A Novel Algorithm for Predicting Phycocyanin Concentrations in Cyanobacteria: A Proximal Hyperspectral Remote Sensing Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains of Cyanobacteria, Green Algae, and Culture Condition
2.2. Data Collection
| Exp. | Pigment | Mean | Std. Dev. | Range | Min | Max | N |
|---|---|---|---|---|---|---|---|
| I | PC (cells mL–1) | 85529.82 | 75586.96 | 240910.00 | 7050.00 | 247960.00 | 11 |
| Chl-a (µg L–1) | 3.40 | 2.39 | 7.10 | 0.70 | 7.80 | 8 | |
| II | PC (cells mL–1) | 50409.40 | 41727.17 | 126064.00 | 506.00 | 126570.00 | 20 |
| Chl-a (µg L–1) | 2.48 | 0.78 | 1.90 | 1.80 | 3.70 | 5 | |
| III | PC (cells mL–1) | 118360.00 | 100771.72 | 269788.00 | 4095.00 | 273883.00 | 12 |
| Chl-a (µg L–1) | 13.32 | 7.12 | 19.80 | 2.10 | 21.90 | 12 | |
| IV | PC (cells mL–1) | 94137.09 | 76424.23 | 239950.00 | 4550.00 | 244500.00 | 11 |
3. Results and Discussion
3.1. Analysis of Reflectance Spectra
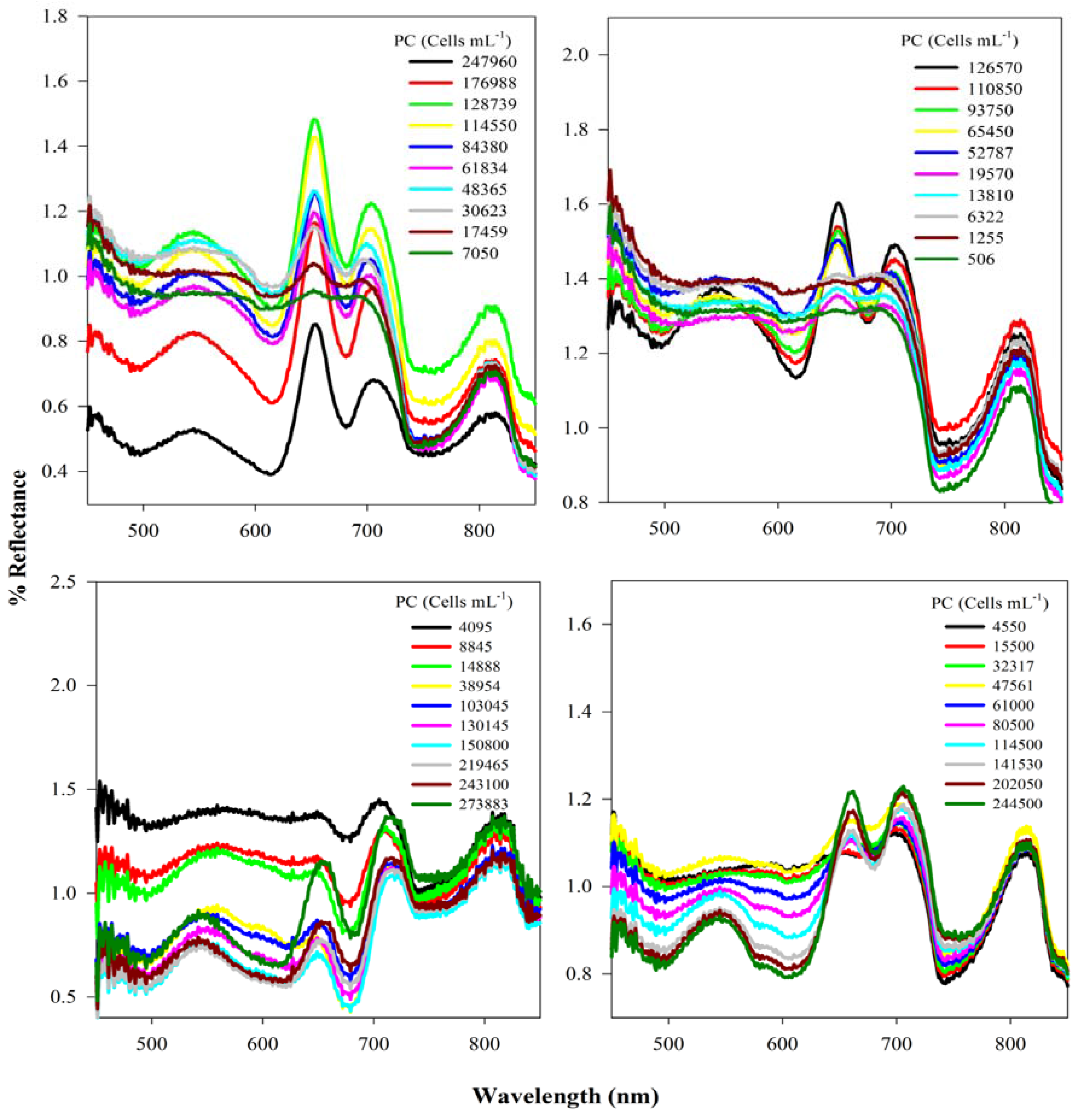
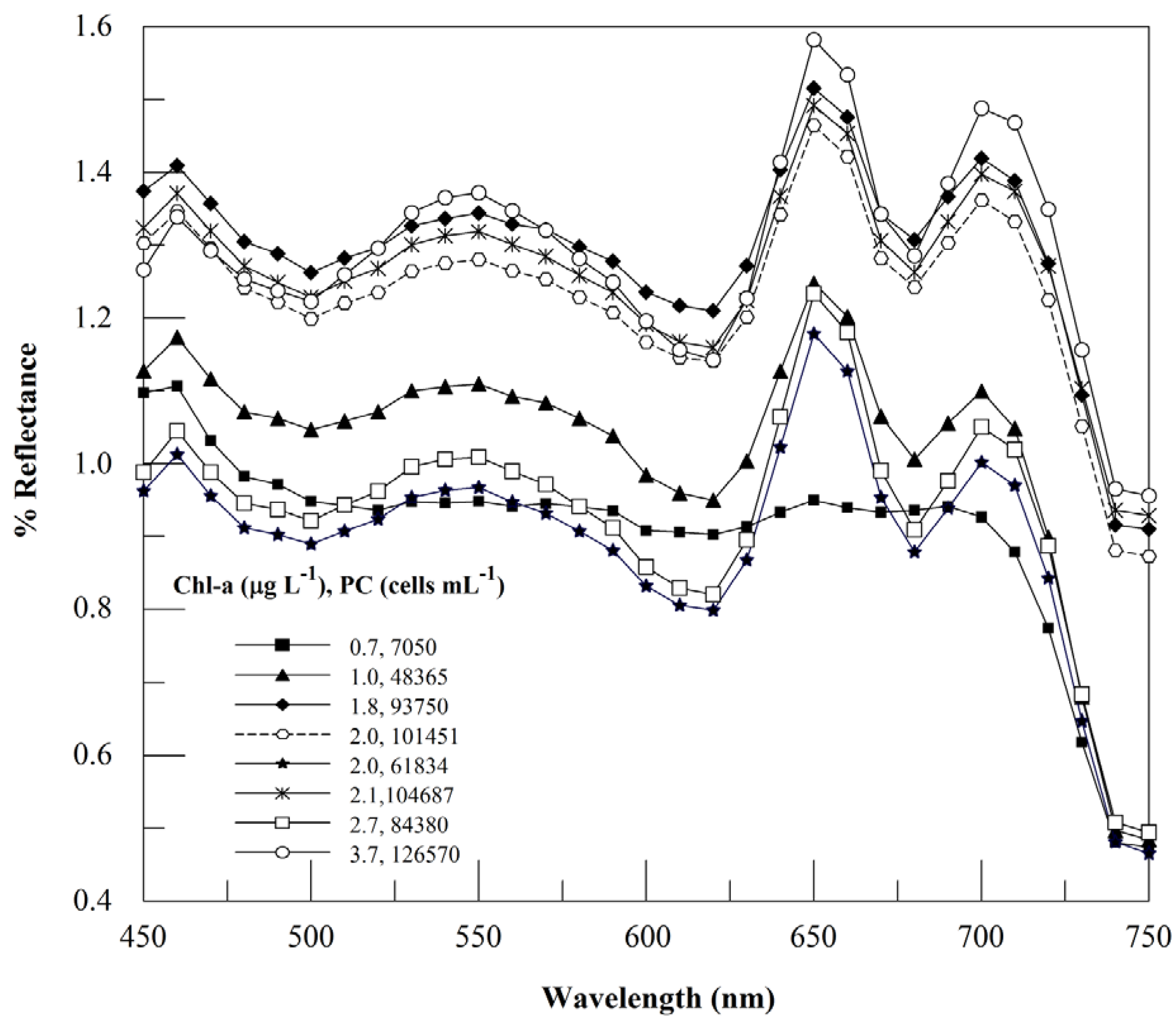
3.2. Context for Model Development

