Crop Sensor-Based In-Season Nitrogen Management of Wheat with Manure Application
Abstract
:1. Introduction
2. Materials and Methods
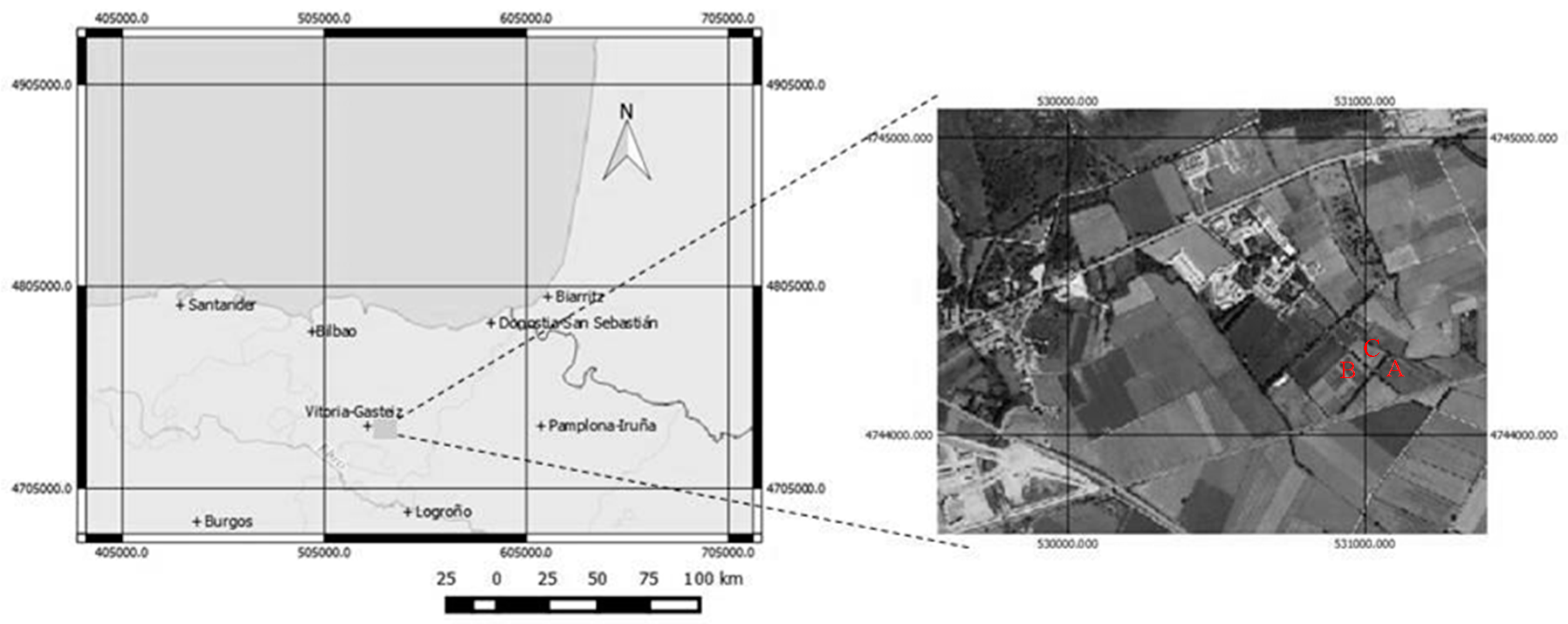
2.1. Experimental Setup and Treatments
2.2. Mineral N Samples (Nmin) and Biomass Samples for the Nitrogen Nutrition Index (NNI)
2.3. Proximal Sensing Tools for Adjusting the Optimum N Rate at GS30
2.4. Grain Yield
2.5. Statistical Analysis
3. Results
3.1. Grain Yield
Optimum N Rate at GS30
3.2. Economically Optimal Dose
3.3. Soil Mineral Nitrogen (Nmin) and Total Rainfall
3.4. NNI
3.5. Proximal Sensing Tools
3.5.1. Absolute Values
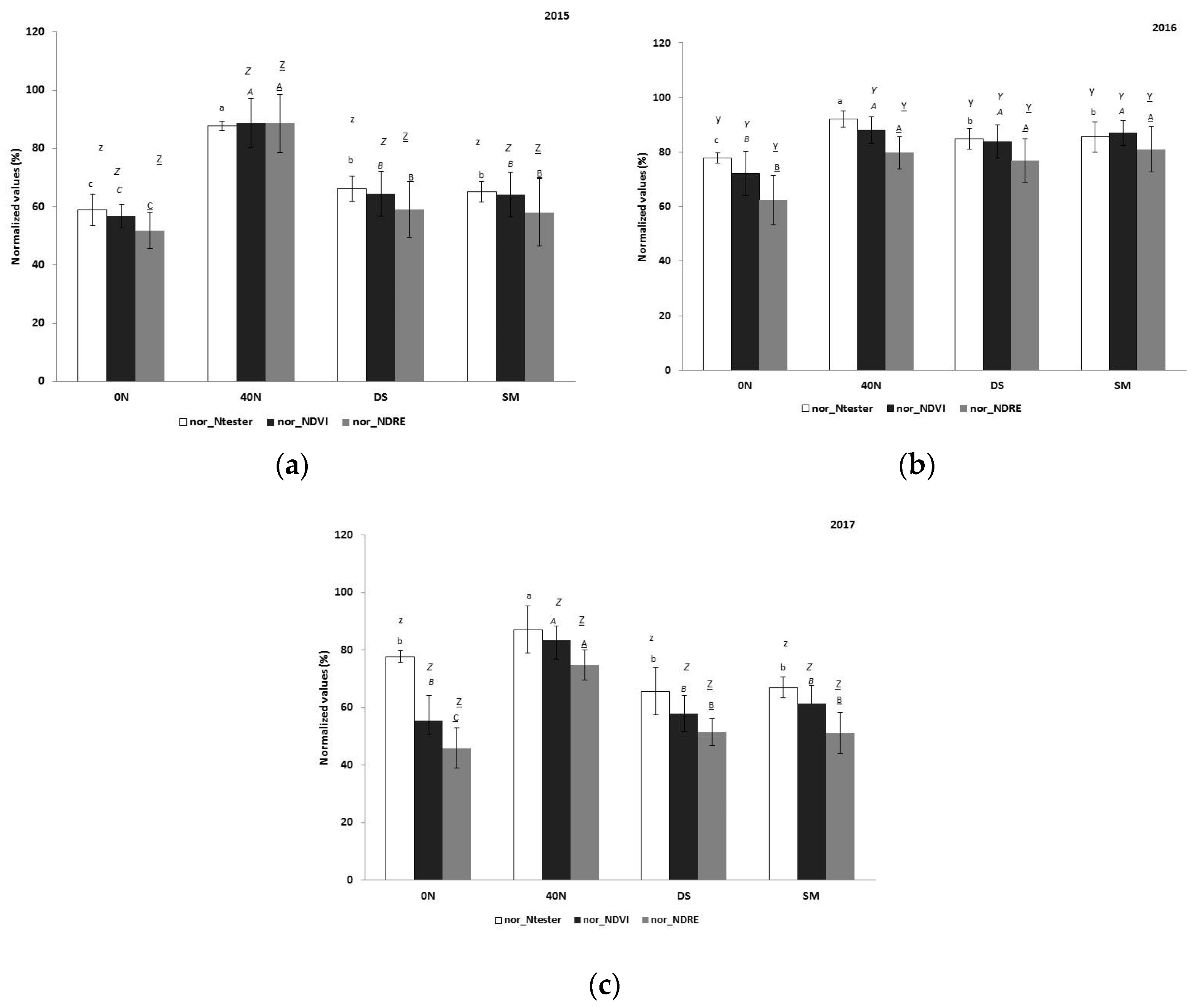
3.5.2. Normalized Values
4. Discussion
4.1. Mineral N Fertilizer Reduction When Organic Fertilizer Was Applied before Sowing
4.2. Soil N Availability
4.3. Nitrogen Nutrition Index (NNI)
4.4. Proximal Sensing Tools and Vegetation Indices
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: a review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef] [Green Version]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- FAO. Pollution from Industrialized Livestock Production; Agriculture and Consumer Protection Department: Rome, Italy, 2005. [Google Scholar]
- Whalen, J.K.; Thomas, B.W.; Sharifi, M. Novel Practices and Smart Technologies to Maximize the Nitrogen Fertilizer Value of Manure for Crop Production in Cold Humid Temperate Regions. Adv. Agron. 2019, 153, 1–85. [Google Scholar]
- Defra. Nutrient Management Guide (RB209). Available online: https://ahdb.org.uk/documents/RB209/RB209_Section2_WEB_2001.pdf (accessed on 11 January 2017).
