Spectral Reflectance Modeling by Wavelength Selection: Studying the Scope for Blueberry Physiological Breeding under Contrasting Water Supply and Heat Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Trial and Plant Material
2.2. Measurements
2.2.1. SWP
2.2.2. Chl Content
2.2.3. Leaf Gas Exchange
2.2.4. Modulated Chlorophyll Fluorescence:
2.2.5. Spectral reflectance
2.3. Modeling Analysis
2.4. Determining the Environmental Effects in the Leaf Spectral Signature
3. Results
3.1. Stem Water Potential (SWP)
3.2. Chlorophyll Content (Chl a, Chl b, Chl total, and Chl a/b)
3.3. Leaf gas Exchange (A, gs, E, and Ci)
3.4. Modulated Chlorophyll a Fluorescence [Y(II), qN, qP, ETRmax, IK, and Alpha]
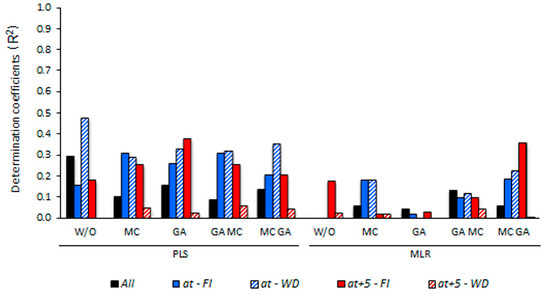
3.5. Determining the Environmental Effects in the Leaf Spectral Signature
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Osborne, T.; Rose, G.; Wheeler, T. Variation in the global-scale impacts of climate change on crop productivity due to climate model uncertainty and adaptation. Agric. For. Meteorol. 2013, 170, 183–194. [Google Scholar] [CrossRef]
- Lobos, G.A.; Hancock, J.F. Breeding blueberries for a changing global environment: A review. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Lobos, G.A. Latin America: A development pole for phenomics. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Passioura, J.B. Phenotyping for drought tolerance in grain crops: When is it useful to breeders? Funct. Plant Biol. 2012, 39, 851–859. [Google Scholar] [CrossRef]
- Araus, J.L.; Elazab, A.; Vergara, O.; Cabrera-Bosquet, L.; Serret, M.D.; Zaman-Allah, M.; Cairns, J.E. New technologies for phenotyping. In Phenomics: How Next-Generation Phenotyping Is Revolutionizing Plant Breeding; Fritsche-Neto, R., Borém, A., Eds.; Springer: New York, NY, USA, 2015; pp. 1–14. [Google Scholar]
- Houle, D.; Govindaraju, D.R.; Omholt, S. Phenomics: The next challenge. Nat. Rev. Genet. 2010, 11, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Garriga, M.; Retamales, J.B.; Romero-Bravo, S.; Caligari, P.D.; Lobos, G.A. Chlorophyll, anthocyanin, and gas exchange changes assessed by spectroradiometry in Fragaria chiloensis under salt stress. J. Integr. Plant Biol. 2014, 56, 505–515. [Google Scholar] [CrossRef]
- Hernandez, J.; Lobos, G.A.; Matus, I.; del Pozo, A.; Silva, P.; Galleguillos, M. Using ridge regression models to estimate grain yield from field spectral data in bread wheat (Triticum aestivum L.) grown under three water regimes. Remote Sens. 2015, 7, 2109–2126. [Google Scholar] [CrossRef]
- Lopes, M.S.; Araus, J.L.; Van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Araus, J.L.; Crossa, J.; Cairns, J.E.; Palacios, N.; Mahuku, G.; Das, B.; Magorokosho, C. High-throughput and precision phenotyping in cereal breeding programs. In Cereal Genomics-II; Gupta, P.K., Varshney, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 341–374. [Google Scholar]
- Cabrera-Bosquet, L.; Crossa, J.; von Zitzewitz, J.; Serret, M.D.; Araus, J.L. High-throughput phenotyping and genomic selection: The frontiers of crop breeding converge. J. Integr. Plant Biol. 2012, 54, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Silva-Perez, V.; Molero, G.; Serbin, S.P.; Condon, A.G.; Reynolds, M.P.; Furbank, R.T.; Evans, J.R. Hyperspectral reflectance as a tool to measure biochemical and physiological traits in wheat. J. Exp. Bot. 2018, 69, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Lobos, G.A.; Poblete-Echeverría, C.; Ahumada, L.; Zúñiga, M.; Romero, S.; Escobar, A.; Caligari, P.D.S. Fast and non-destructive prediction of gas exchange in olive orchards (Olea europaea L.) under different soil water conditions. Acta Hortic. 2014, 1057, 329–334. [Google Scholar] [CrossRef]
