Hyperspectral Identification of Chlorophyll Fluorescence Parameters of Suaeda salsa in Coastal Wetlands
Abstract
:1. Introduction
2. Study Area and Methods
2.1. Study Area
2.2. Experimental Design
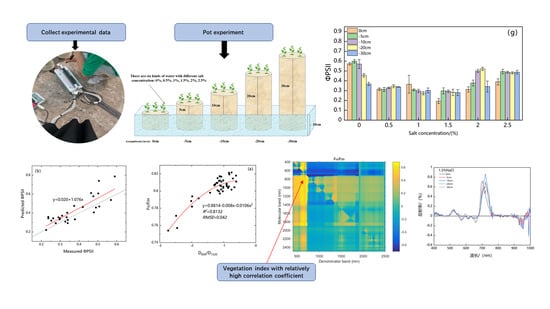
2.2.1. Field Survey and Experiment Design
2.2.2. Measurement of Chlorophyll Fluorescence Parameters of Suaeda salsa
2.2.3. Measurements of the Suaeda salsa Canopy Reflectance Spectra
2.3. Analytical Method
3. Results and Analysis
3.1. Response Analysis of Chlorophyll Fluorescence Parameters of Suaeda salsa to Water and Salt Stress
3.2. Response Analysis of Suaeda salsa Canopy Reflectance Spectra to Water and Salt Stress
Analysis of the Response of First-Order Differential Spectrum of Suaeda salsa Canopy to Water and Salt Stress
3.3. Correlation Analysis of Suaeda salsa Chlorophyll Fluorescence Parameters and Spectrum Ratio Vegetation Index
3.4. Correlation Analysis of Suaeda salsa Chlorophyll Fluorescence Parameters and First-Order Differential Spectrum Ratio Vegetation Index
3.5. Hyperspectral Index Identification Sensitive to Suaeda salsa Chlorophyll Fluorescence Parameters
3.6. Construction of Hyperspectral Recognition Model of Suaeda salsa Chlorophyll Fluorescence Parameters
3.7. Verification and Evaluation of the Accuracy of the Hyperspectral Recognition Model of Suaeda salsa Chlorophyll Fluorescence Parameters
4. Discussion
5. Conclusions
- (1)
- The chlorophyll fluorescence parameters , of Suaeda salsa showed significant relationships with the vegetation index under water and salt. The spectra under different groundwater levels, salt concentrations, and water–salt interactions exhibited a higher raw reflectance within the 500–600 nm, 680–760 nm, 760–920 nm, 1000–1100 nm, 1200–1300 nm, 1370–1400 nm, 1500–1800 nm, and 1800–2350 nm spectral regions. However, when we measured the reflectance of the canopy spectrum, we found that the reflectance of the canopy spectrum may also be related to light conditions, soil types, and crop varieties [41]. More studies are needed to verify our findings [42].
- (2)
- We constructed thirteen new vegetation indices. In addition, we discovered that the model using hyperspectral vegetation index D690/D1320 (the simple ratio of the derivative) to retrieve the Suaeda chlorophyll fluorescence parameter was the most accurate, with a multiple determination coefficient R2 of 0.813 and an RMSE of 0.042. D725/D1284 (the simple ratio of the derivative) retrieved the Suaeda chlorophyll fluorescence parameter model with the highest accuracy, with a multiple determination coefficient R2 of 0.848 and an RMSE of 0.096. However, it remains to be seen whether the newly proposed vegetation index can be applied to the model of UAV hyperspectral remote sensing imagery being constructed to estimate the chlorophyll fluorescence parameters of Suaeda salsa over a large area under water and salt conditions. Thus, subsequent verification is needed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, H.; Zhao, B.; Sun, L.; Dan, C. Discussion on the Understanding of Problems in the Management of Coastal Wetlands and the Solutions. Ocean Dev. Manag. 2014, 31, 57–60. [Google Scholar]
- Ball, M. Mangrove Species Richness in Relation to Salinity and Waterlogging: A Case Study along the Adelaide River Floodplain, Northern Australia. Glob. Ecol. Biogeogr. Lett. 1998, 7, 73–82. [Google Scholar] [CrossRef]