- Ros, G.H.; Hanegraaf, M.C.; Hoffland, E.; Van Riemsdijk, W.H. Predicting soil N mineralization: Relevance of organic matter fractions and soil properties. Soil Boil. Biochem. 2011, 43, 1714–1722. [Google Scholar] [CrossRef]
- Eghball, B. Nitrogen Mineralization from Field-Applied Beef Cattle Feedlot Manure or Compost. Soil Sci. Soc. J. 2000, 64, 2024–2030. [Google Scholar] [CrossRef]
- Eghball, B.; Wienhold, B.J.; Gilley, J.E.; Eigenberg, R.A. Mineralization of Manure Nutrients. Biol. Syst. Eng. Pap. Publ. 2002, 139. [Google Scholar]
- Arregui, L.; Lasa, B.; Lafarga, A.; Irañeta, I.; Baroja, E.; Quemada, M. Evaluation of chlorophyll meters as tools for N fertilization in winter wheat under humid Mediterranean conditions. Eur. J. Agron. 2006, 24, 140–148. [Google Scholar] [CrossRef]
- Basso, B.; Fiorentino, C.; Cammarano, D.; Cafiero, G.; Dardanelli, J. Analysis of rainfall distribution on spatial and temporal patters of wheat yield in Mediterranean environment. Eur. J. Agron. 2012, 41, 52–65. [Google Scholar] [CrossRef]
- Ravier, C.; Jeuffroy, M.-H.; Meynard, J.-M. Mismatch between a science-based decision tool and its use: The case of the balance-sheet method for nitrogen fertilization in France. NJAS-Wagening. J. Life Sci. 2016, 79, 31–40. [Google Scholar] [CrossRef]
- Ravier, C. Conception innovante d’une méthode de fertilization azotée: Ariculation entre diagnostic des usages, ateliers participatifs et modélisation. Ph.D. Thesis, University of Paris-Saclay, Saint-Aubin, France, 2017. [Google Scholar]
- Antille, D.L.; Lobsey, C.R.; McCarthy, C.L.; Thomasson, J.A.; Baillie, C.P. A review of the state of the art in agricultural automation. Part IV: Sensor-based nitrogen management technologies. ASABE Annu. Int. Meet. 2018. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Mullen, R.W.; Freeman, K.W.; Thomason, W.E.; Lukina, E.V. Improving Nitrogen Use Efficiency in Cereal Grain Production with Optical Sensing and Variable Rate Application. Agron. J. 2002, 94, 815. [Google Scholar] [CrossRef] [Green Version]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to Make Use of Plant Sensors-Based Diagnostic Information for Nitrogen Recommendations. Agron. J. 2009, 101, 800. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, G.; Jeufroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Teory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Ortuzar-Iragorri, M.A.; Alonso, A.; Castellón, A.; Besga, G.; Estavillo, J.M.; Aizpurua, A. N-Tester use in soft winter wheat: evaluation of nitrogen status and grain yield prediction. Agron. J. 2005, 97, 1380–1389. [Google Scholar] [CrossRef]
- Piekelek, W.P.; Fox, R.H. Use of a chlorophyll meter to predict sidedress nitrogen requirements for maize. Agron. J. 1992, 84, 59–65. [Google Scholar] [CrossRef]
- Ortuzar-Iragorri, M.A.; Aizpurua, A.; Castellón, A.; Alonso, A.; José, M.; Estavillo, J.M.; Besga, G. Use of an N-Tester chlorophyll meter to tune a late third nitrogen application to wheat under humid Mediterranean conditions. J. Plant Nutr. 2017, 41, 6635. [Google Scholar] [CrossRef]
- Prost, L.; Jeuffroy, M.-H. Replacing the nitrogen nutrition index by the chlorophyll meter to assess wheat N status. Agron. Sustain. Dev. 2007, 27, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship Between the Normalized SPAD Index and the Nitrogen Nutrition Index: Application to Durum Wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Ravier, C.; Meynard, J.-M.; Cohan, J.-P.; Gate, P.; Jeuffroy, M.-H. Early nitrogen deficiencies favor high yield, grain protein content and N use efficiency in wheat. Eur. J. Agron. 2017, 89, 16–24. [Google Scholar] [CrossRef]
