Remote Detection of the Fluorescence Spectrum of Natural Pollens Floating in the Atmosphere Using a Laser-Induced-Fluorescence Spectrum (LIFS) Lidar
Abstract
:1. Introduction
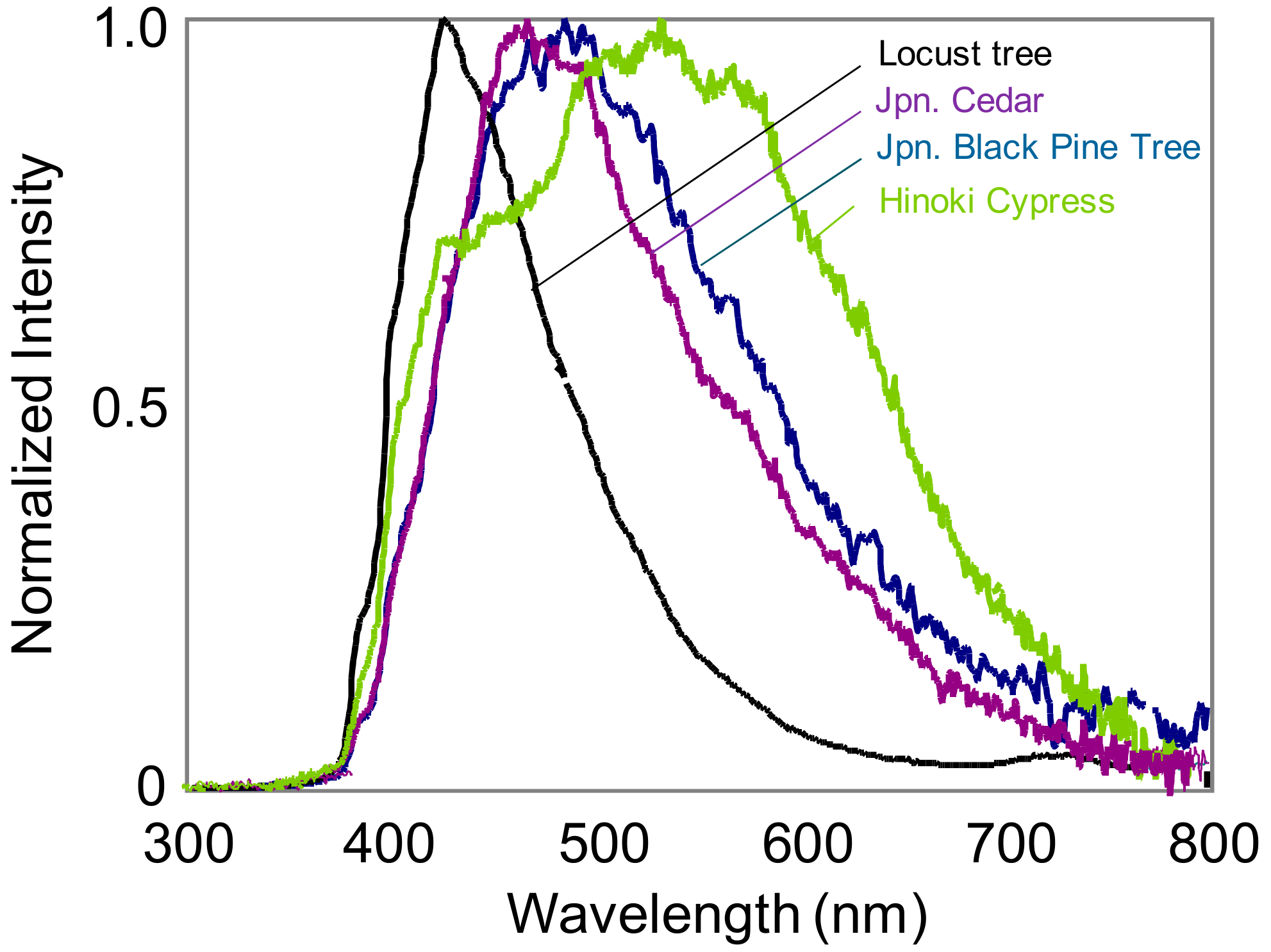
