. Sci. 2010, 277, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- Wermelinger, B.; Rigling, A.; Mathis, D.S.; Kenis, M.; Gossner, M.M. Climate change effects on trophic interactions of bark beetles in inner alpine scots pine forests. Forests 2021, 12, 136. [Google Scholar] [CrossRef]
- Robbins, Z.J.; Xu, C.; Aukema, B.H.; Buotte, P.C.; Chitra-Tarak, R.; Fettig, C.J.; Goulden, M.L.; Goodsman, D.W.; Hall, A.D.; Koven, C.D. Warming increased bark beetle-induced tree mortality by 30% during an extreme drought in California. Glob. Chang. Biol. 2022, 28, 509–523. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; Mcnulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J. Climate change and forest disturbances climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. Bioscience 2001, 51, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Lange, H.; Økland, B.; Krokene, P. Thresholds in the life cycle of the spruce bark beetle under climate change. Inter. J. Complex Syst. 2006, 1648, 1–10. [Google Scholar]
- Wermelinger, B.; Seifert, M. Analysis of the temperature dependent development of the spruce bark beetle Ips typographus (L.) (Col., Scolytidae). J. Appl. Entomol. 1998, 122, 185–191. [Google Scholar] [CrossRef]
- Ungerer, M.J.; Ayres, M.P.; Lombardero, M.J. Climate and the northern distribution limits of Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). J. Biogeogr. 1999, 26, 1133–1145. [Google Scholar] [CrossRef] [Green Version]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Régnière, J.; Bentz, B.J. Modeling cold tolerance in the mountain pine beetle, Dendroctonus ponderosae. Insect Physiol. 2007, 53, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Aukema, B.H.; Erbilgin, N.; Klepzig, K.D.; Wallin, K.F. Interactions among conifer terpenoids and bark beetles across multiple levels of scale: An attempt to understand links between population patterns and physiological processes. Recent Adv. Phytochem. 2005, 39, 80–118. [Google Scholar]
- Hebertson, E.G.; Jenkins, M.J. Climate factors associated with historic spruce beetle (Coleoptera: Curculionidae) outbreaks in Utah and Colorado. Environ. Entomol. 2008, 37, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Negron, J.F.; Popp, J.B. The flight periodicity, attack patterns, and life history of D. confusus confusus Swaine Coleoptera: Curculionidae: Scolytinae), the western balsam bark beetle, in north central Colorado. West. N. Am. Natural. 2009, 69, 447–458. [Google Scholar] [CrossRef]
- Hart, S.J.; Veblen, T.T.; Mietkiewicz, N.; Kulakowski, D. Negative feedbacks on bark beetle outbreaks: Widespread and severe spruce beetle infestation restricts subsequent infestation. PLoS ONE 2015, 10, e0127975. [Google Scholar] [CrossRef] [Green Version]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Kane, J.M.; Kolb, T.E. Importance of resin ducts in reducing ponderosa pine mortality from bark beetle attack. Oecologia 2010, 164, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.P.; Allen, C.D.; Millar, C.I.; Swetnam, T.W.; Michaelsen, J.; Still, C.J.; Leavitt, S.W. Forest responses to increasing aridity and warmth in the southwestern united states. Proc. Natl. Acad. Sci. USA 2010, 107, 21289–21294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffa, K.F.; Smalley, E.B. Interaction of pre-attack and induced monoterpene concentrations in host conifer defense against bark beetle fungal complexes. Oecologia 1995, 102, 285–295. [Google Scholar] [CrossRef]
