Improvements in Soil Carbon and Nitrogen Capacities after Shrub Planting to Stabilize Sand Dunes in China’s Horqin Sandy Land
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Site Selection and Location
2.3. Soil Sampling
2.4. Laboratory Analyses
2.5. Data Analyses
3. Results
3.1. Changes in Soil Particle-Size Distributions and Bulk Density
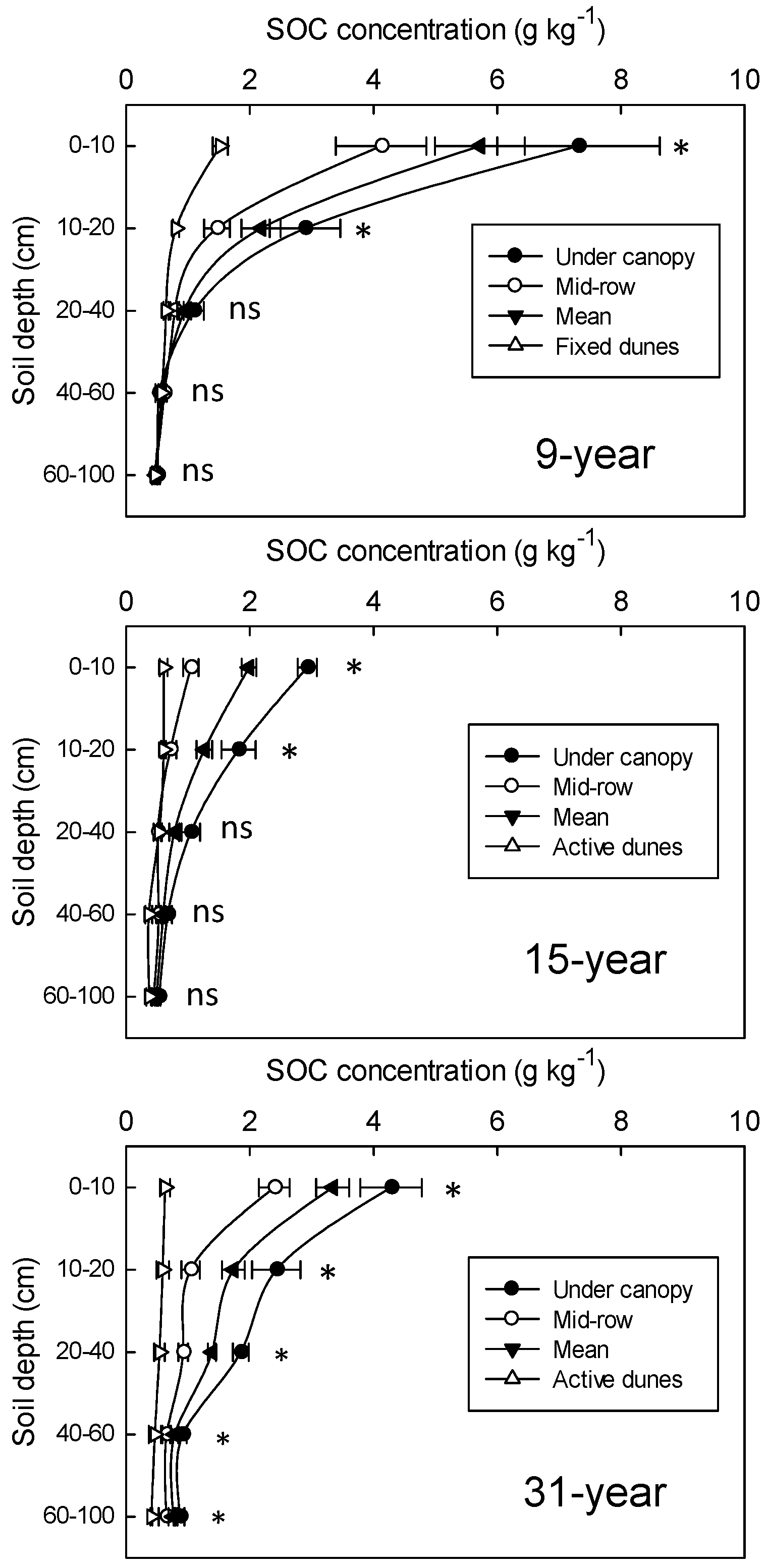
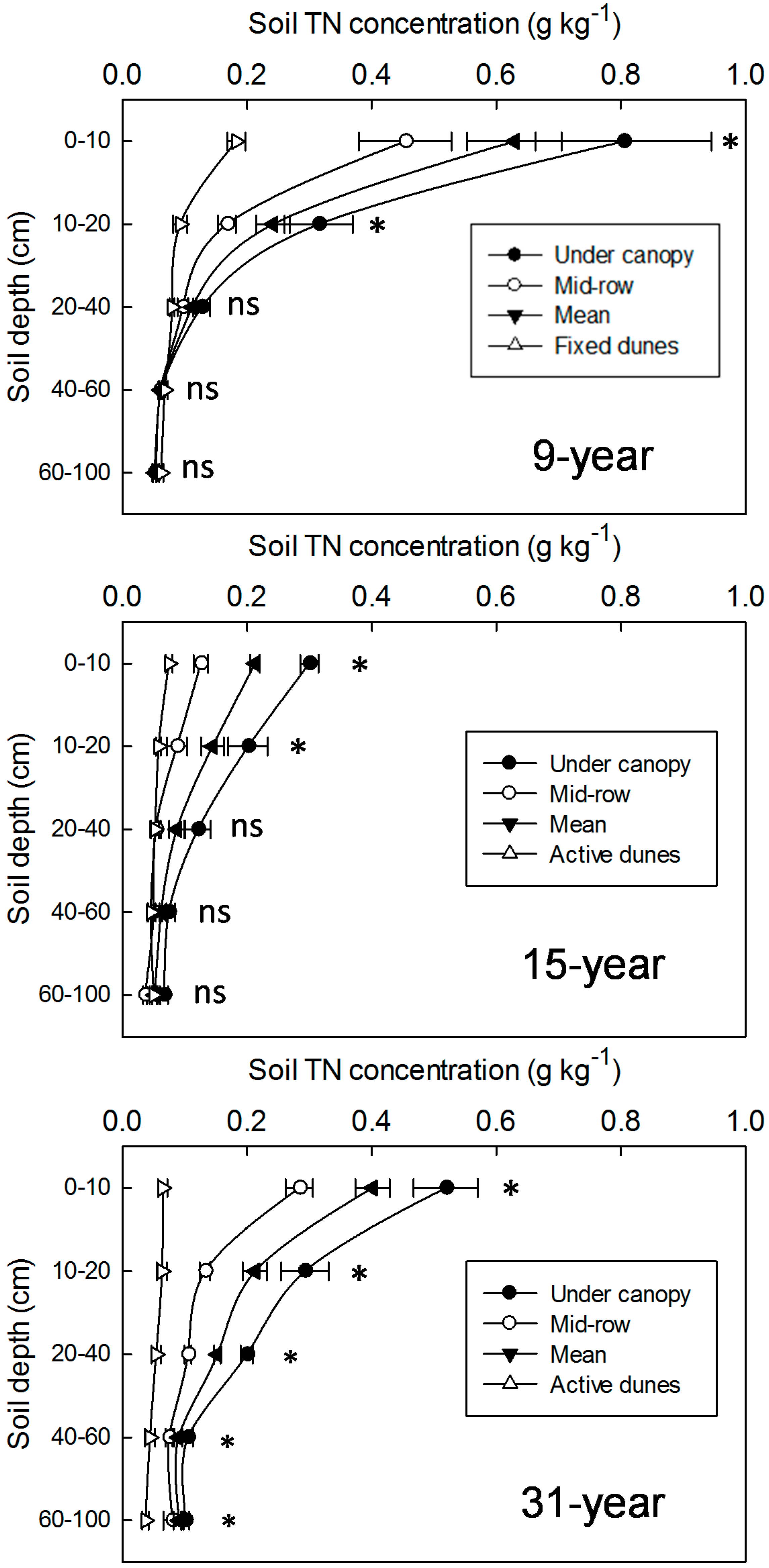
3.2. Changes in SOC and TN Concentrations
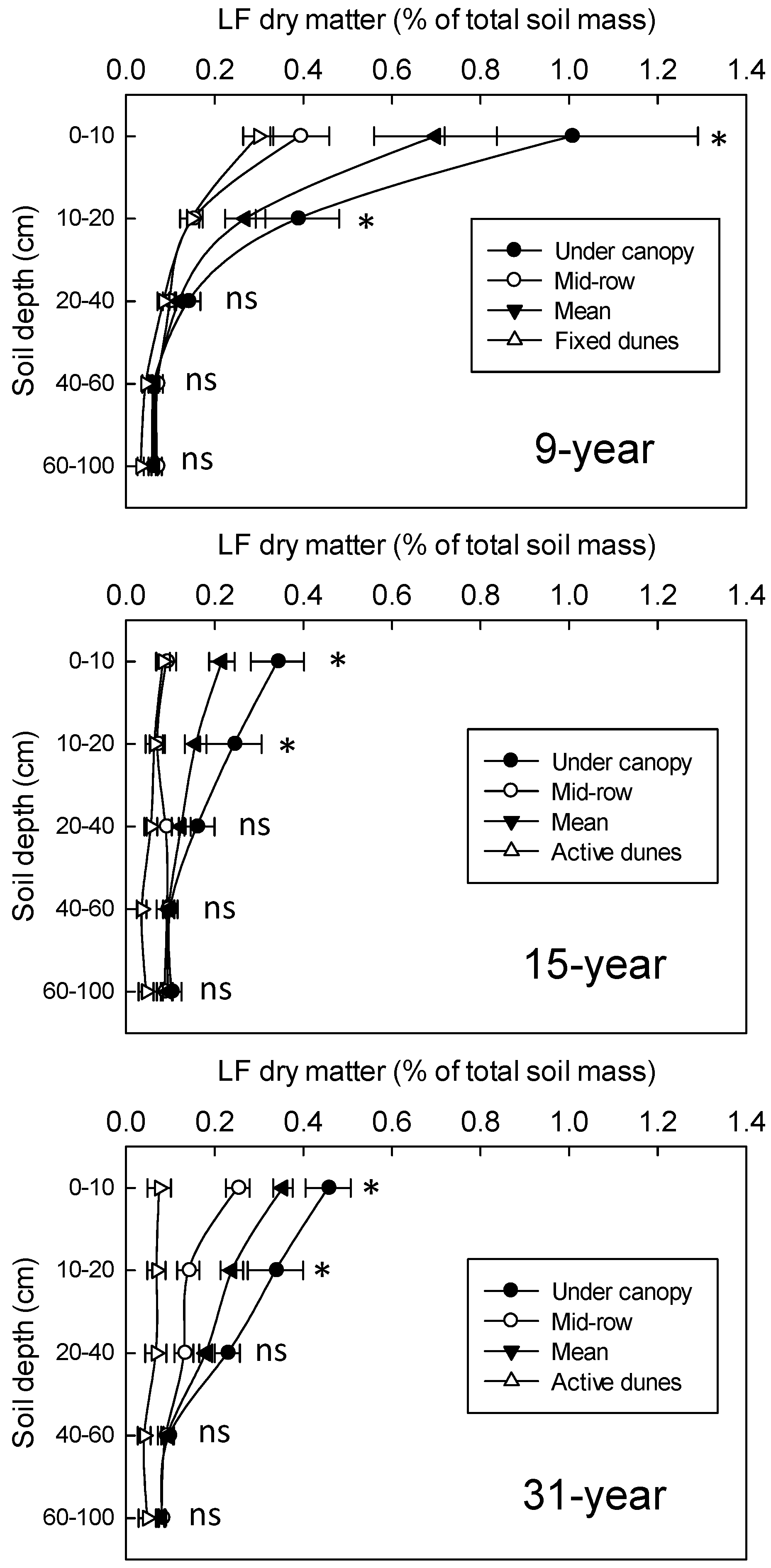
3.3. Changes in Soil LF Organic Matter
3.4. Changes in Soil C and N Storage and Accumulation Rates
4. Discussion
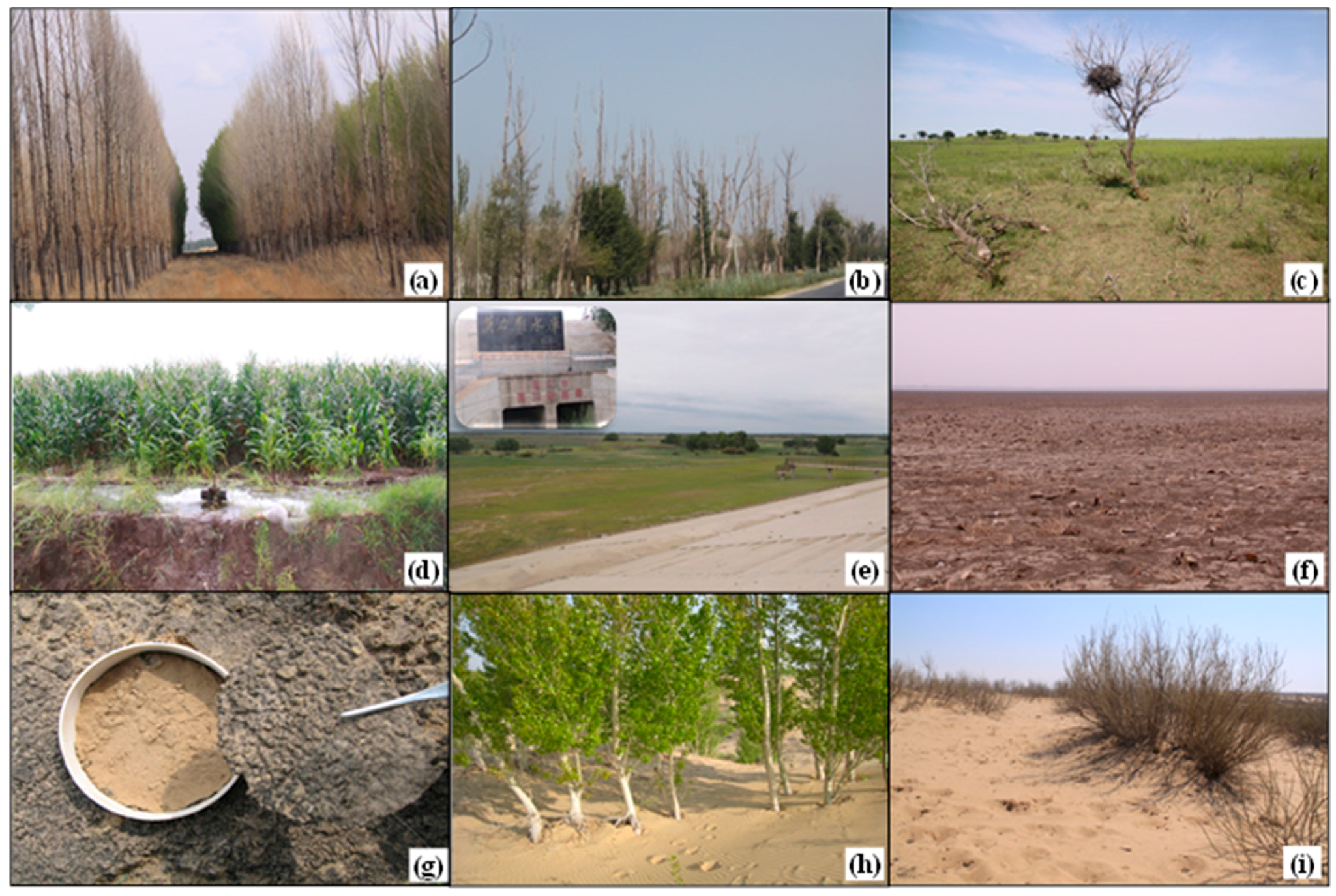
4.1. Challenges for Afforestation in Arid and Semiarid Ecosystems
4.2. Carbon Sequestration Potential of Desertification Control in China’s Horqin Sandy Land
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lal, R. Sequestering carbon in soils of arid ecosystems. Land Degrad. Dev. 2009, 20, 441–454. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Carbon Sequestration in Drylands; FAO: Rome, Italy, 2004. [Google Scholar]
- Lal, R. Potential of desertification control to sequester carbon and mitigate the greenhouse effect. Clim. Chang. 2001, 51, 35–72. [Google Scholar] [CrossRef]
- Nosetto, M.D.; Jobbágy, E.G.; Paruelo, J.M. Carbon sequestration in semi-arid rangelands: Comparison of Pinus ponderosa plantations and grazing exclusion in NW Patagonia. J. Arid Environ. 2006, 67, 142–156. [Google Scholar] [CrossRef]
- Malagnoux, M. Arid land forests of the world: Global environmental perspectives. In Proceedings of the International Conference on Afforestation and Sustainable Forests as a Means to Combat Desertification, Jerusalem, Israel, 16–19 April 2007. [Google Scholar]