- Shanahan, J.; Kitchen, N.; Raun, W.; Schepers, J.; Raun, W. Responsive in-season nitrogen management for cereals. Comput. Electron. Agric. 2008, 61, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Thind, H.S.; Varinderpal-Singh, B.-S. A framework for refining nitrogen management in dry direct-seeded rice using GreenSeeker™ optical sensor. Comput. Electron. Agric. 2015, 110, 114–120. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.L.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Marti, J.; Bort, J.; Slafer, G.A.; Araus, J.L. Can wheat yield be assessed by early measurements of Normalized Difference Vegetation Index? Ann. Appl. Biol. 2007, 150, 225. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Yuan, F. Evaluating different approaches to non-destructive nitrogen status diagnosis of rice using portable RapidSCAN active canopy sensor. Sci. Rep. 2017, 7, 14073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Ding, X.; Kuang, Q.; Ata-Ui-Karim, S.T.; Cheng, T.; Liu, X.; Tian, Y.; Zhu, Y.; Cao, W.; Cao, Q. Potential of UAV-Based Active Sensing for Monitoring Rice Leaf Nitrogen Status. Front. Plant Sci. 2018, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, D.J. Monitoring wheat fields by RapidScan: Accuracy and limitations. In Proceedings of the Conference on Precision Agriculture (ECPA 2017), Edinburgh, UK, 16–20 July 2017. [Google Scholar]
- Zhang, K.; Ge, X.; Shen, P.; Li, W.; Liu, X.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. Predicting Rice Grain Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages. Remote Sens. 2019, 11, 387. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellon, A.; Aizpurua, A. Topdressing nitrogen recommendation in wheat after applying organic manures: the use of field diagnostic tools. Nutr. Cycl. Agroecosyst. 2018, 110, 89–103. [Google Scholar] [CrossRef]
- Papadakis, J. Climates of the World and Their Agricultural Potentialities; Libro de Edicion Argentina: Buenos Aires, Argentina, 1966. [Google Scholar]
- Euskalmet. Euskal Meteorologia Agentzia. 2018. Available online: http://www.euskalmet.euskadi.eus/ (accessed on 21 November 2018).
- IUSS Working Group, WRB. Base referencial mundial del recurso suelo 2014, Actualización 2015. Sistema internacional de clasificación de suelos para la nomenclatura de suelos y la creación de leyendas de mapas de suelos. In Informes Sobre Recursos Mundiales de Suelos 106; FAO: Roma, Italy, 2014. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1. Physical and Mineralogical Methods; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service. U.S. Department of Agriculture: Washington, DC, USA, 1999.
- MAPA. Métodos oficiales de análisis. In Tomo III; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1994. [Google Scholar]
- Walkey, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic and titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Cawse, P.A. The determination of nitrate in soil solutions by ultraviolet spectrophotometry. Analyst 1967, 92, 311–315. [Google Scholar] [CrossRef]
- Nelson, D.W. Determination of ammonium in KCl extracts of soils by the salicylate method. Commun. Soil Sci. Plant Anal. 1983, 14, 1051–1062. [Google Scholar] [CrossRef]
- AOAC, Association of Official Analytical Chemists International. Plants, 24, 127. In Official Methods of AOAC International, 16th ed.; Patricia, C., Ed.; AOAC International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Lemaire, G.; Salette, J.; Sigogne, M.; Terrasson, J.P. Relation entre dynamique de croissance et dynamique de prélèvement d’azote pour un peuplement de graminées fourragéres. I: Etude de l’effet du milieu. Agronomie 1984, 4, 423–430. [Google Scholar] [CrossRef]