- Theuerkauff, D.; Rivera-Ingraham, G.A.; Roques, J.A.C.; Azzopardi, L.; Bertini, M.; Lejeune, M.; Farcy, E.; Lignot, J.; Sucré, E. Salinity variation in a mangrove ecosystem: A physiological investigation to assess potential consequences of salinity disturbances on mangrove crabs. Zool. Stud. 2018, 57, e36. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Zhou, W.; Wu, A. Current Situation and Prospect of Suaeda Development in China. J. Beijing Technol. Bus. Univ. Nat. Sci. Ed. 2005, 1, 1–4. [Google Scholar]
- Li, X.; Yuan, H.; Xu, S.; Duan, L.; Li, N.; Zhang, M.; Song, J. Study on the Accumulation and Dispersion Characteristics of Heavy Metals in the Saline Soil of the Coastal Wetland in Jiaozhou Bay. Mar. Sci. 2011, 35, 88–95. [Google Scholar]
- Guan, B.; Yu, J.; Lu, Z.; Zhang, Y.; Wang, X. Effects of Water-Salt Stress on Seedling Growth and Activities of Antioxidative Enzyme of Suaeda Salsa in Coastal Wetlands of the Yellow River Delta. Environ. Sci. 2011, 32, 2422–2429. [Google Scholar]
- Datt, B. Visible/near infrared reflectance and chlorophyll content in Eucalyptus leaves. Int. J. Remote Sens. 1999, 20, 2741–2759. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Shan, L. Effect of Water Stress on Root Water Uptake and Photosynthetic Characteristics of Alfalfa. Acta Grassl. 2007, 15, 207–209. [Google Scholar]
- Guo, S.; Zhao, K. Possible Mechanism of NaCl Stress Inhibiting Photosynthesis of Maize Seedlings. Acta Plant Physiol. 2001, 27, 461–466. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, J. Study on the Fluorescence-spectrum Characteristics of Vegetable Chlorophyll under Different Fertilization Conditions. Spectrosc. Spectr. Anal. 2020, 40, 2427–2433. [Google Scholar]
- Li, C. Study on Spectral Monitoring of “Cotton-Soil” Water and Salt Changes in Jiangsu Coastal Saline Alkali Land; Nanjing Agricultural College: Nanjing, China, 2016. [Google Scholar]
- Horler, D.N.H.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Miehé, J.A. Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 1997, 2, 316–320. [Google Scholar] [CrossRef]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H.K. Imaging of the blue, green, and red fluorescence emission of plants: An overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L. Chlorophyll fluorescence effects on vegetation apparent reflectance: I. Leaf-level measurements and model simulation. Remote Sens. Environ. 2000, 74, 582–595. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Chlorophyll fluorescence effects on vegetation apparent reflectance: II. Laboratory and airborne canopy-level measurements with hyperspectral data. Remote Sens. Environ. 2000, 74, 596–608. [Google Scholar] [CrossRef]
- Tan, C.; Huang, W.; Jin, X.; Wang, J.; Tong, L.; Wang, J.; Guo, W. Using Hyperspectral Vegetation Index to Monitor the Chlorophyll Fluorescence Parameters Fv/Fm of Compact Corn. Spectrosc. Spectr. Anal. 2012, 32, 1287–1291. [Google Scholar]
- Zhang, H.; Zhua, L.; Hu, H.; Zhen, K.; Jina, Q. Monitoring leaf chlorophyll fluorescence with spectral reflectance in rice (Oryza sativa L.). Procedia Eng. 2011, 15, 4403–4408. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yu, X.; Li, K.; Jiang, Y.; Zhang, R. Effects of Sea Salt on the Reflectance Spectra and Chlorophyll Fluorescence Parameters of Green Bamboo Leaves. Acta Ecol. Sin. 2014, 34, 4920–4930. [Google Scholar]
- Zhu, Y.; Tian, Y.; Ma, J.; Yao, X.; Liu, X.; Cao, W. Relationship between chlorophyll fluorescence parameters and reflectance spectrum characteristics of wheat leaves. Acta Crop. 2007, 8, 1286–1292. [Google Scholar]
- Xue, H.; Zhang, Y.; Liu, L.; Sun, H.; Li, C. Effects of drought stress and rewatering on cotton leaf spectrum, photosynthesis and fluorescence parameters. China Agric. Sci. 2013, 46, 2386–2393. [Google Scholar]