- Le Houérou, H.N. Restoration and rehabilitation of arid and semiarid Mediterranean ecosystems in North Africa and West Asia: A review. Arid Soil Res. Rehabil. 2000, 14, 3–14. [Google Scholar] [CrossRef]
- Wofsy, S.C. Where has all the carbon gone? Science 2001, 292, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Grünzweig, J.M.; Lin, T.; Rotenberg, E.; Schwartz, A.; Yakir, D. Carbon sequestration in arid-land forest. Glob. Chang. Biol. 2003, 9, 791–799. [Google Scholar] [CrossRef]
- Nair, P.K.R. Methodological challenges in estimating carbon sequestration potential of agroforestry systems. In Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges, Advances in Agroforestry 8; Kumar, B.M., Nair, P.K.R., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Zhang, W.T.; Hu, G.Q.; Dang, Y.; Weindorf, D.C.; Sheng, J.D. Afforestation and the impacts on soil and water conservation at decadal and regional scales in Northwest China. J. Arid Environ. 2016, 130, 98–104. [Google Scholar] [CrossRef]
- Li, X.J.; Li, X.R.; Wang, X.P.; Yang, H.T. Changes in soil organic carbon fractions after afforestation with xerophytic shrubs in the Tengger Desert, northern China. Eur. J. Soil Sci. 2016, 67, 184–195. [Google Scholar] [CrossRef]
- Scott, N.A.; Tate, K.R.; Ford-Robertson, J.; Giltrap, D.J.; Smith, C.T. Soil carbon storage in plantation forests and pastures: Land-use change implications. Tellus 1999, 51B, 326–335. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Cunningham, S.C.; Thomson, J.R.; Baker, P.J.; Beringer, J.; Cavagnaro, T.R. Does afforestation of pastures increase sequestration of soil carbon in Mediterranean climates? Agric. Ecosyst. Environ. 2012, 159, 176–183. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, C.X.; Hasi, E.; Dong, Z.B. Has the Three Norths Forest Shelterbelt Program solved the desertification and dust storm problems in arid and semiarid China? J. Arid Environ. 2010, 74, 13–22. [Google Scholar] [CrossRef]
- Li, Y.Q.; Brandle, J.; Awada, T.; Chen, Y.P.; Han, J.J.; Zhang, F.X.; Luo, Y.Q. Accumulation of carbon and nitrogen in the plant-soil system after afforestation of active sand dunes in China’s Horqin Sandy Land. Agric. Ecosyst. Environ. 2013, 177, 75–84. [Google Scholar] [CrossRef]
- Zhou, R.L.; Li, Y.Q.; Zhao, H.L.; Drake, S. Desertification effects on C and N content of sandy soils under grassland in Horqin, northern China. Geoderma 2008, 145, 370–375. [Google Scholar] [CrossRef]
- Yan, Q.L.; Zhu, J.J.; Hu, Z.B.; Sun, O.J. Environmental impacts of the shelter forests in Horqin Sandy Land, Northeast China. J. Environ. Qual. 2011, 40, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, X.H.; Brandle, J.; Zhang, T.H.; Chen, Y.P.; Han, J.J. Temporal progress in improving carbon and nitrogen storage by grazing exclosure practice in a degraded land area of China’s Horqin Sandy Grassland. Agric. Ecosyst. Environ. 2012, 159, 55–61. [Google Scholar] [CrossRef]
- Cao, C.Y.; Jiang, S.Y.; Ying, Z.; Zhang, F.X.; Han, X.S. Spatial variability of soil nutrients and microbiological properties after the establishment of leguminous shrub Caragana microphylla Lam. plantation on sand dune in the Horqin Sandy Land of Northeast China. Ecol. Eng. 2011, 37, 1467–1475. [Google Scholar] [CrossRef]