- Yue, S.; Zhao, R.; Zhang, F.; Cui, Z.; Meng, Q.; Li, F.; Chen, X. Critical Nitrogen Dilution Curve for Optimizing Nitrogen Management of Winter Wheat Production in the North China Plain. Agron. J. 2012, 104, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Justes, E.; Mary, B.; Meynard, J.-M.; Machet, J.-M.; Thelier-Huche, L. Determination of a Critical Nitrogen Dilution Curve for Winter Wheat Crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Follett, R.; Follett, R.; Halvorson, A. Use of a chlorophyll meter to evaluate the nitrogen status of dryland winter wheat. Commun. Soil Sci. Plant Anal. 1992, 23, 687–697. [Google Scholar] [CrossRef]
- Ortuzar-Iragorri, M.A.; Castellón, A.; Alonso, A.; Besga, G.; Estavillo, J.M.; Aizpurua, A. Estimation of optimum nitrogen fertilizer rates in winter wheat in humid Mediterranean conditions, I: Selection of yield and protein response models. Commun. Soil Sci. Plant Anal. 2010, 41, 2293–2300. [Google Scholar] [CrossRef]
- SAS Institute. SAS version 8; SAS Institute: Cary, NC, USA, 1998. [Google Scholar]
- Cerrato, M.E.; Blackmer, A.M. Comparison of Models for Describing; Corn Yield Response to Nitrogen Fertilizer. Agron. J. 1990, 82, 138. [Google Scholar] [CrossRef]
- Roberts, D.F.; Ferguson, R.B.; Kitchen, N.R.; Adamchuk, V.I.; Shanahan, J.F. Relationships between Soil-Based Management Zones and Canopy Sensing for Corn Nitrogen Management. Agron. J. 2012, 104, 119. [Google Scholar] [CrossRef]
- Scharf, P.C.; Kitchen, N.R.; Sudduth, K.A.; Davis, J.G. Spatially Variable Corn Yield is a Weak Predictor of Optimal Nitrogen Rate Peter C. Soil Sci. Soc. Am. J. 2006, 70, 2154–2160. [Google Scholar] [CrossRef]
- Aizpurua, A.; Estavillo, J.M.; Castellón, A.; Alonso, A.; Besga, G.; Ortuzar-Iragorri, M.A. Estimation of optimum nitrogen fertilizer rates in winter wheat in humid mediterranean conditions. II: Economically optimal dose nitrogen. Commun. Soil Sci. Plant Anal. 2010, 41, 301–307. [Google Scholar] [CrossRef]
- MAPAMA. Publicación de indicadores de precios y salarios agrarios; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013.
- De Mendiburu, F. Una herramienta de análisis estadístico para la investigación agrícola. Ph.D. Thesis, Universidad Nacional de Ingeniería, Lima, Peru, 2009. [Google Scholar]
- Mohanty, M.; Sinha, N.K.; Reddy, K.S.; Chaudhary, R.S.; Rao, A.S.; Dalal, R.C.; Menzies, N.W. How important is the quality of organic amendments in relation to mineral N availability in soils? Agric. Res. 2013, 2, 99–110. [Google Scholar] [CrossRef]
- Walley, F.; Yates, T.; Van Groenigen, J.-W.; Van Kessel, C. Relationships between Soil Nitrogen Availability Indices, Yield, and Nitrogen Accumulation of Wheat. Soil Sci. Soc. J. 2002, 66, 1549. [Google Scholar] [CrossRef]
- Arregui, L.M.; Quemada, M. Drainage and nitrate leaching in a crop rotation under different N-fertilizer strategies: application of capacitance probes. Plant Soil 2006, 288, 57–69. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Angers, D.A.; Morvan, T.; Pomar, C. Dynamics of Pig Slurry Nitrogen in Soil and Plant as Determined with N. Soil Sci. Soc. J. 2004, 68, 637. [Google Scholar] [CrossRef]
- Shah, G.M.; Rashid, M.I.; Shah, G.A.; Groot, J.C.J.; Lantinga, E.A. Mineralization and herbage recovery of animal manure nitrogen after application to various soil types. Plant Soil 2013, 365, 69–79. [Google Scholar] [CrossRef]
- INTIA. Manual del cultivo de colza de otoño. Instituto Navarro de Tecnologías e Infraestructuras agroalimentarias. Available online: https://intiasa.es/repositorio/images/docs/ManualCOLZA2012.pdf (accessed on 11 January 2017).