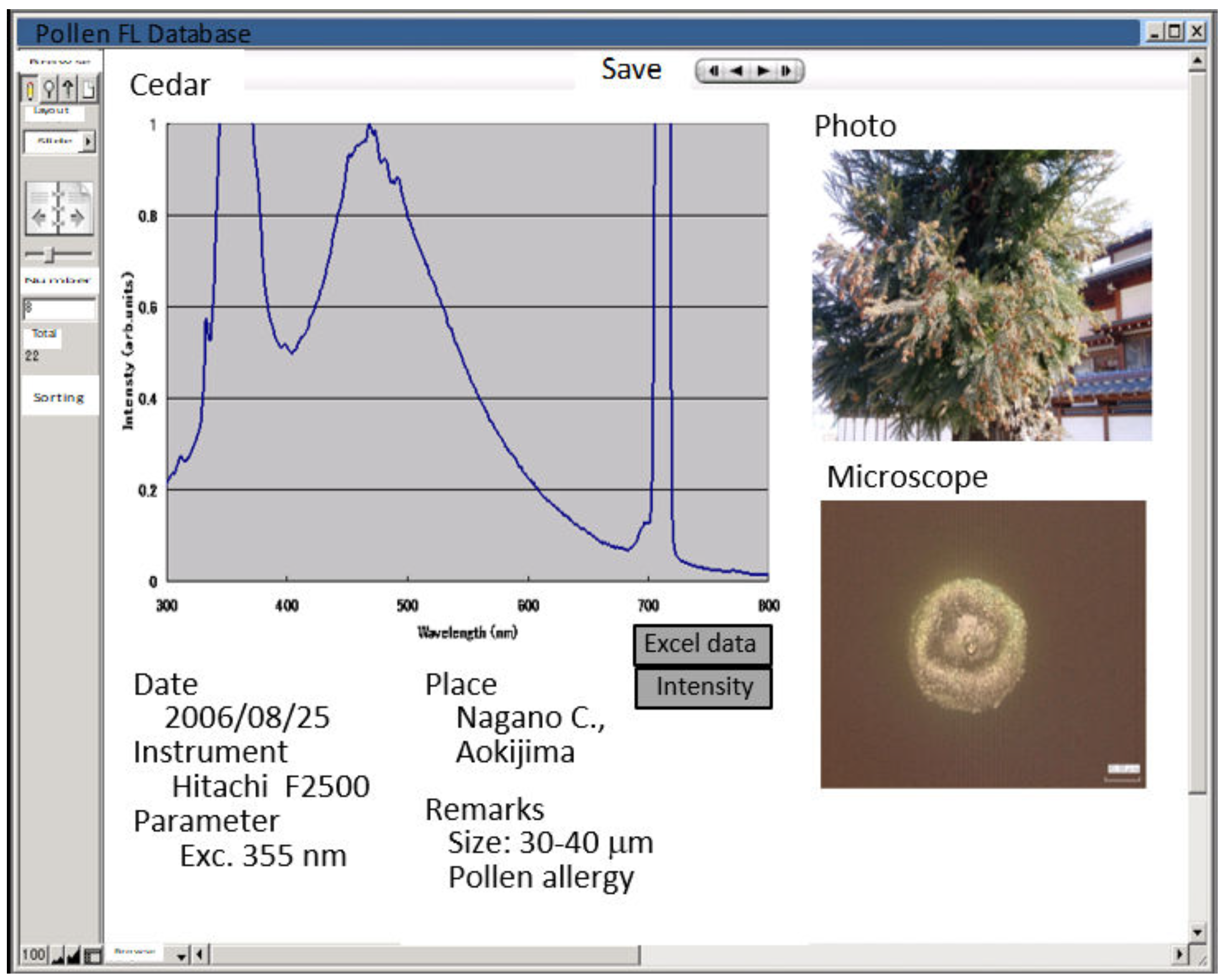
2. Spectral Characteristics of Pollen Fluorescence
3. LIFS Lidar System
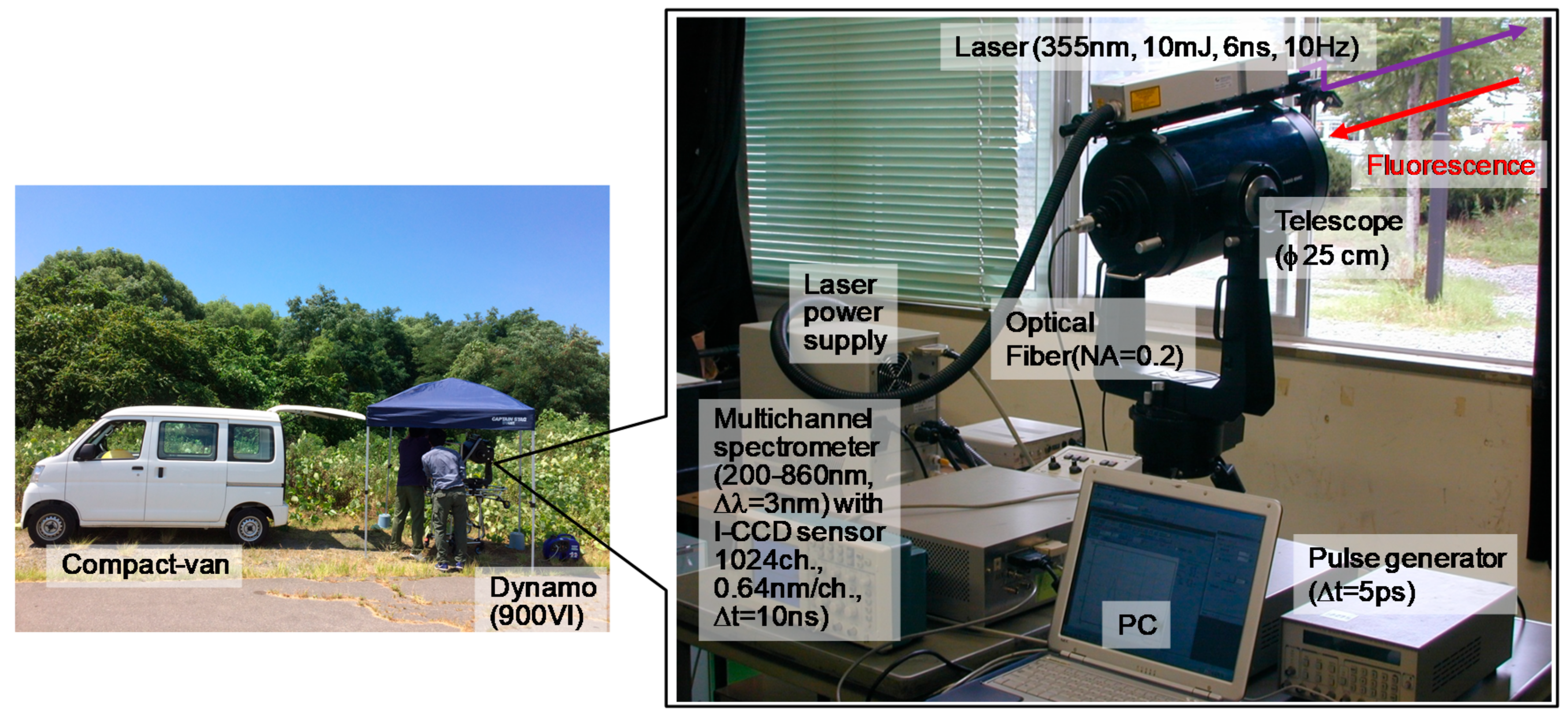
3.1. Design and Construction
3.2. Performance Check of LIFS Lidar
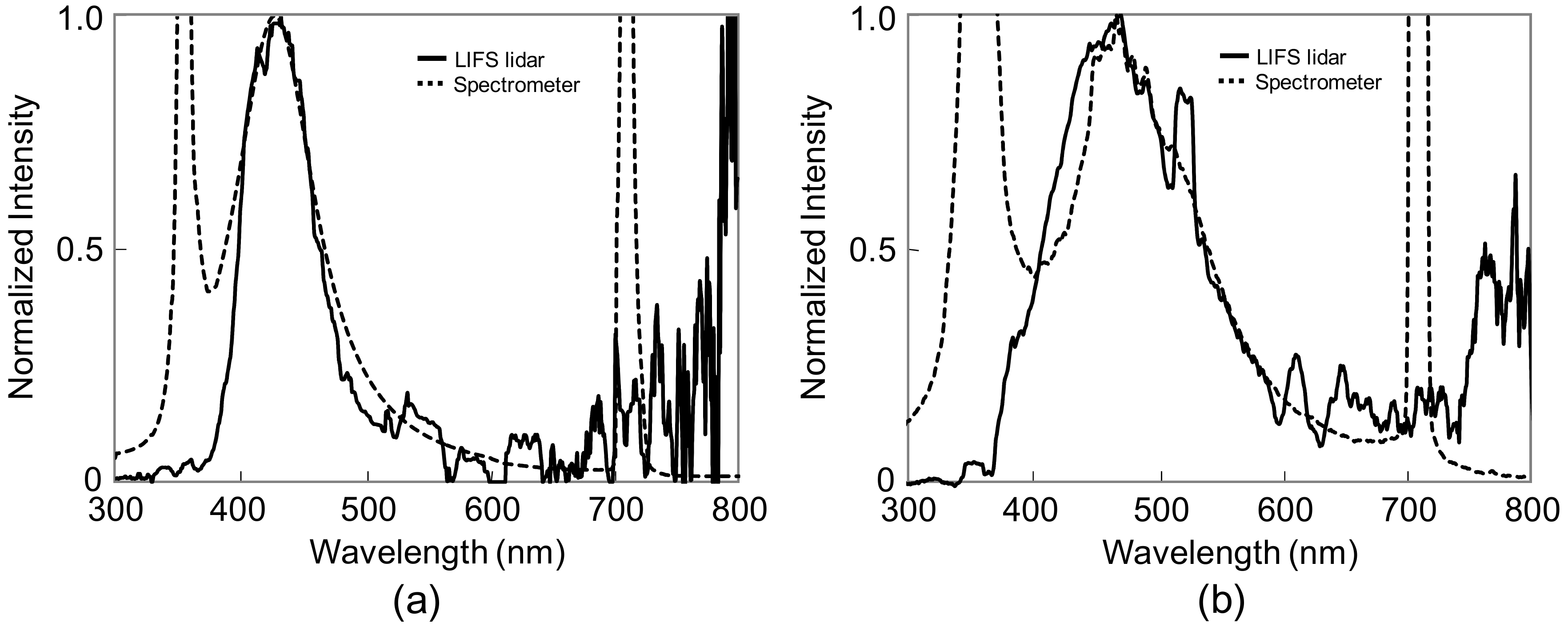