- Jiang, D.M.; Cao, C.Y.; Zhang, Y.; Cui, Z.B.; Han, X.S. Plantations of native shrub species restore soil microbial diversity in the Horqin Sandy Land, Northeastern China. J. Arid Land 2014, 6, 445–453. [Google Scholar] [CrossRef]
- Su, Y.Z.; Zhao, H.L. Soil properties and plant species in an age sequence of Caragana microphylla plantations in the Horqin Sandy Land, North China. Ecol. Eng. 2003, 20, 223–235. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhao, X.Y.; Wang, S.K.; Zhang, F.X.; Lian, J.; Huang, W.D.; Mao, W. Carbon accumulation in the bulk soil and different soil fractions during the rehabilitation of desertified grassland in Horqin Sandy Land, northern China. Pol. J. Ecol. 2015, 63, 88–101. [Google Scholar] [CrossRef]
- Murage, E.W.; Voroney, P.; Beyaert, R.P. Turnover of carbon in the free light fraction with and without charcoal as determined using the 13C natural abundance method. Geoderma 2007, 138, 133–143. [Google Scholar] [CrossRef]
- Sequeira, C.H.; Alley, M.M.; Jones, B.P. Evaluation of potentially labile soil organic carbon and nitrogen fractionation procedures. Soil Biol. Biochem. 2011, 43, 438–444. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Land Use, Land-Use Change, and Forestry: A Special Report of the Intergovernmental Panel on Climate Change; Watson, R.T., Noble, I.R., Bolin, B., Ravindranath, N.H., Verardo, D.J., Dokken, D.J., Eds.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- FAO (FAO/IUSS Working Group WRB). World Reference Base for Soil Resources 2006; World Soil Resources Report; FAO: Rome, Italy, 2006; p. 103. [Google Scholar]