- Gallejones, P.; Castellón, A.; del Prado, A.; Unamunzaga, O.; Aizpurua, A. Nitrogen and sulphur fertilization effect on leaching losses, nutrient balance and plant quality in a wheat–rapeseed rotation under a humid Mediterranean climate. Nutr. Cycl. Agroecosyst. 2012, 93, 3355. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Wiltshire, J.J.J.; Kindred, D.R.; Hatley, D.L.J.; Clarke, S. Detecting Soil Nitrogen Supplies by Canopy Sensing—Project Report 460; HGCA: London, UK, 2009. [Google Scholar]
- Cilia, C.; Panigada, C.; Rossini, M.; Meroni, M.; Busetto, L.; Amaducci, S.; Boschetti, M.; Picchi, V.; Colombo, R. Nitrogen Status Assessment for Variable Rate Fertilization in Maize through Hyperspectral Imagery. Remote Sens. 2014, 6, 6549–6565. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.L.; Lenz-Wiedemann, V.I.; Rascher, U.; et al. Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Miao, Y.; Wu, D.; Shao, H.; Khosla, R.; Mi, G. Active Optical Sensing of Spring Maize for In-Season Diagnosis of Nitrogen Status Based on Nitrogen Nutrition Index. Remote Sens. 2016, 8, 605. [Google Scholar]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Claessens, A.; Tremblay, N.; Cambouris, A.N.; Nolin, M.C.; Parent, L.-É. Chlorophyll Measurements and Nitrogen Nutrition Index for the Evaluation of Corn Nitrogen Status. Agron. J. 2008, 100, 1264. [Google Scholar] [CrossRef] [Green Version]
- Peltonen, J.; Virtanen, A.; Haggrèn, E. Using a Chlorophyll Meter to Optimize Nitrogen Fertilizer Application for Intensively-Managed Small-Grain Cereals. J. Agron. Sci. 1995, 174, 309–318. [Google Scholar] [CrossRef]
- Bundy, L.G.; Andraski, T.W. Diagnostic Tests for Site-Specific Nitrogen Recommendations for Winter Wheat. Agron. J. 2004, 96, 608–614. [Google Scholar] [CrossRef]
- Ravier, C.; Quemada, M.; Jeuffroy, M.-H. Use of a chlorophyll meter to assess nitrogen nutrition index during the growth cycle in winter wheat. Field Crop. Res. 2017, 214, 73–82. [Google Scholar] [CrossRef]
- Ravier, C.; Jeuffroy, M.H.; Gate, P.; Cohan, J.P.; Meynard, J.M. Combining user involvement with innovative design to develop a radical new method for managing N fertilization. Nutr. Cycl. Agroecosyst. 2018, 110, 117. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, Y.; Cao, Q.; Wang, H.; Gnyp, M.L.; Bareth, G.; Khosla, R.; Yang, W.; Liu, F.; Liu, C. In-Season Estimation of Rice Nitrogen Status with an Active Crop Canopy Sensor. IEEE J. Sel. Top. Appl. Earth Obs. Sens. 2014, 7, 4403–4413. [Google Scholar] [CrossRef]
- Sharifi, M.; Zebarth, B.J.; Burton, D.L.; Rodd, V.; Grant, C.A. Long-Term Effects of Semisolid Beef Manure Application to Forage Grass on Soil Mineralizable Nitrogen. Soil Sci. Soc. J. 2011, 75, 649. [Google Scholar] [CrossRef]
- Moreno-García, B.; Casterad, M.A.; Guillén, M.; Quílez, D. Agronomic and Economic Potential of Vegetation Indices for Rice N Recommendations under Organic and Mineral Fertilization in Mediterranean Regions. Remote Sens. 2018, 10, 1908. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, S.T.; Liu, P.; Zhang, J.W.; Zhao, B. Effects of long-term mixed application of organic and inorganic fertilizers on canopy apparent photosynthesis and yield of winter wheat. Chin. J. Appl. Ecol. 2015, 26, 2362–2370. [Google Scholar]