3.3. Usefulness of 654 nm Peak in PC Band Ratio Models
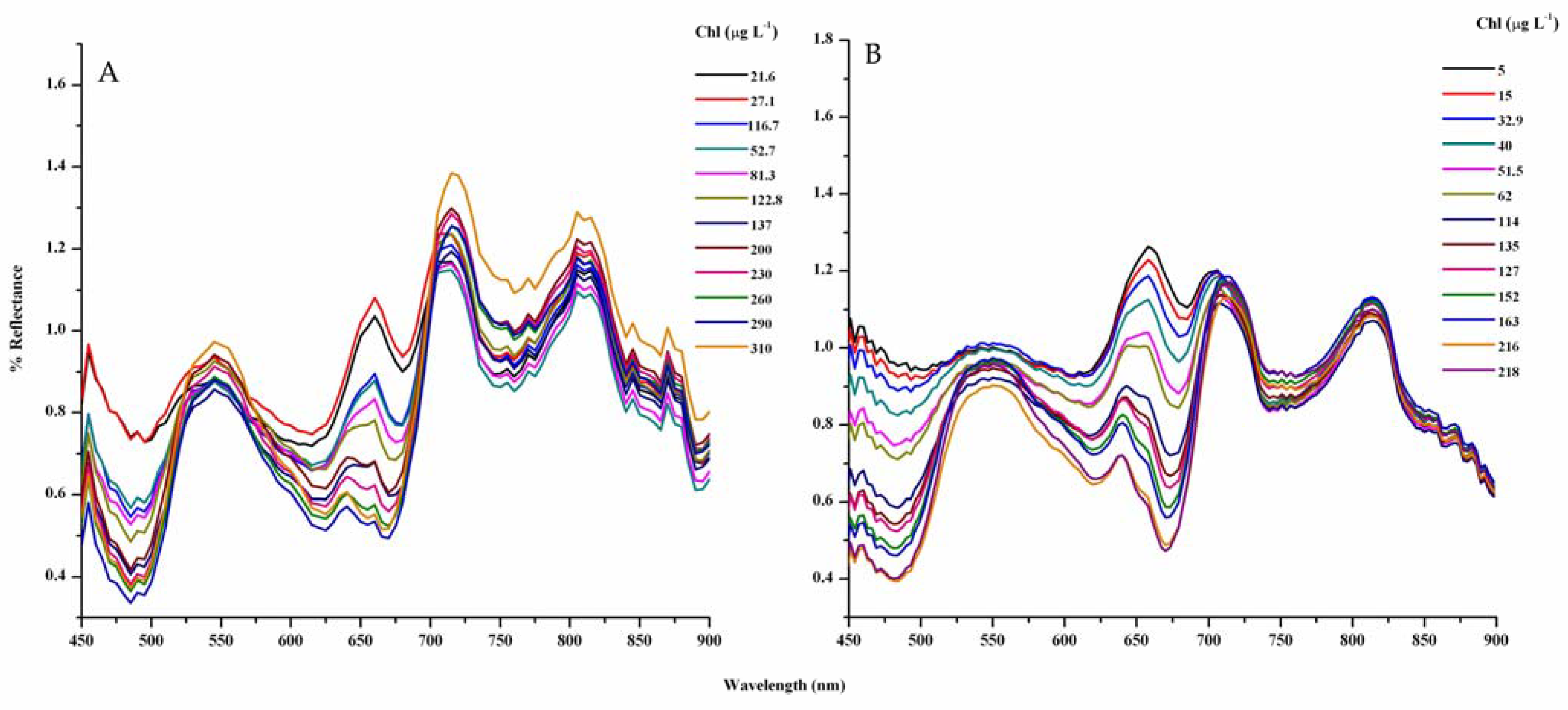
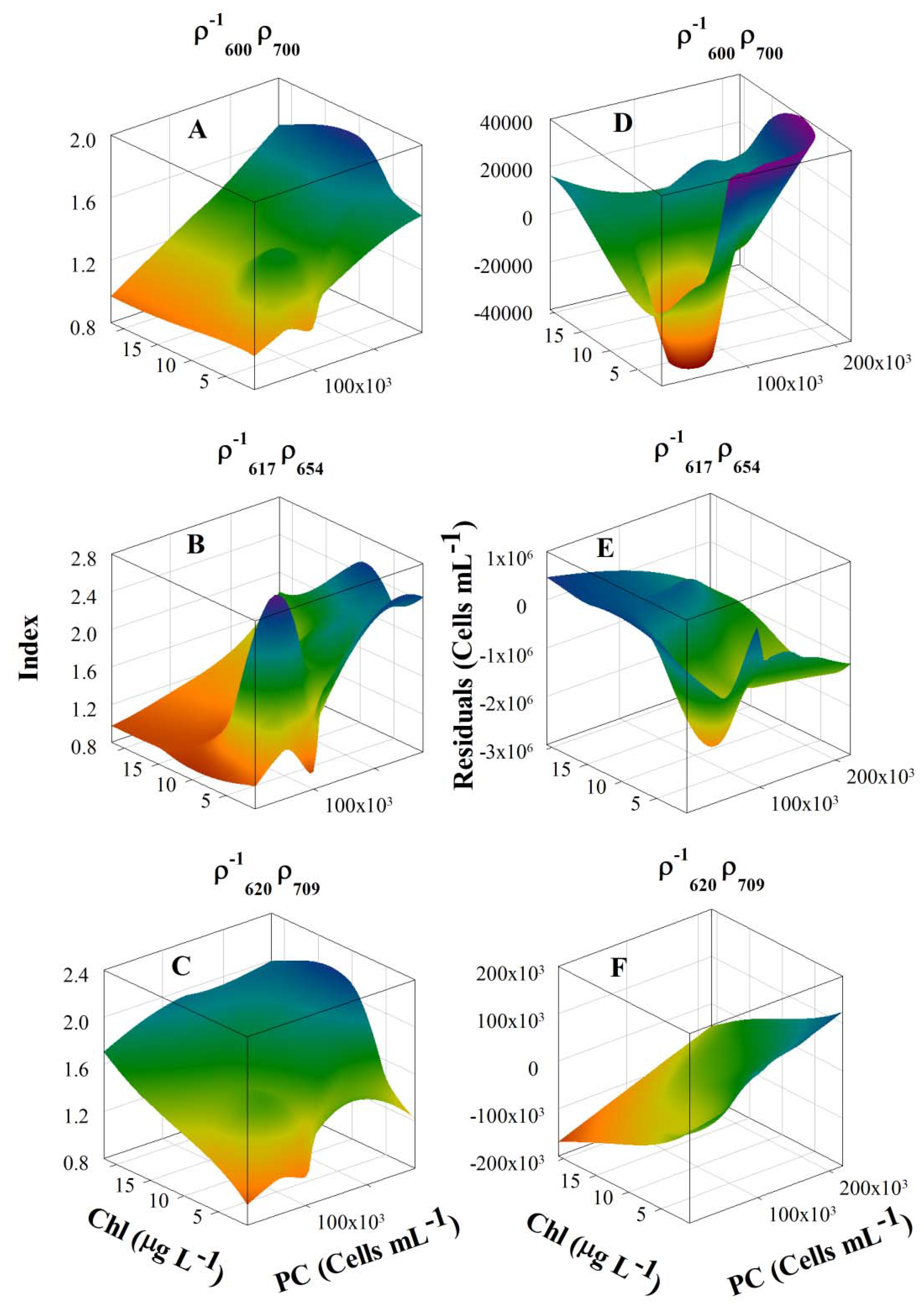
3.4. Model Development and Calibration

| Band Combination | a0 (STE) | a1 (STE) | R2 | Adj. R2 | STE |
|---|---|---|---|---|---|
| Synechocystis dataset (Exp I, II and III) | |||||
| 1.0176 (0.0526) | 3.1921 × 10−7 (4.3934 × 10−7) | 0.71 | 0.70 | 0.1732 | |
| 0.9270 (0.0365) | 3.8154 × 10−6 (3.0458 × 10−7) | 0.88 | 0.87 | 0.1201 | |
| 0.9773 (0.0098) | 2.4985 × 10−6 (8.1863 × 10−8) | 0.97 | 0.97 | 0.0323 | |
| −0.0044 (0.006) | 1.9394 × 10−6 (5.0150 × 10−8) | 0.98 | 0.98 | 0.0198 | |
| Synechocystis and Anabaena dataset (Exp I, II, III and IV) | |||||
| 1.0085 (0.0101) | 2.2589 × 10−6 (9.3327 × 10−8) | 0.95 | 0.95 | 0.0387 | |
| 0.0199 (0.0083) | 1.7889 × 10−6 (7.616 × 10−8) | 0.95 | 0.95 | 0.0316 | |
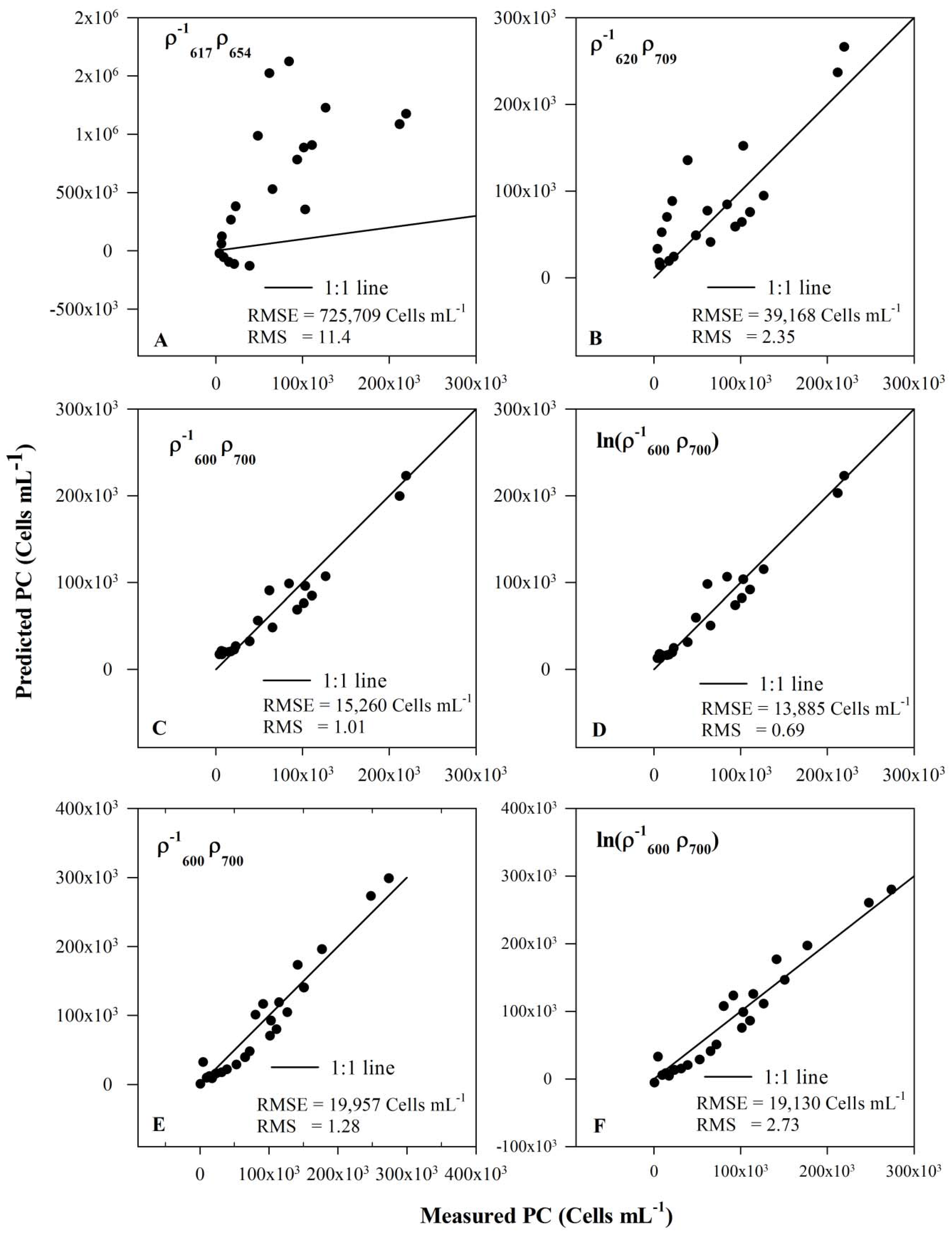
3.5. Model Validation
| Band Combination | RMSE | RMS | R2 |
|---|---|---|---|
| Synechocystis data set (Exp I, II and III) | |||
| 725,709 | 11.4 | 0.45 | |
| 39,168 | 2.35 | 0.70 | |
| 15,260 | 1.01 | 0.94 | |