- Zhu, Z.D.; Chen, G.T. Sandy Desertification in China: Status and Trends; Science Press: Beijing, China, 1994. (In Chinese) [Google Scholar]
- Li, Y.Q.; Han, J.J.; Wang, S.K.; Brandle, J.; Lian, J.; Luo, Y.Q.; Zhang, F.X. Soil organic carbon and total nitrogen storage under different land uses in the Naiman Banner, a semiarid degraded region of northern China. Can. J. Soil. Sci. 2014, 94, 9–20. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Soil Quality–Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–577. [Google Scholar]
- McGill, W.B.; Figueiredo, C.T. Total nitrogen. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Canadian Society of Soil Science/Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 201–211. [Google Scholar]
- Perez-Quezada, J.F.; Delpiano, C.A.; Snyder, K.A.; Johnson, D.A.; Franck, N. Carbon pools in an arid shrub land in Chile under natural and afforested conditions. J. Arid Environ. 2011, 75, 29–37. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Metzeling, K.J.; MacNally, R.; Thomson, J.R.; Cavagnaro, T.R. Changes in soil carbon of pastures after afforestation with mixed species: Sampling, heterogeneity and surrogates. Agric. Ecosyst. Environ. 2012, 158, 58–65. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, W.; Cao, J.; Liu, X.; Shen, H.; Zhao, X. Changes in soil organic carbon, nitrogen, phosphorus, and bulk density after afforestation of the “Beijing–Tianjin Sandstorm Source Control” program in China. Catena 2014, 118, 186–194. [Google Scholar] [CrossRef]