- Siband, P.; Loyce, C.; Witt, C.; Dingkuhn, M. Evaluer le statut azote du riz irrigué. In Modélisation des Agroécosystémes et Aide á la Decision; Malézieux, E., Trébuil, G., Jaeger, M., Eds.; CIRAD: Montpellier, France, 2001; pp. 95–106. [Google Scholar]
- Broge, N.; Mortensen, J. Deriving green crop area index and canopy chlorophyll density of winter wheat from spectral reflectance data. Remote Sens. Environ. 2002, 81, 45–57. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Gonzalez-Murua, C.; González-Moro, M.B.; Estavillo, J.M. Late nitrogen fertilization affects nitrogen remobilization in wheat. J. Plant Nutr. Soil Sci. 2012, 175, 115–124. [Google Scholar] [CrossRef]
- Mullen, R.W.; Freeman, K.W.; Raun, W.R.; Johnson, G.V.; Stone, M.L.; Solie, J.B. Identifying an In-Season Response Index and the Potential to Increase Wheat Yield with Nitrogen. Agron. J. 2003, 95, 347. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Stone, M.L.; Martin, K.L.; Freeman, K.W.; Mullen, R.W.; Zhang, H.; Schepers, J.S.; Johnson, G.V.; Raun, W.; et al. Optical Sensor-Based Algorithm for Crop Nitrogen Fertilization. Commun. Soil Sci. Plant Anal. 2005, 36, 2759–2781. [Google Scholar] [CrossRef]
- Calvo, N.I.R.; Rozas, H.S.; Echeverría, H.; Diovisalvi, N. Using Canopy Indices to Quantify the Economic Optimum Nitrogen Rate in Spring Wheat. Agron. J. 2015, 107, 459–465. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Use of field diagnostic tools for top dressing nitrogen recommendation when organic manures are applied in humid Mediterranean conditions. In Proceedings of the 14th International Conference on Precision Agriculture, Montreal, QC, Canada, 24–27 June 2018. [Google Scholar]
- Soenen, B.; Cohan, J.P.; Jeuffroy, M.H.; Meynard, J.M.; Ravier, C. Fertilisation azotée du blé: raisonner sans objectif de rendement? Perspect. Agric. 2017, 445, 40–42. [Google Scholar]


| Initial Fertilization | 2015 | 2016 | 2017 | 2015–2016–2017 | Treatment Identification | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total Na (kg ha−1) | N–NH4+b (kg ha−1) | Total Na (kg ha−1) | N–NH4+b (kg ha−1) | Total Na (kg ha−1) | N–NH4+b (kg ha−1) | Topdressing at GS21 (kg N ha−1) | Topdressing at GS30 (kg N ha−1) | ||
| Conventional [--] | -- | -- | -- | -- | -- | -- | 40 | 0 | 40N + 0N |
| 40 | 40N + 40N | ||||||||
| 80 | 40N + 80N | ||||||||
| 120 | 40N + 120N | ||||||||
| 160 | 40N + 160N | ||||||||
| Dairy Slurry (DS) [40 t ha−1] | 192 | 104 | 144 | 80 | 120 | 68 | -- | 0 | DS + 0N |
| 40 | DS + 4N | ||||||||
| 80 | DS + 80 N | ||||||||
| 120 | DS + 120N | ||||||||
| 160 | DS + 160N | ||||||||
| Sheep manure (SM) [40 t ha−1] | 336 | 0 | 592 | 200 | 448 | -- | -- | 0 | SM + 0N |
| 40 | SM + 40N | ||||||||
| 80 | SM + 80N | ||||||||
| 120 | SM + 120N | ||||||||
| 160 | SM + 120N | ||||||||
| Control | -- | -- | -- | -- | -- | -- | 0N | ||
| Overfertilized | -- | -- | -- | -- | 80 | 200 | 280N | ||
| Growing Season | Treatments | Nmin (0–30cm; kg N ha−1) | ||||
|---|---|---|---|---|---|---|
| Initial | GS21 | GS30 | ||||