| 13,885 | 0.69 | 0.95 | |
| Synechocystis and Anabaena dataset (Exp I, II, III and IV) | |||
| 19,957 | 1.28 | 0.94 | |
| 19,130 | 2.73 | 0.94 | |

4. Conclusions
Acknowledgements
References and Notes
- Carmichael, W.W. The cyanotoxins. Adv. Bot. Res. 1997, 27, 211–256. [Google Scholar]
- Iwasa, M.; Yamamoto, M.; Tanaka, Y.; Kaito, M.; Adachi, Y. Spirulina-associated hepatotoxicity. Am. J. Gastroenterol. 2002, 97, 3212–3213. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Stephan, P.; Wiegand, C.; Kotut, K.; Krienitz, L. Cyanobacterial toxins in Lake Baringo, Kenya. Limnologica. 2003, 33, 2–9. [Google Scholar] [CrossRef]
- Backer, L.C. Cyanobacterial harmful algal blooms: developing a public health response. Lake Reserv. Manage. 2002, 18, 20–31. [Google Scholar] [CrossRef]
- Golbeck, J.H.; Bryant, D.A. Photosytem I. In Current Topics in Bioenergetics, Light Driven Reactions in Bioenergetics; Lee, C.P., Ed.; Academic Press: New York, NY, USA, 1991; pp. 83–177. [Google Scholar]
- Richardson, L.L. Remote sensing of algal bloom dynamics. BioSci. 1996, 46, 492–501. [Google Scholar] [CrossRef]