- Han, X.; Zhao, F.; Tong, X.; Deng, J.; Yang, G.; Chen, L.; Kang, D. Understanding soil carbon sequestration following the afforestation of former arable land by physical fractionation. Catena 2017, 150, 317–327. [Google Scholar] [CrossRef]
- Davis, M.R.; Condron, L.M. Impact of grassland afforestation on soil carbon in New Zealand: A review of paired-site studies. Aust. J. Soil Res. 2002, 40, 675–690. [Google Scholar] [CrossRef]
- Blujdea, V. Challenges and trade-offs in environmental and financial approaches of the afforestation of degraded lands. In Climate and Land Degradation; Sivakumar, M.V.K., Ndiang’ui, N., Eds.; Springer: Berlin, Germany, 2007. [Google Scholar]
- CCTV-2 (China Central Television). The fall of Shelter Forest, Half-Hour Economy. Available online: http://jingji.cntv.cn/2013/11/21/VIDE1385044320483135.shtml (accessed on 21 November 2013).
- Zhang, G.Y. Analysis of temperature and precipitation nearly 10 years and over year in Naiman Banner. J. Inn. Mong. Univ. Natl. 2010, 25, 517–518. (In Chinese) [Google Scholar]
- Zhao, H.L.; Zhao, X.Y.; Zhang, T.H.; Li, Y.S.; Han, F.L.; Zhou, R.L. The time-space variation of groundwater and its causes in the central desertified area of the Naiman Banner of Mongolia in the past 20 years. J. Desert Res. 1999, 19 (Suppl. 1), 7–11. (In Chinese) [Google Scholar]
- Chassany, J.P. Economic and social appraisal of the feasibility of land restoration, rehabilitation, and reallocation in arid and semiarid zones: A holistic approach. Arid Soil Res. Rehabil. 1999, 13, 383–395. [Google Scholar] [CrossRef]