- Lobos, G.A.; Matus, I.; Rodriguez, A.; Romero-Bravo, S.; Araus, J.L.; del Pozo, A. Wheat genotypic variability in grain yield and carbon isotope discrimination under Mediterranean conditions assessed by spectral reflectance. J. Integr. Plant Biol. 2014, 56, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Echeverría, C.; Ortega-Farías, S.; Lobos, G.A.; Romero, S.; Ahumada, L.; Escobar, A.; Fuentes, S. Non-invasive method to monitor plant water potential of an olive orchard using visible and near infrared spectroscopy analysis. Acta Hortic. 2014, 1057, 363–368. [Google Scholar] [CrossRef]
- Lobos, G.A.; Poblete-Echeverría, C. Spectral Knowledge (SK-UTALCA): Software for exploratory analysis of high-resolution spectral reflectance data on plant breeding. Front. Plant Sci. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Garriga, M.; Romero-Bravo, S.; Estrada, F.; Escobar, A.; Matus, I.A.; del Pozo, A.; Astudillo, C.A.; Lobos, G.A. Assessing wheat traits by spectral reflectance: Do we really need to focus on predicted trait-values or directly identify the elite genotypes group? Front. Plant Sci. 2017, 8, 280. [Google Scholar] [CrossRef]
- Leardi, R.; Seasholtz, M.B.; Pell, R.J. Variable selection for multivariate calibration using a genetic algorithm: prediction of additive concentrations in polymer films from Fourier transform-infrared spectral data. Anal. Chim. Acta-Comp. 2002, 461, 189–200. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Lee, B.W. Assessment of rice leaf growth and nitrogen status by hyperspectral canopy reflectance and partial least square regression. Eur. J. Agron. 2006, 24, 349–356. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Hawkins, D. The problem of overfitting. J. Chem. Inf. Comput. Sci. 1990, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, L.; Saudland, J.; Wagner, J.; Nielsen, J.P.; Munck, L.; Engelsen, S.B. Interval partial least-squares regression (iPLS): A comparative chemometric study with an example from near-infrared spectroscopy. Appl. Spectrosc. 2000, 54, 413–419. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Balabin, R.M.; Safieva, R.Z.; Lomakina, E.I. Comparison of linear and nonlinear calibration models based on near infrared (NIR) spectroscopy data for gasoline properties prediction. Chemometr. Intell. Lab. 2007, 88, 183–188. [Google Scholar] [CrossRef]
- Næs, T.; Mevik, B.H. Understanding the collinearity problem in regression and discriminant analysis. J. Chemom. 2001, 15, 413–426. [Google Scholar] [CrossRef]
- Wu, D.; Chen, J.; Lu, B.; Xiong, L.; He, Y.; Zhang, Y. Application of near infrared spectroscopy for the rapid determination of antioxidant activity of bamboo leaf extract. Food Chem. 2012, 135, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Jouan-Rimbaud, D.; Massart, D.L.; Leardi, R.; De Noord, O.E. Genetic algorithms as a tool for wavelength selection in multivariate calibration. Anal. Chem. 1995, 67, 4295–4301. [Google Scholar] [CrossRef]
- Leardi, R. Application of genetic algorithm-PLS for feature selection in spectral data sets. J. Chemom. 2000, 14, 643–655. [Google Scholar] [CrossRef]
- Leardi, R.; Norgaard, L. Sequential application of backward interval partial least squares and genetic algorithms for the selection of relevant spectral regions. J. Chemom. 2004, 18, 486–497. [Google Scholar] [CrossRef]
- Li, L.; Ustin, S.L.; Riano, D. Retrieval of fresh leaf fuel moisture content using genetic algorithm partial least squares (GA-PLS) modeling. IEEE Geosci. Remote Sens. Lett. 2007, 4, 216–220. [Google Scholar] [CrossRef]
- Arakawa, M.; Yamashita, Y.; Funatsu, K. Genetic algorithm-based wavelength selection method for spectral calibration. J. Chemometr. 2011, 25, 10–19. [Google Scholar] [CrossRef]
- Sratthaphut, L.; Ruangwises, N. Genetic algorithms-based approach for wavelength selection in spectrophotometric determination of vitamin B12 in pharmaceutical tablets by partial least-squares. Procedia Eng. 2012, 32, 225–231. [Google Scholar] [CrossRef]
- Givianrad, M.H.; Saber-Tehrani, M.; Zarin, S. Genetic algorithm-based wavelength selection in multicomponent spectrophotometric determinations by partial least square regression: Application to a sulfamethoxazole and trimethoprim mixture in bovine milk. J. Serb. Chem. Soc. 2013, 78, 555–564. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Holland, J.H. Genetic algorithms and machine learning. Mach. Learn. 1988, 3, 95–99. [Google Scholar] [CrossRef]