- Glazer, A.N. Light guides: directional energy transfer in a photosynthetic antenna. J. Biol. Chem. 1989, 264, 1–4. [Google Scholar] [PubMed]
- Simis, S.; Peters, S.; Gons, H. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Schalles, J.; Yacobi, Y. Remote detection and seasonal patterns of phycocyanin, carotenoid and Chl-a pigments in eutrophic waters. Archive Hydrobiol. Sp. Issues Adv. Limnol. 2000, 55, 153–168. [Google Scholar]
- Dekker, A.G. Detection of the Optical Water Quality Parameters for Eutrophic Waters by High Resolution Remote Sensing. Ph.D. Thesis, Free Universit, Amsterdam, The Netherlands, 1993. [Google Scholar]
- Simis, S.; Ruiz-Verdú, A.; Domínguez-Gómez, J.; Peña-Martinez, R.; Peters, S.; Gons, H. Influence of phytoplankton pigment composition on remote sensing cyanobacterial biomass. Remote Sens. Environ. 2007, 106, 414–427. [Google Scholar] [CrossRef]
- Kutser, T.; Metsamaa, L.; Strombeck, N.; Vahtmae, E. Monitoring cyanobacterial blooms by satellite remote sensing. Estuar. Coast. Shelf S. 2006, 67, 303–312. [Google Scholar] [CrossRef]