| Mean Values | Mean Values | sd | Mean Values | sd | ||
| 2015 | 0N | 50 | 22 A | 5 | 12 b | 5 |
| 40 + 0N | 22 A | 5 | 13 b | 4 | ||
| DS + 0N | 4 B b | 1 | 13 | 9 | ||
| SM + 0N | 9 B b | 3 | 12 | 5 | ||
| 2016 | 0N | 42 | 30 | 9 | 1 c | 1 |
| 40 + 0N | 30 | 9 | 3 b | 1 | ||
| DS + 0N | 32 a | 4 | 4 | 0 | ||
| SM + 0N | 30 a | 2 | 1 | 4 | ||
| 2017 | 0N | 34 | 33 | 12 | 26 a | 3 |
| 40 + 0N | 33 | 12 | 32 a | 13 | ||
| DS + 0N | 36 a | 11 | 16 | 3 | ||
| SM + 0N | 16 ab | 12 | 14 | 11 | ||
| Growing Season | Treatments | NNI | |||
|---|---|---|---|---|---|
| GS21 | GS30 | ||||
| Mean Values | sd | Mean Values | sd | ||
| 2015 | OverFert | 0.37 b | 0.02 | ND | ND |
| 0N | 0.37 b | 0.02 | 0.26 b | 0.03 | |
| Conventional | 0.37 b | 0.02 | 0.35 b | 0.09 | |
| Dairy Slurry | 0.34 b | 0.05 | 0.37 | 0.08 | |
| Sheep manure | 0.38 b | 0.06 | 0.29 b | 0.02 | |
| 2016 | OverFert | 0.60 B a | 0.08 | 0.77 A a | 0.10 |
| 0N | 0.60 B a | 0.08 | 0.42 B a | 0.04 | |
| Conventional | 0.60 B a | 0.08 | 0.55 B a | 0.01 | |
| Dairy Slurry | 0.67 A a | 0.03 | 0.51 B | 0.03 | |
| Sheep manure | 0.68 A a | 0.05 | 0.51 B a | 0.07 | |
| 2017 | OverFert | 0.23 c | 0.02 | 0.80 A | 0.05 |
| 0N | 0.23 c | 0.02 | 0.33 C b | 0.03 | |
| Conventional | 0.23 c | 0.02 | 0.53 B a | 0.05 | |
| Dairy Slurry | 0.26 c | 0.02 | 0.38 C | 0.04 | |
| Sheep manure | 0.23 c | 0.04 | 0.37 C b | 0.05 | |
| Growing Season | Treatments | abs_Ntester | RapidScan CS-45 | ||||
|---|---|---|---|---|---|---|---|
| abs_NDVI | abs_NDRE | ||||||
| Mean Values | sd | Mean Values | sd | Mean Values | sd | ||
| 2015 | 0N | 304b z | 34 | 0.35 B Z | 0.04 | 0.12 B Z | 0.01 |
| 40N | 460 a | 22 | 0.54 A Z | 0.04 | 0.20 A Z | 0.02 | |
| DS | 334 b z | 16 | 0.39 B Z | 0.04 | 0.14 B Z | 0.02 | |
| SM | 332 b z | 10 | 0.39 B Z | 0.05 | 0.13 B Z | 0.02 | |
| 2016 | 0N | 403 c y | 8 | 0.55 B Y | 0.05 | 0.18 B Y | 0.02 |
| 40N | 477 a | 14 | 0.67 AY | 0.02 | 0.24 A Y | 0.15 | |
| DS | 438 b y | 15 | 0.65 AY | 0.04 | 0.23 A Y | 0.02 | |
| SM | 442 b y | 28 | 0.66 AY | 0.03 | 0.23 A Y | 0.02 | |
| 2017 | 0N | 377 b z | 13 | 0.39 C Y | 0.06 | 0.12 B | 0.01 |
| 40N | 507 a | 54 | 0.59 A Z | 0.03 | 0.19 A | 0.01 | |
| DS | 382 b z | 53 | 0.43 B Z | 0.04 | 0.13 B | 0.01 | |
| SM | 389 b z | 12 | 0.41 C Z | 0.04 | 0.13 B | 0.01 | |
| Initial Fertilization | Proximal Tool Readings at GS30 (%) | Optimal N Application at GS30 (kg N ha−1) |
|---|---|---|
| Dairy Slurry | 60–65 | 118–128 |
| Dairy Slurry/Conventional | 85–90 | 100–110 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranguren, M.; Castellón, A.; Aizpurua, A. Crop Sensor-Based In-Season Nitrogen Management of Wheat with Manure Application. Remote Sens. 2019, 11, 1094. https://doi.org/10.3390/rs11091094
Aranguren M, Castellón A, Aizpurua A. Crop Sensor-Based In-Season Nitrogen Management of Wheat with Manure Application. Remote Sensing. 2019; 11(9):1094. https://doi.org/10.3390/rs11091094
Chicago/Turabian StyleAranguren, Marta, Ander Castellón, and Ana Aizpurua. 2019. "Crop Sensor-Based In-Season Nitrogen Management of Wheat with Manure Application" Remote Sensing 11, no. 9: 1094. https://doi.org/10.3390/rs11091094