- Goicoechea, H.C.; Olivieri, A.C. Wavelength selection for multivariate calibration using a genetic algorithm: A novel initialization strategy. J. Chem. Inf. Comp. Sci. 2002, 42, 1146–1153. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Xiong, Y.; Li, Z.; Feng, S. The determination of the fatty acid content of sea buckthorn seed oil using near infrared spectroscopy and variable selection methods for multivariate calibration. Vib. Spectrosc. 2016, 84, 24–29. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Yang, C.; Du, S.; Yang, W. Photosynthetic performance of soybean plants to water deficit under high and low light intensity. S. Afr. J. Bot. 2016, 105, 279–287. [Google Scholar] [CrossRef]
- Fang, M.; Li, H.; Liu, Z.; Xian, X. Online evaluation of yellow peach quality by visible and near-infrared spectroscopy. Adv. J. Food Sci. Technol. 2013, 5, 606–612. [Google Scholar] [CrossRef]
- Bryla, D.R.; Strik, B.C. Effects of cultivar and plant spacing on the seasonal water requirements of highbush blueberry. J. Am. Soc. Hortic. Sci. 2007, 132, 270–277. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Cobo, N.G.; del Pozo, A. Spectral irradiance, gas exchange characteristics and leaf traits of Vaccinium corymbosum L. ‘Elliott’ grown under photo-selective nets. Environ. Exp. Bot. 2012, 75, 142–149. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N,N-dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Estrada, F.; Escobar, A.; Romero-Bravo, S.; González-Talice, J.; Poblete-Echeverría, C.; Caligari, P.D.; Lobos, G.A. Fluorescence phenotyping in blueberry breeding for genotype selection under drought conditions, with or without heat stress. Sci. Hortic. 2015, 181, 147–161. [Google Scholar] [CrossRef]
- Atzberger, C.; Guérif, M.; Baret, F.; Werner, W. Comparative analysis of three chemometric techniques for the spectroradiometric assessment of canopy chlorophyll content in winter wheat. Comput. Electron. Agric. 2010, 73, 165–173. [Google Scholar] [CrossRef]
- Herrmann, I.; Pimstein, A.; Karnieli, A.; Cohen, Y.; Alchanatis, V.; Bonfil, D.J. LAI assessment of wheat and potato crops by VENUS and Sentinel-2 bands. Remote Sens. Environ. 2011, 115, 2141–2151. [Google Scholar] [CrossRef]
- Gredilla, A.; de Vallejuelo, S.F.O.; Elejoste, N.; de Diego, A.; Madariaga, J.M. Non-destructive spectroscopy combined with chemometrics as a tool for green chemical analysis of environmental samples: A review. Trends Anal. Chem. 2016, 76, 30–39. [Google Scholar] [CrossRef]
- Reynolds, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant. Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Non-destructive assessment of chlorophyll carotenoid and anthocyanin content in higher plant leaves: principles and algorithms. Pap. Nat. Resour. 2004, 263, 78–94. [Google Scholar]
- Vergara-Díaz, O.; Zaman-Allah, M.A.; Masuka, B.; Hornero, A.; Zarco-Tejada, P.; Prasanna, B.M.; Araus, J.L. A novel remote sensing approach for prediction of maize yield under different conditions of nitrogen fertilization. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Tumbo, S.D.; Wagner, D.G.; Heinemann, P.H. Hyperspectral based neural network for predicting chlorophyll status in corn. Trans. ASABE 2002, 45, 825–832. [Google Scholar] [CrossRef]
- Doughty, C.E.; Asner, G.P.; Martin, R.E. Predicting tropical plant physiology from leaf and canopy spectroscopy. Oecologia 2011, 165, 289–299. [Google Scholar] [CrossRef]