- Kichas, N.E.; Hood, S.M.; Pederson, G.T.; Everett, R.G.; McWethy, D.B. Whitebark pine (Pinus albicaulis) growth and defense in response to mountain pine beetle outbreaks. For. Ecol. Manag. 2020, 457, 117736. [Google Scholar] [CrossRef]
- Amman, G.D.; Cole, W.E. Mountain pine beetle dynamics in lodgepole pine forests. In Part II: Population Dynamics. USDA Forest Service General Technical Report GTR-INT-145; Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1983. [Google Scholar]
- Wermelinger, B. Ecology and management of the spruce bark beetle, Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Arias, M.; Robertson, L.; Garcia, A.; Arcos, S.C.; Escuer, M.; Sanz, R.; Mansilla, J.P. Bursaphelenchus fungivorus (Nematoda: Aphelenchida) associated with Orthotomicus erosus (Coleoptera: Scolitydae) in Spain. For. Pathol. 2010, 35, 375–383. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Linares, J.C.; Camarero, J.J. Reduced growth sensitivity to climate in bark-beetle infested Aleppo pines: Connecting climatic and biotic drivers of forest dieback. For. Ecol. Manag. 2015, 357, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Pernek, M.; Matošević, D.; Lacković, N.; Cota, E. When a native species behaves like invasive—Bark beetle Orthotomicus erosus in the Mediterranean pine forest in Croatia. In Proceedings of the 3rd Croatian Symposium on Invasive Species/Jelaska, Zagreb, Croatia, 26–27 November 2018. [Google Scholar]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Langstrom, B. Life cycles and shoot-feeding of pine shoot beetles. Studia For. Suec. 1983, 163, 1–29. [Google Scholar]
- Schroeder, L.M.; Lindelöw, Å. Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J. Chem. Ecol. 1989, 15, 807–817. [Google Scholar] [CrossRef]
- Schroeder, L.M. Attraction of the bark beetle Tomicus piniperda to Scots pine trees in relation to tree vigor and attack density. Entomol. Exp. Appl. 2011, 44, 53–58. [Google Scholar] [CrossRef]
- Haack, R.A.; Kucera, D. New Introduction–Common Pine Shoot Beetle, Tomicus piniperda L.; Northeastern Area; Pest Alert NATP-05–93; U.S. Department Agricultural Forest Service (USDA): Washington, DC, USA, 1993. [Google Scholar]
- Haack, R.A.; Lawrence, R.K.; Heaton, G.C. Seasonal shoot-feeding by Tomicus piniperda (Coleoptera: Scolytidae) in Michigan. Great Lakes Entomol. 2000, 33, 1–8. [Google Scholar]
- Haack, R.A.; Lawrence, R.K.; Heaton, G.C. Tomicus piniperda (Coleoptera:Scolytidae) shoot-feeding characteristics and overwintering behavior in Scots pine christmas trees. J. Econ. Entomol. 2001, 94, 422–429. [Google Scholar] [CrossRef]
- Horn, A.; Kerdelhué, C.; Lieutier, F.; Rossi, J.P. Predicting the distribution of the two bark beetles Tomicus destruens and Tomicus piniperda in Europe and the Mediterranean region. Agric. For. Entomol. 2012, 14, 358–366. [Google Scholar] [CrossRef]
- Bakke, A. Ecological studies on bark beetles (Coleoptera: Scolytidae) associated with Scots Pine (Pinus sylvestris L.) in Norway with particular reference to the influence of temperature. Medd. Nor. Skogforsok 1968, 21, 443–602. [Google Scholar]