3.2.1. Detection of False Pollen (Flour Powder)
3.2.2. Detection of Natural Tree Pollen (Japanese Black Pine Tree)
4. Field Observations
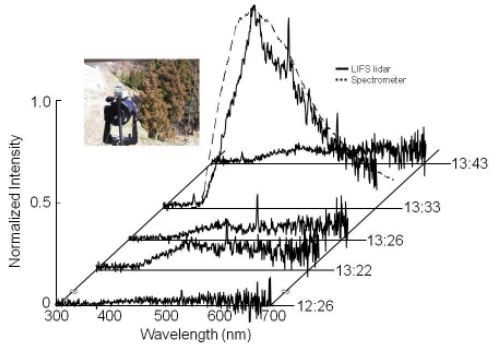
4.1. Cedar Pollen Observations
4.2. Ragweed Pollen Observations
5. Discussions
5.1. System Operations
5.2. Pollen Observations in Field
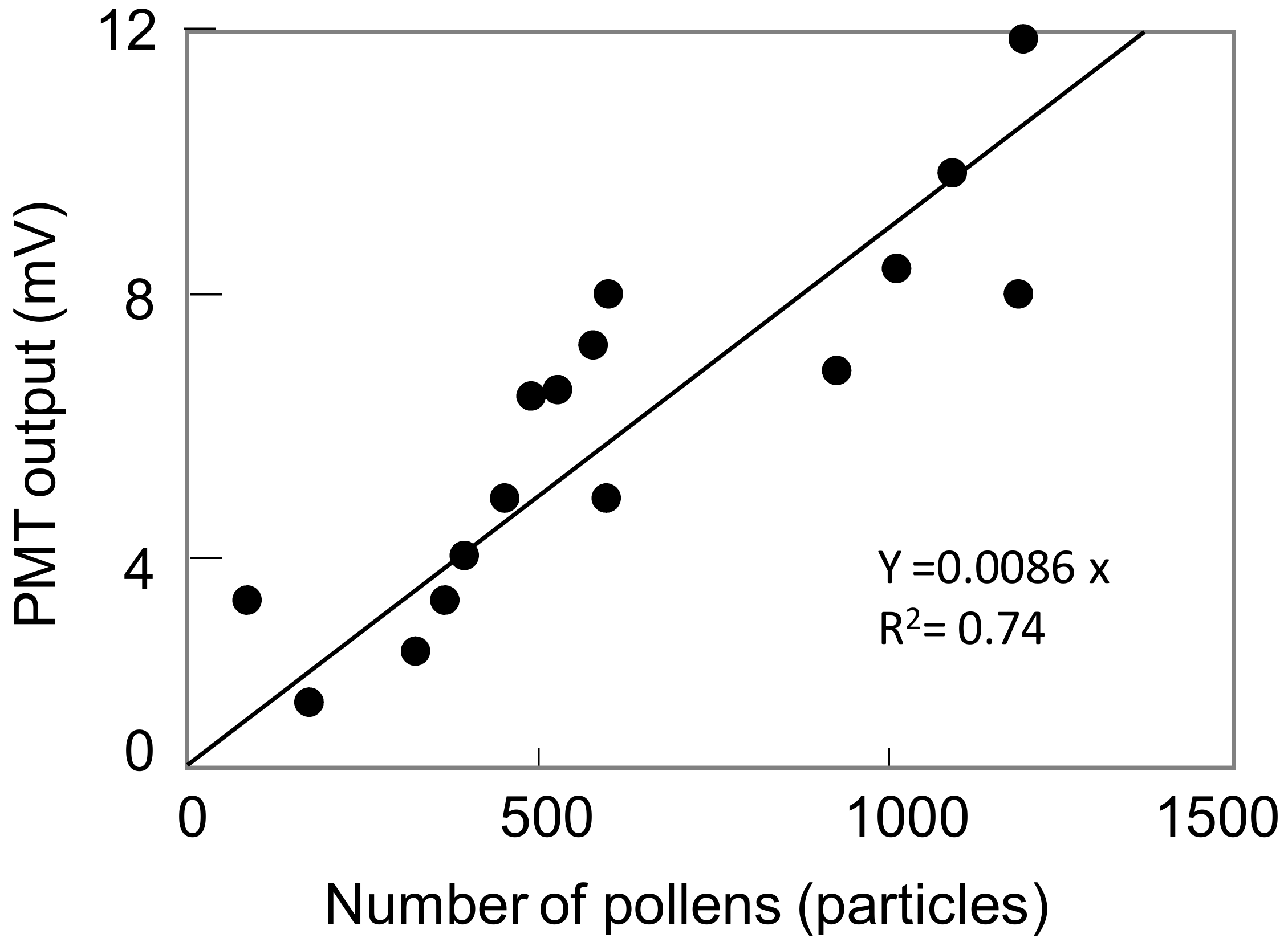
5.3. Estimation of Detected Particle Numbers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pollen Allergy-Australasian Society of Clinical Immunology and Allergy (ASCIA). Available online: https://www.allergy.org.au/patients/allergic-rhinitis-hay-fever-and-sinusitis/pollen-allergy (accessed on 17 August 2018).