- Chen, B.; Wang, K.; Li, S.; Jin, X.; Chen, J.; Zhang, D. Effect of disease stress on the spectral reflectance and chlorophyll fluorescence characteristics of cotton leaves. J. Agric. Eng. 2011, 27, 86–93. [Google Scholar]
- Hu, Z.; Wang, Y.; Lan, H.; Guo, R.; Chen, G.; Chen, C. Correlation analysis of spectra, fluorescence parameters and chlorophyll content of cowpea leaves sprayed with cytokinin. Hubei Agric. Sci. 2016, 55, 2290–2294. [Google Scholar]
- Zhang, H.; Hu, H.; Zhang, X.; Wang, K.; Song, T.; Zeng, F. Detecting Suaeda salsa L. chlorophyll fluorescence response to salinity stress by using hyperspectral reflectance. Acta Physiol. Plant. 2012, 34, 581–588. [Google Scholar] [CrossRef]
- Reis, L.A.C.; de Oliveira, J.A.; dos Santos Farnese, F.; Rosado, A.M.; Reis, L.A.C. Chlorophyll fluorescence and water content parameters are good biomarkers for selecting drought tolerant eucalyptus clones. For. Ecol. Manag. 2020, 481, 118682. [Google Scholar]
- Qi, Z. Response of Photosynthesis and Growth of Phragmites communis to Soil Water and Salt Factors in Coastal Reclamation Wetland of Chongming Dongtan; East China Normal University: Shanghai, China, 2017. [Google Scholar]
- Zhang, Y.; Zhou, Y.; Shi, Y.; Li, C.; Pan, J.; Han, X.; Zhang, L. Effect of Water Stress on Chlorophyll Content in Ground Cover Plants. Shelter. Technol. 2012, 02, 42–44. [Google Scholar]
- Zhang, J.; Jiang, C.; Ping, J. Research Progress on the Effects of Salt Stress on Plant Photosynthesis. Agric. Sci. Res. 2008, 03, 74–80. [Google Scholar]
- Rapacz, M. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica 2007, 45, 409–418. [Google Scholar] [CrossRef]
- Yin, H.; Tian, C. Photosynthetic Physiological and Ecological Characteristics of Suaeda salsa Seedlings in Different Salinity Environments. Arid Zone Res. 2014, 31, 850–855. [Google Scholar]
- El-Hendawy, S.; Al-Suhaibani, N.; Elsayed, S.; Alotaibi, M.; Hassan, W.; Schmidhalter, U. Performance of optimized hyperspectral reflectance indices and partial least squares regression for estimating the chlorophyll fluorescence and grain yield of wheat grown in simulated saline field conditions. Plant Physiol. Biochem. 2019, 144, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Guérif, M.; Baret, F.; Skidmore, A.; Gitelson, A.; Schlerf, M.; Darvishzadeh, R.; Olioso, A. Simple and robust methods for remote sensing of canopy chlorophyll content: A comparative analysis of hyperspectral data for different types of vegetation. Plant Cell Environ. 2016, 39, 2609–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.Z.; Ustin, S.L. Steady state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Envron. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S. Significance and discussion of chlorophyll fluorescence kinetic parameters. Bot. Bull. 1999, 16, 444–448. [Google Scholar]
- Li, S.W.; Chan, B.K.K.; Tam, N.T.Y. Effect of barnacle fouling on leaf stomata density and chlorophyll concentration of Kandelia obovata, a dominant mangrove species in Hong Kong and Taiwan. Hydrobiologia 2009, 618, 199–203. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Yu, X.P. A new species of apple snail in the genus Pomacea (Gastropoda: Caenogastropoda: Ampullariidae). Zool. Stud. 2019, 58, e13. [Google Scholar] [CrossRef]
- Dumidae, A.; Janthu, P.; Subkrasae, C.; Polsee, R.; Mangkit, B.; Thanwisai, A.; Vitta, P. Population Genetics Analysis of a Pomacea Snail (Gastropoda: Ampullariidae) in Thailand and its Low Infection by Angiostrongylus cantonensis. Zool. Stud. 2021, 60, e31. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, S.; Tian, Y.; Li, Y.; Wen, R.; Tsou, J.Y.; Zhang, Y. Monitoring Suaeda salsa spectral response to salt conditions in coastal wetlands: A case study in Dafeng Elk National nature reserve, China. Remote Sens. 2020, 12, 2700. [Google Scholar] [CrossRef]