- Salonen, K. On the life-cycle, especially on the reproduction biology of Blastopbagus piniperda, L. (Col., Scolytidae). Acta For. Fenn. 1973, 127, 1–72. [Google Scholar]
- Langstrom, B. Wind-thrown Scots pines as brood material for Tomicus piniperda and T minor. Silva Fenn. 1984, 18, 187–198. [Google Scholar]
- Yu, L.F.; Huang, J.X.; Zong, S.X.; Huang, H.G.; Luo, Y.Q. Detect shoot-beetle damages of Yunnan Pine using Landsat time-series data. Forests 2018, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.F.; Zhan, Z.Y.; Ren, L.L.; Liu, Y.J.; Huang, H.G.; Luo, Y.Q. Effects of stand and landscape level variables on shoot damage ratios caused by shoot beetles in Southwest China. For. Ecol. Manag. 2022, 507, 120030. [Google Scholar] [CrossRef]
- Saarenmaa, H. Within-tree population dynamics models for integrated management of Tomicus piniperda (coleoptera, scolytidae). Commun. Inst. For. Fenn. 1985, 118, 40. [Google Scholar]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Drought-Induced Multifactor Decline of Scots Pine in the Pyrenees and Potential Vegetation Change by the Expansion of Co-occurring Oak Species. Ecosystems 2010, 13, 978–991. [Google Scholar] [CrossRef]
- Li, L.S.; Wang, H.L.; Cai, X.S.; Yang, D.S. Study on Relationship between Damage of Tomicus piniperda and Its Environment. Yunnan For. Sci. Technol. 1997, 2, 11–16. [Google Scholar]
- Huai, K.Y.; Li, L.S.; Zhou, N.; Zhu, G.S. Study on Forecasting Occurring Time of Tomicus piniperda with the Phenological Phase of Pinus yunnanensis. Yunnan For. Sci. Technol. 1997, 2, 72–77. [Google Scholar]
- Guo, L.; Dai, J.; Ranjitkar, S.; Xu, J.; Luedeling, E. Response of chestnut phenology in China to climate variation and change. Agric. For. Meteorol. 2013, 180, 164–172. [Google Scholar] [CrossRef]
- Wold, S.; Albano, C.; Dunn, W.J.; Esbensen, K.; Johansson, E. Modelling data tables by principal components and pls: Class patterns and quantitative predictive relations. Analusis 1989, 12, 477–485. [Google Scholar]
- Wehrens, R.; Mevik, B.H. The pls package: Principal component and partial least squares regression in r. J. Stat. Softw. 2007, 18, 1–24. [Google Scholar]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Luedeling, E.; Gassner, A. Partial least squares regression for analyzing walnut phenology in California. Agric. For. Meteorol. 2012, 158–159, 43–52. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kramer, D. Using Cellular Automata to Model Southern Pine Beetle Regional Patterns. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 1993. [Google Scholar]
- Gumpertz, M.L.; Wu, C.-t.; Pye, J.M. Logistic regression for southern pine beetle outbreaks with spatial and temporal autocorrelation. For. Sci. 2000, 46, 95–107. [Google Scholar]
- Duehl, A.J.; Koch, F.H.; Hain, F.P. Southern pine beetle regional outbreaks modeled on landscape, climate and infestation history. For. Ecol. Manag. 2011, 261, 473–479. [Google Scholar] [CrossRef]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Meyer, C.W. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preisler, H.K.; Hicke, J.A.; Ager, A.A.; Hayes, J.L. Climate and weather influences on spatial temporal patterns of mountain pine beetle populations in Washington and Oregon. Ecology 2012, 93, 2421–2434. [Google Scholar] [CrossRef]