- Nyongesah, M.J.; Wang, Q.; Li, P. Effectiveness of photochemical reflectance index to trace vertical and seasonal chlorophyll a/b ratio in Haloxylon ammodendron. Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant. Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Lopes, J.I.; Gonçalves, B.C.; Ferreira, T.C.; Correia, C.M. Physiological behaviour, oxidative damage and antioxidative protection of olive trees grown under different irrigation regimes. Plant Soil 2007, 292, 1–12. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Fang, Z.; Bouwkamp, J.C.; Solomos, T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I Canopy characteristics. Aust. J. Plant. Physiol. 1993, 20, 55–67. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Tardaguila, J.; Fernández-Novales, J.; Diago, M.P. Data mining and NIR spectroscopy in viticulture: Applications for plant phenotyping under field conditions. Sensors 2016, 16, 236. [Google Scholar] [CrossRef]
- Santos, A.O.; Kaye, O. Grapevine leaf water potential based upon near infrared spectroscopy. Sci. Agric. 2009, 66, 287–292. [Google Scholar] [CrossRef]
- De Bei, R.; Cozzolino, D.; Sullivan, W.; Cynkar, W.; Fuentes, S.; Dambergs, R.; Tyerman, S. Non-destructive measurement of grapevine water potential using near infrared spectroscopy. Aust. J. Grape Wine R. 2011, 17, 62–71. [Google Scholar] [CrossRef]
- Vila, H.; Hugalde, I.; Di Filippo, M. Estimación de potencial hídrico en vid por medio de medidas termográficas y espectrales. Rev. Inv. Agropec. 2011, 37, 46–52. [Google Scholar]
- Valenzuela-Estrada, L.R.; Vera-Caraballo, V.; Ruth, L.E.; Eissenstat, D.M. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am. J. Bot. 2008, 95, 1506–1514. [Google Scholar] [CrossRef]
- Valenzuela-Estrada, L.R.; Richards, J.H.; Diaz, A.; Eissensat, D.M. Patterns of nocturnal rehydration in root tissues of Vaccinium corymbosum L. under severe drought conditions. J. Exp. Bot. 2009, 60, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Pallozzi, E.; Cocozza, C.; Tognetti, R.; Giovannelli, A.; Cantini, C.; Centritto, M. Assessing gas exchange, sap flow and water relations using tree canopy spectral reflectance indices in irrigated and rainfed Olea europaea L. Environ. Exp. Bot. 2014, 99, 43–52. [Google Scholar] [CrossRef]
- Tsonev, T.; Wahbi, S.; Sun, P.; Sorrentino, G.; Centritto, M. Gas exchange, water relations and their relationships with photochemical reflectance index in Quercus ilex plants during water stress and recovery. Int. J. Agric. Biol. 2014, 16, 335–341. [Google Scholar]
- Fréchette, E.; Wong, C.Y.; Junker, L.V.; Chang, C.Y.Y.; Ensminger, I. Zeaxanthin-independent energy quenching and alternative electron sinks cause a decoupling of the relationship between the photochemical reflectance index (PRI) and photosynthesis in an evergreen conifer during spring. J. Exp. Bot. 2015, 66, 7309–7323. [Google Scholar] [CrossRef]
- Fréchette, E.; Chang, C.Y.Y.; Ensminger, I. Photoperiod and temperature constraints on the relationship between the photochemical reflectance index and the light use efficiency of photosynthesis in Pinus strobus. Tree Physiol. 2016, 36, 311–324. [Google Scholar] [CrossRef]
- Poblete-Echeverría, C.; Sepulveda-Reyes, D.; Ortega-Farias, S.; Zuñiga, M.; Fuentes, S. Plant water stress detection based on aerial and terrestrial infrared thermography: A study case from vineyard and olive orchard. Acta Hortic. 2016, 1112, 141–146. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L. Chlorophyll fluorescence effects on vegetation apparent reflectance: I. Leaf-level measurements and model simulation. Remote Sens. Environ. 2000, 74, 582–595. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.; Miller, J.; Mohammed, G.; Noland, T.; Sampson, P. Estimation of chlorophyll fluorescence under natural illumination from hyperspectral data. Int. J. Appl. Earth Obs. 2001, 3, 321–327. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Picchi, V.; Panigada, C.; Cogliati, S.; Nali, C.; Colombo, R. Assessing steady-state fluorescence and PRI from hyperspectral proximal sensing as early indicators of plant stress: The case of ozone exposure. Sensors 2008, 8, 1740–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhua, L.; Hu, H.; Zhen, K.; Jina, Q. Monitoring leaf chlorophyll fluorescence with spectral reflectance in rice (Oryza sativa L.). Procedia Eng. 2011, 15, 4403–4408. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef]