| Chlorophyll Fluorescence Parameters | Salt Concentration | Groundwater Level | Salt Concentration * Groundwater Level |
|---|---|---|---|
| 2.129(0.065) | 1.612(0.174) | 2.191 ** | |
| 2.044(0.076) | 2.891 * | 1.681 * | |
| 14.293 *** | 21.583 *** | 4.928 *** | |
| 57.43 *** | 6.475 *** | 2.079 ** | |
| 68.43 *** | 10.953 *** | 3.593 *** | |
| 42.381 *** | 2.077(0.087) | 5.207 *** | |
| 136.38 *** | 9.231 *** | 11.073 *** | |
| 42.526 *** | 4.032 ** | 2.623 *** | |
| 67.371 *** | 1.118(0.35) | 1.952 * | |
| 60.245 *** | 11.63 *** | 2.387 ** |
| Vegetation Index/Chlorophyll Fluorescence Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| −0.706 ** | 0.188(0.076) | 0.142(0.182) | −0.231 * | −0.296 ** | −0.302 ** | −0.057(0.596) | −0.412 ** | |
| 0.016(0.881) | 0.529 ** | 0.643 ** | 0.082(0.441) | 0.084(0.432) | 0.103(0.335) | −0.527 ** | −0.150(0.158) | |
| −0.328 ** | −0.007(0.948) | 0.112(0.293) | 0.228 * | 0.189(0.074) | 0.119(0.263) | 0.146(0.170) | −0.653 ** | |
| 0.716 ** | −0.077(0.472) | −0.020(0.854) | 0.225 * | 0.325 ** | 0.365 ** | 0.019(0.862) | 0.325 ** | |
| −0.168(0.114) | 0.697 ** | 0.631 ** | −0.350 ** | −0.357 ** | −0.255 * | −0.589 ** | 0.070(0.514) | |
| 0.135(0.204) | 0.454 ** | 0.705 ** | 0.340 ** | 0.356 ** | 0.316 ** | −0.368 ** | −0.407 ** | |
| −0.283 ** | 0.365 ** | 0.085(0.425) | −0.722 ** | −0.753 ** | −0.688 ** | v0.306 ** | 0.245 * | |
| 0.046(0.666) | −0.617 ** | −0.566 ** | 0.185(0.080) | 0.166(0.119) | 0.094(0.381) | 0.745 ** | −0.007(0.945) | |
| 0.343 ** | −0.053(0.619) | −0.197(0.063) | −0.270 * | −0.204(0.053) | −0.110(0.302) | 0.014(0.896) | 0.736 ** | |
| 0.710 ** | −0.070(0.515) | 0.026(0.810) | 0.310 ** | 0.386 ** | 0.402 ** | 0.022(0.837) | 0.252 * | |
| 0.708 ** | −0.066(0.536) | 0.024(0.822) | 0.300 ** | 0.380 ** | 0.401 ** | 0.016(0.878) | 0.268 * | |
| 0.698 ** | -0.216 * | −0.174(0.101) | 0.228 * | 0.294 ** | 0.294 ** | 0.082(0.445) | 0.404 ** | |
| 0.080(0.453) | −0.063(0.557) | −0.123(0.247) | −0.070(0.510) | −0.079(0.460) | −0.086(0.422) | 0.054(0.612) | −0.017(0.871) | |
| 0.352 ** | −0.250 * | 0.005(0.959) | 0.666 ** | 0.706 ** | 0.678 ** | 0.178(0.093) | −0.139(0.190) | |
| 0.038(0.723) | −0.630 ** | −0.575 ** | 0.192(0.070) | 0.164(0.122) | 0.083(0.435) | 0.740 ** | 0.000(0.998) |
| Numerical Comparison/Correlation Analysis | Pearson Correlation | Significance | RMSEP |
|---|---|---|---|
| D690/D1320 calculates the value of (predicted value) | 0.548 ** | 0.002 | 0.334 |
| measured value | |||
| D725/D1284 calculates the value of (predicted value) measured value | 0.779 ** | 0.000 | 0.388 |
| Measured value of |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Lu, X.; Li, Y.; Li, S.; Zhang, Y. Hyperspectral Identification of Chlorophyll Fluorescence Parameters of Suaeda salsa in Coastal Wetlands. Remote Sens. 2021, 13, 2066. https://doi.org/10.3390/rs13112066
Zheng W, Lu X, Li Y, Li S, Zhang Y. Hyperspectral Identification of Chlorophyll Fluorescence Parameters of Suaeda salsa in Coastal Wetlands. Remote Sensing. 2021; 13(11):2066. https://doi.org/10.3390/rs13112066
Chicago/Turabian StyleZheng, Wei, Xia Lu, Yu Li, Shan Li, and Yuanzhi Zhang. 2021. "Hyperspectral Identification of Chlorophyll Fluorescence Parameters of Suaeda salsa in Coastal Wetlands" Remote Sensing 13, no. 11: 2066. https://doi.org/10.3390/rs13112066