- Seidl, R.; Müller, J.; Hothorn, T.; Bässler, C.; Heurich, M.; Kautz, M. Small beetle, large-scale drivers: How regional and landscape factors affect outbreaks of the European spruce bark beetle. J. Appl. Ecol. 2015, 53, 530–540. [Google Scholar] [CrossRef]
- Buotte, P.C.; Hickem, J.A.; Preisler, H.K.; Abatzoglou, J.T.; Raffa, K.F.; Logan, J.A. Climate influences on whitebark pine mortality from mountain pine beetle in the Greater Yellowstone Ecosystem. Ecol. Appl. 2016, 26, 2505–2522. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, M.L.; Kolb, T.E.; Pockman, W.T.; Plaut, J.A.; Yepez, E.A.; Macalady, A.K.; Pangle, R.E.; McDowell, N.G. Drought predisposes pinon-juniper woodlands to insect attacks and mortality. New Phytol. 2013, 198, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, M.L.; Kolb, T.E.; Wallin, K.F.; Wagner, M.R. Seasonal dynamics of tree growth, physiology, and resin defenses in a Northern Arizona Ponderosa Pine Forest. Can. J. For. Res. 2007, 37, 1173–1183. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Ye, H.; Dang, C.L. Study on the feature of the bark beetle (Blastophagus piniperdal) injuring the pine (Pinus yunnanensis). J. Yunnan Univ. 1986, 8, 218–222. [Google Scholar]
- Lieutier, F.; Garcia, J.; Yart, A. Wound reactions of Scots pine (Pinus sylvestris L.) to attacks by Tomicus piniperda L. and Ips sexdentatus Boern. (Col., Scolytidae). J. Appl. Ent. 1995, 9, 591–600. [Google Scholar] [CrossRef]
- Cao, Y.X.; Zhang, Z.; Liu, S.C.; Li, L.Z.; Li, D.M. Advances in the research on the resistance of conifer resin to pests. Chin. Bull. Entomol. 2007, 44, 804–810. [Google Scholar]
- Schroeder, L.M.; Eidmann, H.H. Gallery initiation by Tomicus piniperda (Coleoptera: Scolytidae) on scots pine trees baited with host volatiles. J. Chem. Ecol. 1987, 13, 1591–1599. [Google Scholar] [CrossRef]
- Yin, H.F.; Huang, F.S.; Li, Z.L. Economic Entomology of China (Volume 29): Coleoptera (Coleoptera); Science Press: Beijing, China, 1984. [Google Scholar]
- Wu, X.B. Analysis of Climate Factors Influencing the Outbreak of Tomicus minor Hartig in Ninghua County, Fujian Province. J. Green Sci. Technol. 2012, 7, 55–57. [Google Scholar]
- Li, Y. Causes of Tomicus piniperda, L. Disaster in Yuxi of Yunnan Province and Countermeasures of Prevention and Control. For. Resour. Manag. 2013, 3, 40–42. [Google Scholar]
- Su, J.J. Forests Response to Drought over the Yunnan-Guizhou Plateau; Lanzhou University: Lanzhou, China, 2021. [Google Scholar]
- Lévesque, M.; Rigling, A.; Bugmann, H.; Weber, P.; Brang, P. Growth response of five co-occurring conifers to drought across a wide climatic gradient in central Europe. Agric. For. Meteorol. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- Chen, Y. Drought Study in the Central and Western Yunnan by Tree-Ring Proxies; Lanzhou University: Lanzhou, China, 2017. [Google Scholar]
- Yang, R.Q.; Fan, Z.X.; Li, Z.S.; Wen, Q.Z. Radial growth of Pinus yunnanensis at different elevations and their responses to climatic factors in the Yulong Snow Mountain, Northwest Yunnan, China. Acta Ecol. Sin. 2018, 38, 8983–8991. [Google Scholar]