- Lideman, L.; Nishihara, G.N.; Noro, T.; Terada, R. Effect of temperature and light on the photosynthesis as measured by chlorophyll fluorescence of cultured Eucheuma denticulatum and Kappaphycus sp. (Sumba strain) from Indonesia. J. Appl. Phycol. 2013, 25, 399–406. [Google Scholar] [CrossRef]
- Percival, D.C.; Sharpe, S.; Maqbool, R.; Zaman, Q. Narrow band reflectance measurements can be used to estimate leaf area index, flower number, fruit set and berry yield of the wild blueberry (Vaccinium angustifolium Ait.). Acta Hortic. 2012, 926, 363–369. [Google Scholar] [CrossRef]
- Glass, V.M.; Percival, D.C.; Proctor, J.T.A. Tolerance of lowbush blueberries (Vaccinium angustifolium Ait.) to drought stress. I. Soil water and yield component analysis. Can. J. Plant Sci. 2005, 85, 911–917. [Google Scholar] [CrossRef]
- Rho, H.; Yu, D.J.; Kim, S.J.; Lee, H.J. Limitation factors for photosynthesis in ‘Bluecrop’ highbush blueberry (Vaccinium corymbosum) leaves in response to moderate water stress. J. Plant Biol. 2012, 55, 450–457. [Google Scholar] [CrossRef]
- Hancock, J.F.; Lyrene, P.; Finn, C.E.; Vorsa, N.; Lobos, G.A. Blueberry and cranberry. In Temperate Fruit Crop Breeding: Germplasm to Genomics; Hancock, J.F., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2008; pp. 115–149. [Google Scholar]
- Yendrek, C.R.; Tomaz, T.; Montes, C.M.; Cao, Y.; Morse, A.M.; Brown, P.J.; McIntyre, L.M.; Leakey, A.D.B.; Ainsworth, E.A. High-throughput phenotyping of maize leaf physiological and biochemical traits using hyperspectral reflectance. Plant Physiol. 2017, 173, 614. [Google Scholar] [CrossRef] [PubMed]
- Voogt, W.; van Dijk, P.; Douven, F.; van der Maas, R. Development of a soilless growing system for blueberries (Vaccinium corymbosum): Nutrient demand and nutrient solution. Acta Hortic. 2014, 1017, 215–221. [Google Scholar] [CrossRef]
- Kingston, P.H.; Scagel, C.F.; Bryla, D.R.; Strik, B. Suitability of sphagnum moss, coir, and douglas fir bark as soilless substrates for container production of highbush blueberry. HortScience 2017, 52, 1692–1699. [Google Scholar] [CrossRef]
- Lobos, G.A.; Camargo, A.; del Pozo, A.; Araus, J.L.; Ortiz, R.; Doonan, J.H. Editorial: Plant phenotyping and phenomics for plant breeding. Front. Plant Sci. 2017, 8, 2181. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobos, G.A.; Escobar-Opazo, A.; Estrada, F.; Romero-Bravo, S.; Garriga, M.; del Pozo, A.; Poblete-Echeverría, C.; Gonzalez-Talice, J.; González-Martinez, L.; Caligari, P. Spectral Reflectance Modeling by Wavelength Selection: Studying the Scope for Blueberry Physiological Breeding under Contrasting Water Supply and Heat Conditions. Remote Sens. 2019, 11, 329. https://doi.org/10.3390/rs11030329
Lobos GA, Escobar-Opazo A, Estrada F, Romero-Bravo S, Garriga M, del Pozo A, Poblete-Echeverría C, Gonzalez-Talice J, González-Martinez L, Caligari P. Spectral Reflectance Modeling by Wavelength Selection: Studying the Scope for Blueberry Physiological Breeding under Contrasting Water Supply and Heat Conditions. Remote Sensing. 2019; 11(3):329. https://doi.org/10.3390/rs11030329
Chicago/Turabian StyleLobos, Gustavo A., Alejandro Escobar-Opazo, Félix Estrada, Sebastián Romero-Bravo, Miguel Garriga, Alejandro del Pozo, Carlos Poblete-Echeverría, Jaime Gonzalez-Talice, Luis González-Martinez, and Peter Caligari. 2019. "Spectral Reflectance Modeling by Wavelength Selection: Studying the Scope for Blueberry Physiological Breeding under Contrasting Water Supply and Heat Conditions" Remote Sensing 11, no. 3: 329. https://doi.org/10.3390/rs11030329
APA StyleLobos, G. A., Escobar-Opazo, A., Estrada, F., Romero-Bravo, S., Garriga, M., del Pozo, A., Poblete-Echeverría, C., Gonzalez-Talice, J., González-Martinez, L., & Caligari, P. (2019). Spectral Reflectance Modeling by Wavelength Selection: Studying the Scope for Blueberry Physiological Breeding under Contrasting Water Supply and Heat Conditions. Remote Sensing, 11(3), 329. https://doi.org/10.3390/rs11030329