- Vincent, R.K.; Qin, X.M.; McKay, R.M.L.; Miner, J.; Czajkowski, K.; Savino, J.; Bridgeman, T. Phycocyanin detection from LANDSAT TM data for mapping cyanobacterial blooms in Lake Erie. Remote Sens. Environ. 2004, 89, 381–392. [Google Scholar] [CrossRef]
- Randolph, K.; Wilson, J.; Tedesco, L.; Li, L.; Pascual, D.; Soyeux, M. Hyperspectral remote sensing of cyanobacteria in turbid productive water using optically active pigments, chlorophyll a and phycocyanin. Remote Sens. Environ. 2008, 112, 4009–4019. [Google Scholar] [CrossRef]
- Metsamaa, L.; Kutser, T.; Strombeck, N. Recognising cyanobacterial blooms based on their optical signature: a modeling study. Boreal Environ. Res. 2006, 11, 493–506. [Google Scholar]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [PubMed]
- Gitelson, A.A.; Schalles, J.F.; Rundquist, D.C.; Schiebe, F.R.; Yacobi, Y.Z. Comparative reflectance properties of algal cultures with manipulated densities. J. Appl. Phycol. 1999, 11, 345–354. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Yacobi, Y.Z.; Schalles, J.F.; Rundquist, D.C.; Han, L.; Stark, R.; Etzion, D. Remote estimation of phytoplankton density in productive waters. Archive Hydrobiol. Sp. Issues Adv. Limnol. 2000, 55, 121–136. [Google Scholar]
- Lemasson, C.; Marsac, N.T.D.; Cohen-Bazire, G. Role of allophycocyanin as a light-harvesting pigment in cyanobacteria. P. Natl. Acad. Sci. USA 1973, 70, 3130–3133. [Google Scholar] [CrossRef]
- Rowan, K.S. Photosynthetic Pigments of Algae, 1st ed.; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Glazer, A.N. Phycobiliproteins. Method. Enzymol. 1988, 167, 291–303. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Curtis, H. Biology of Plants; Worth Publishers: New York, NY, USA, 1976. [Google Scholar]
- Emerson, R.; Lewis, C.M. The photosynthetic efficiency of phycocyanin in Chroococcus, and the problem of carotenoid participation in photosynthesis. J. Gen. Physiol. 1942, 25, 579–595. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Lazzara, L.; Prieur, L. Variations in the spectral values of specific absorption of phytoplankton. Limnol. Oceanogr. 1987, 32, 403–415. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the Chl-a specific absorption coefficients of natural phytoplankton-analysis and parameterization. J. Geophys. Res.—Oceans. 1995, 100, 13321–13332. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mishra, S.; Mishra, D.R.; Schluchter, W.M. A Novel Algorithm for Predicting Phycocyanin Concentrations in Cyanobacteria: A Proximal Hyperspectral Remote Sensing Approach. Remote Sens. 2009, 1, 758-775. https://doi.org/10.3390/rs1040758
Mishra S, Mishra DR, Schluchter WM. A Novel Algorithm for Predicting Phycocyanin Concentrations in Cyanobacteria: A Proximal Hyperspectral Remote Sensing Approach. Remote Sensing. 2009; 1(4):758-775. https://doi.org/10.3390/rs1040758
Chicago/Turabian StyleMishra, Sachidananda, Deepak R. Mishra, and Wendy M. Schluchter. 2009. "A Novel Algorithm for Predicting Phycocyanin Concentrations in Cyanobacteria: A Proximal Hyperspectral Remote Sensing Approach" Remote Sensing 1, no. 4: 758-775. https://doi.org/10.3390/rs1040758