- Wang, S.J.; Chen, Y.P.; Chen, F.; Zhang, H.L. Responses of radial growth of Pinus yunnanensis to climatic and hydrological factors at different altitudes in Western Yunnan, China. Chin. J. Appl. Ecol. 2021, 32, 3557–3566. [Google Scholar]
- Liu, H.P.; Jiang, Z.L.; Li, L.S.; Zhao, X.G.; Liang, X.Y.; Ma, S.Z.; Jiang, Y.H. Study on Forecasting the Occurring Quantity of Adults of Tomicus piniperda. Yunnan For. Sci. Technol. 1997, 2, 60–69. [Google Scholar]
- Ye, H. The influence of temperature and light on the taking-off of Tomicus piniperda. Entomol. Knowl. 2000, 37, 342–344. [Google Scholar]
- Liu, H.; Zhang, Z.; Ye, H.; Wang, H.; Clarke, S.R.; Lu, J. Response of Tomicus yunnanensis (Coleoptera: Scolytinae) to infested and uninfested Pinus yunnanensis bolts. J. Econ. Entomol. 2010, 103, 95–100. [Google Scholar] [CrossRef]
- Lü, J.; Hu, S.J.; Ma, X.Y.; Chen, J.M.; Li, Q.Q.; Ye, H. Origin and expansion of the yunnan shoot borer, Tomicus yunnanensis (Coleoptera: Scolytinae): A mixture of historical natural expansion and contemporary human-mediated relocation. PLoS ONE 2014, 9, e111940. [Google Scholar] [CrossRef] [Green Version]
- Gokturk, A. Use of pheromone traps against Tomicus piniperda and Tomicus minor in the Kazbegi National Park, Georgian Republic. Afr. J. Agr. Res. 2011, 6, 2430–2435. [Google Scholar]
- Chen, P.; Zhou, N.; Zhao, T.; Hu, G.H.; Feng, Z.W.; Li, L.S. Relationship between Stand Status of Pinus yunnanensis and Damage of Tomicus piniperda. J. Northeast. For. Univ. 2004, 32, 13–15. [Google Scholar]
- Wang, H.L.; Chen, S.W.; Wu, Y.; Pu, M.G. Preliminary studies on the bionomics and management of the pine bark beetle (Blastophagus piniperdal L.) in Kunming district, China. J. Southwest For. Coll. 1987, 2, 33–42. [Google Scholar]
- Li, L.S.; Liu, H.P.; Zhou, N.J.; Zhao, L.; Huai, K.Y.; Lang, N.J.; Wang, H.L.; Wang, H.Q. Study on the Forecasting Method for Tomicus piniperda. Yunnan For. Sci. Technol. 1997, 2, 44–56. [Google Scholar]
- Liu, H.P.; Jiang, Z.L.; Zhao, X.G.; Liang, X.Y.; Ma, S.Z.; Jiang, Y.H. Study on Forecasting the Flying Period of Adults of Tomicus piniperda. Yunnan For. Sci. Technol. 1997, 2, 70–74. [Google Scholar]
- Zhu, Z.H. Present Condition of Population Ecological Study on Tomicus piniperda of Pinus yunnanensis. Yunnan For. Sci. Technol. 2003, 1, 52–55. [Google Scholar]
- Anderbrant, O. Reemergence and second brood in the bark beetle Ips typographus. Holarct. Ecol. 1989, 12, 494–500. [Google Scholar] [CrossRef]





| Year | Month/Day | Julian Day of Year | Sensor | Cloud Cover | |
|---|---|---|---|---|---|
| 1 | 2000 | November/16 | 321 | ETM+ | 6% |
| 2 | 2001 | December/05 | 339 | ETM+ | 1% |
| 3 | 2002 | December/24 | 358 | ETM+ | 0% |
| 4 | 2003 | November/17 | 321 | TM5 | 0% |
| 5 | 2004 | November/11 | 316 | ETM+ | 0% |
| 6 | 2005 | November/6 | 310 | TM5 | 0% |
| 7 | 2006 | November/17 | 321 | ETM+ | 0% |
| 8 | 2007 | November/20 | 324 | ETM+ | 0% |
| 9 | 2008 | November/14 | 319 | TM5 | 3% |
| 10 | 2009 | November/09 | 313 | ETM+ | 0% |
| 11 | 2010 | December/14 | 348 | ETM+ | 4% |
| 12 | 2011 | November/15 | 319 | ETM+ | 14% |
| 13 | 2012 | November/01 | 306 | ETM+ | 13% |
| 14 | 2013 | November/12 | 316 | OLI | 1.65% |