- Zhao, H.L.; Guo, Y.R.; Zhou, R.L.; Drake, S. Biological soil crust and surface soil properties in different vegetation types of Horqin Sand Land, China. Catena 2010, 82, 70–76. [Google Scholar] [CrossRef]
- Liu, Y.M.; Yang, H.Y.; Li, X.R.; Xing, Z.S. Effects of biological soil crusts on soil enzyme activities in revegetated areas of the Tengger Desert, China. Appl. Soil Ecol. 2014, 80, 6–14. [Google Scholar] [CrossRef]
- Belnap, J.; Gillette, D.A. Vulnerability of desert biological crusts to wind erosion: The influences of crust development, soil texture, and disturbance. J. Arid Environ. 1998, 39, 133–142. [Google Scholar] [CrossRef]
- Housman, D.C.; Powers, H.H.; Collins, A.D.; Belnap, J. Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J. Arid Environ. 2006, 66, 620–634. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, P.; Su, Y.G.; Jia, R.L. Carbon fixation by biological soil crusts following revegetation of sand dunes in arid desert regions of China: A four-year field study. Catena 2012, 97, 119–126. [Google Scholar] [CrossRef]
- Reubens, B.; Moeremans, C.; Poesen, J.; Nyssen, J.; Tewoldeberhan, S.; Franzel, S.; Deckers, J.; Orwa, C.; Muys, B. Tree species selection for land rehabilitation in Ethiopia: From fragmented knowledge to an integrated multi-criteria decision approach. Agrofor. Syst. 2011, 82, 303–330. [Google Scholar] [CrossRef]
- Liu, W.H.; Zhu, J.J.; Jia, Q.Q.; Zheng, X.; Li, J.S.; Lou, X.D.; Hu, L.L. Carbon sequestration effects of shrub lands in Three-North Shelterbelt Forest region, China. Chin. J. Geogr. Sci. 2014, 24, 444–453. [Google Scholar] [CrossRef]