- Baba, K.; Nakatsu, K. National epidemiological survey of nasal allergy in 2008. Prog. Med. 2008, 28, 145–156. (In Japanese) [Google Scholar]
- Ishizaki, T.; Koizumi, K.; Ikemori, R.; Ishiyma, Y.; Kushibiki, V. Studies of prevalence of Japanese cedar pollinosis among the residents in a densely cultivated area. Ann. Allergy 1987, 58, 265–270. [Google Scholar] [PubMed]
- Saito, Y. Japanese Cedar Pollinosis: Discovery, Nomenclature, and Epidemiological Trends. Available online: https://www.jstage.jst.go.jp/article/pjab/90/6/90_203/_article (accessed on 24 September 2018).
- American College of Allergy. Asthma and Immunology, The Year 2040: Double the pollen, Double the Allergy Suffering. Available online: https://acaai.org/news/year-2040-double-pollen-double-allergy-suffering (accessed on 24 September 2018).
- Pollen Monitoring System. Available online: http://kafun.taiki/go/jp (accessed on 17 August 2018). (In Japanese).
- Lee, J.; Kronborg, G.; O’Hehir, R.E.; Hew, M. Who’s risk of thunderstorm asthma? The ryegrass pollen trifecta and lessons learnt from the Melbourne thunderstorm epidemic. Respir. Med. 2017, 132, 46–148. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.D.; Schevin, P.; Ferrier, J.; Jolly, D.; Andreasen, T.; Ascanius, S.E.; Hendriksen, S.E.; Poulsen, U. Long-distance pollen transport from North America to Greenland in spring. J. Geophys. Res. 2008, 113, G02013. [Google Scholar] [CrossRef]
- Moss, P.T.; Kershaw, A.P.; Grindrod, J. Pollen transport and deposition in riverine and marine environments within the humid tropics of northeastern Australia. Rev. Palaeob. Palyn. 2005, 134, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Frei, T. Pollen distribution at high elevation in Switzerland: Evidence for medium range transport. Grana 1997, 36, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Immler, F.; Engelbart, D.; Schrems, O. Fluorescence from atmospheric aerosol detected by a lidar indicates biogenic particles in the lower stratosphere. Atmos. Chem. Phys. 2005, 5, 345–355. [Google Scholar] [CrossRef]
- Noh, Y.M.; Lee, H.; Mueller, D.; Lee, K.; Shin, D.; Shin, S.; Cho, T.J.; Choi, Y.J.; Kin, K.R. Investigation of the diurnal pattern of the vertical distribution of pollen in the lower troposphere using LIDAR. Atmos. Chem. Phys. 2013, 13, 7619–7629. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Roy, G.; Bernier, R. Lidar polarization discrimination of bioaerosols. Opt. Eng. 2010, 49, 116201. [Google Scholar]
- Sassen, K. Boreal tree pollen sensed by polarization lidar: Depolarizing biogenic chaff. Geophys. Res. Lett. 2008, 35, L18810. [Google Scholar] [CrossRef]
- Yee, E.; Kosteniuk, P.R.; Roy, G.; Evans, B.T.N. Remote biodetection performance of a pulsed monostatic lidar system. Appl. Opt. 1992, 31, 2900–2913. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.R.; Roy, G.; Mathieu, P.; Larochelle, V.; McFee, J.; Ho, J. Standoff sensing of bioaerosols using intensified range-gated spectral analysis of laser-induced fluorescence. IEEE Trans. Geosci. Remote Sens. 2004, 42, 865–874. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. The Fluorescence of Organic Natural Products. In Moleculaar Luminescence Spectroscopy; Schulman, S.G., Ed.; John Wiley & Sons: New York, NY, USA, 1985. [Google Scholar]