| 15 | 2014 | November/23 | 327 | ETM+ | 3% |
| 16 | 2015 | December/12 | 346 | ETM+ | 12% |
| 17 | 2016 | November/20 | 325 | OLI | 0% |
| 18 | 2017 | November/15 | 319 | ETM+ | 4% |
| Climate Variables | Description | |
|---|---|---|
| Precipitation | Pspring | Cumulative precipitation between March to May. |
| Psummer | Cumulative precipitation between June to August. | |
| Pautumn | Cumulative precipitation between September to November. | |
| Pwinter | Cumulative precipitation between December to February. | |
| P0 | Cumulative precipitation in the current year (0). | |
| P1 | Cumulative precipitation in the previous year (1). | |
| P12 | Cumulative precipitation in previous 1–2 years. | |
| P123 | Cumulative precipitation in previous 1–3 years. | |
| Temperature | MT | Monthly mean temperature. |
| Tspring | Mean temperature between March to May. | |
| Tsummer | Mean temperature between June to August. | |
| Tautumn | Mean temperature between September to November. | |
| Twinter | Mean temperature between December to February. | |
| Tmin | The minimum temperature of the coldest month. | |
| Coldt | The minimum daily temperature of the coldest month. | |
| Tmean | Annual mean temperature. | |
| Solar radiation duration | MS | Monthly solar radiation duration. |
| Sspring | Cumulative solar radiation duration between March to May. | |
| Ssummer | Cumulative solar radiation duration between June to August. | |
| Sautumn | Cumulative solar radiation duration between September to November. | |
| Swinter | Cumulative solar radiation duration between December to February. | |
| Smean | Annual cumulative solar radiation duration. | |
| Estimate | SE | t.Value | Pr (>|t|) | Sig | |
|---|---|---|---|---|---|
| MTMay | 0.084 | 0.015 | 5.602 | 3.1 × 10−05 | *** |
| MTJune | 0.083 | 0.026 | 3.216 | 5.1 × 10−03 | ** |
| Tspring | 0.085 | 0.021 | 4.083 | 7.7 × 10−04 | *** |
| Tsummer | 0.074 | 0.013 | 5.753 | 2.3 × 10−05 | *** |
| Twinter | 0.048 | 0.029 | 1.668 | 1.1 × 10−01 | |
| Tmean | 0.085 | 0.012 | 7.138 | 1.6 × 10−06 | *** |
| MSMay | 0.075 | 0.020 | 3.762 | 1.5 × 10−03 | *** |
| MSNov | 0.063 | 0.022 | 2.822 | 1.2 × 10−02 | * |
| Sspring | 0.068 | 0.026 | 2.590 | 1.9 × 10−02 | * |
| Sautumn | 0.053 | 0.028 | 1.873 | 7.8 × 10−02 | |
| Smean | 0.054 | 0.022 | 2.444 | 2.5 × 10−02 | * |
| Pspring | −0.094 | 0.016 | −5.827 | 2.0 × 10−05 | *** |
| P1 | −0.073 | 0.018 | −3.961 | 1.0 × 10−03 | ** |
| P12 | −0.091 | 0.019 | −4.931 | 1.2 × 10−04 | *** |
| P123 | −0.103 | 0.015 | −7.106 | 1.7 × 10−06 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zhan, Z.; Zhou, Q.; Gao, B.; Ren, L.; Huang, H.; Luo, Y. Climate Drivers of Pine Shoot Beetle Outbreak Dynamics in Southwest China. Remote Sens. 2022, 14, 2728. https://doi.org/10.3390/rs14122728
Yu L, Zhan Z, Zhou Q, Gao B, Ren L, Huang H, Luo Y. Climate Drivers of Pine Shoot Beetle Outbreak Dynamics in Southwest China. Remote Sensing. 2022; 14(12):2728. https://doi.org/10.3390/rs14122728
Chicago/Turabian StyleYu, Linfeng, Zhongyi Zhan, Quan Zhou, Bingtao Gao, Lili Ren, Huaguo Huang, and Youqing Luo. 2022. "Climate Drivers of Pine Shoot Beetle Outbreak Dynamics in Southwest China" Remote Sensing 14, no. 12: 2728. https://doi.org/10.3390/rs14122728