- Pohlker, C.; Huffman, J.A.; Poschl, U. Autofluorescence of atmospheric bioaerosols-fluorescent biomolecules and potential interferences. Atmos. Meas. Tech. 2012, 5, 37–71. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.L. Detection and characterization of biological and other organic-carbon aerosol particles in atmosphere using fluorescence. J. Q. Spec. Radiat. Transfer 2015, 150, 12–35. [Google Scholar] [CrossRef]
- Carrano, J.C.; Collins, C.J. (Eds.) Optically Based Biological and Chemical Detection for Defence V. Proc. SPIE 2009, 7484. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Samson, G.; Morales, F.; Tremblay, N.; Moya, I. Ultraviolet-induced fluorescence for plant monitoring: present state and prospectis. Agronomie 1999, 19, 543–578. [Google Scholar] [CrossRef]
- Saito, Y. Laser-induced fluorescence spectroscopy/technique as a tool for field monitoring of physiological status of living plants. Proc. SPIE 2007. [Google Scholar] [CrossRef]
- Pisano, A.M.; Dominicis, D.; Biamino, W.; Bignami, F.; Gherardi, S.; Colao, F.; Coppini, G.; Marullo, S.; Sprovieri, M.; Trivero, P.; et al. An oceanographic survey for oil spill monitoring and model forecasting validation using remote sensing and in situ data in the Mediterranean sea. Deep-Sea Res II 2016, 133, 132–145. [Google Scholar] [CrossRef]
- Saito, Y.; Kakuda, K.; Yokoyama, M.; Kubota, T.; Tomida, T.; Park, H.D. Design and daytime performance of laser-induced fluorescence spectrum lidar for simultaneous detection of multiple components, dissolved organic matter, phycocyanin, and chlorophyll in river water. Appl. Opt. 2016, 55, 6727–6734. [Google Scholar] [CrossRef] [PubMed]
- Weibring, P.; Johansson, T.; Edner, H.; Svanberg, S.; Sundner, B.; Raimondi, V.; Cecchi, G.; Pantani, L. Fluorescence lidar imaging of historical monuments. Appl. Opt. 2001, 40, 6111–6120. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, V.; Cecchi, G.; Lognoli, D.; Palombi, L.; Gronlund, R.; Johansson, A.; Svanberg, S.; Barcup, K.; Hallstorm, J. The fluorescence lidar technique for the remote senseing of photoautotrophic biodeteriogens in the outdoor cultural heritage: A decade of in situ experiments. Intern. Biodet. Biodeg. 2009, 63, 823–835. [Google Scholar] [CrossRef]
- Hodge, F.E.; Swift, R.N.; Yungel, J.K. Feasibility of airborne detection of laser-induced fluorescence emissions from green terrestrial plants. Appl. Opt. 1983, 22, 2991–3000. [Google Scholar] [CrossRef]
- Brydegaard, M.; Guan, Z.; Wellenreuther, M.; Svanberg, S. Insect monitoring with fluorescence lidar techniques: feasibility study. Appl. Opt. 2009, 48, 5668–5677. [Google Scholar] [CrossRef] [PubMed]
- Brydegaard, M.; Lundin, P.; Guan, Z.; Runemark, A.; Akesson, S.; Svanberg, S. Feasibility study: fluorescence lidar for remote bird classification. Appl. Opt. 2010, 49, 4531–4544. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Huang, Z.; Nishizawa, T.; Matsui, I.; Ttatarov, B. Fluorescence from atmospheric aerosols observed with a multi-channel lidar spectrometer. Opt. Exp. 2012, 20, 20800–20807. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Tomida, T.; Shiraishi, K. Fluorescence lidar seamlessly connecting individual observation of the globl environmental systems. Proceedings of Lidar Remote Sensing for Environmental Monitoring XVI, Honolulu, HI, USA, 24–25 September 2018. [Google Scholar]
- Stepens, J.R. Fluorescence cross section measurements of biological agent simultants. In Proceedings of the 1996 Edgewood RDEC Scientific Conference on Obscuration and Aerosol Research, Aberdeen, MD, USA, 19–22 November 1996. [Google Scholar]
- Weichert, R. Determination of backscatter and fluorescence cross-sections of biological aerosols. Landb. Volk. 2002, 34, 83–87. [Google Scholar]
- Reiner, W.; Klemm, W.; Legenhausen, K.; Pawellek, C. Determination of backscatter and fluorescence ross section of biological aerosols. Part. Syst. Charact. 2002, 19, 216–222. [Google Scholar]
- Baum, W.A.; Dunkelman, L. Horizontal attenuation of ultraviolet by the lower atmosphere. J. Opt. Soc. Amer. 1995, 45, 166–174. [Google Scholar] [CrossRef]
- Bertolotti, M.; Mluzii, L.; Sette, D. On the possibility of measuring optical visibility by using a Ruby laser. Appl. Opt. 1969, 8, 117–120. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Symbol | Value | |
|---|---|---|---|
| For PMA sensitivity calculation | Transmittance of lens | Tl | 92% |
| Optics efficiency | To | 93% | |
| Transmittance of filter | Tf | 92% | |
| Filter spectral width | ΔλF | 10 × 10−9 (m) | |
| PMT anode sensitivity | S | 4.4 × 101 (A/W) at −300 (V) | |
| Resistance | R | 50 (Ω) | |
| PMT output | VPMT | 21.42 × 10−3 (V) at −300 (V) | |
| PMA output | PPMA | 53376 (count/3 nm) at gain 8 | |
| For pollen number calculation | Laser power | P | 10 × 10−3 (/6 ns) |
| Fluorescence cross section of Cedar pollen | σ | 2 × 10−18 (m2 sr−1 nm−1) | |
| Overlapping factor | Y | 1 | |
| Telescope effective area | A | 0.045 (m2) | |
| Range | r | 20 (m) | |
| Velocity of light | c | 3 × 108 (m/s) | |
| Laser pulse width | tL | 6 × 10−9 (s) | |
| Atmospheric transmittance of laser | TL | 0.992 (km−1) at 355 nm for visibility of 20 km [35] | |
| Atmospheric transmittance of fluorescence | TF | 0.995 (km−1) at 460 nm for visibility of 20 km [36] | |
| PMA output | PPMA | 320 (count/3 nm) at gain 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, Y.; Ichihara, K.; Morishita, K.; Uchiyama, K.; Kobayashi, F.; Tomida, T. Remote Detection of the Fluorescence Spectrum of Natural Pollens Floating in the Atmosphere Using a Laser-Induced-Fluorescence Spectrum (LIFS) Lidar. Remote Sens. 2018, 10, 1533. https://doi.org/10.3390/rs10101533
Saito Y, Ichihara K, Morishita K, Uchiyama K, Kobayashi F, Tomida T. Remote Detection of the Fluorescence Spectrum of Natural Pollens Floating in the Atmosphere Using a Laser-Induced-Fluorescence Spectrum (LIFS) Lidar. Remote Sensing. 2018; 10(10):1533. https://doi.org/10.3390/rs10101533
Chicago/Turabian StyleSaito, Yasunori, Kentaro Ichihara, Kenzo Morishita, Kentaro Uchiyama, Fumitoshi Kobayashi, and Takayuki Tomida. 2018. "Remote Detection of the Fluorescence Spectrum of Natural Pollens Floating in the Atmosphere Using a Laser-Induced-Fluorescence Spectrum (LIFS) Lidar" Remote Sensing 10, no. 10: 1533. https://doi.org/10.3390/rs10101533