| Soil Properties | Layer (cm) | 9-Year | 15-Year | 31-Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Under Canopy | Between Rows | Mean | Fixed Dunes | Under Canopy | Between Rows | Mean | Active Dunes | Under Canopy | Between Rows | Mean | Active Dunes | ||
| Coarse sand (2.0–0.1 mm, %) | 0201310 | 71.7 ± 4.3a | 79.6 ± 2.7ab | 75.7 ± 2.7ab | 84.4 ± 2.3b | 84.2 ± 0.8a | 87.5 ± 1.1bc | 85.8 ± 0.6ab | 89.9 ± 1.4c | 72.7 ± 2.8a | 77.5 ± 1.7a | 75.1±1.7a | 94.8 ± 0.8b |
| 10–20 | 86.6 ± 3.5a | 92.0 ± 1.1ab | 89.3 ± 1.8ab | 94.3 ± 1.0b | 86.9 ± 1.4a | 88.3 ± 1.2ab | 87.6 ± 0.8ab | 90.8 ± 1.7b | 86.3 ± 1.3a | 90.1 ± 0.9b | 88.2 ± 0.9ab | 96.8 ± 0.4c | |
| 20–40 | 93.8 ± 0.7a | 94.5 ± 0.4a | 94.2 ± 0.4a | 97.7 ± 0.4b | 87.6 ± 0.9a | 89.4 ± 0.3ab | 88.5 ± 0.4ab | 91.9 ± 2.1b | 88.7 ± 0.9a | 90.3 ± 0.9a | 89.5 ± 0.7a | 97.0 ± 0.3b | |
| 40–60 | 95.3 ± 0.6a | 95.3 ± 0.3a | 95.3 ± 0.4a | 97.1 ± 0.9b | 88.2 ± 0.4a | 89.3 ± 0.4ab | 88.7 ± 0.4ab | 92.2 ± 2.1b | 91.2 ± 0.9a | 90.5 ± 0.9a | 90.8 ± 0.8a | 96.3 ± 0.3b | |
| 60–100 | 95.3 ± 0.7a | 94.5 ± 0.7a | 94.9 ± 0.6a | 95.3 ± 0.7a | 87.4 ± 1.0a | 90.1 ± 0.9a | 88.8 ± 0.7a | 93.7 ± 1.8b | 91.5 ± 0.7a | 91.2 ± 0.7a | 91.4 ± 0.5a | 96.6 ± 0.4b | |
| 0–100 | 88.6 ± 1.8a | 91.2 ± 0.7ab | 89.9 ± 1.0a | 93.7 ± 0.9b | 86.8 ± 0.4a | 88.9 ± 0.3a | 87.9 ± 0.2a | 91.7 ± 1.5b | 86.1 ± 1.0a | 87.9 ± 0.6a | 87.0 ± 0.7a | 96.3 ± 0.3b | |
| Fine sand (0.10–0.05 mm, %) | 0–10 | 12.5 ± 2.0a | 8.7 ± 1.1a | 10.6 ± 1.3a | 10.7 ± 1.5a | 9.3 ± 0.3a | 9.1 ± 0.6a | 9.2 ± 0.4a | 7.6 ± 1.1b | 10.9 ± 1.1a | 8.0 ± 0.7b | 9.4 ± 0.9ab | 2.8 ± 0.6c |
| 10–20 | 6.5 ± 1.4a | 4.1 ± 0.5a | 5.3 ± 0.7a | 4.1 ± 0.8a | 8.7 ± 0.7a | 9.5 ± 0.7a | 9.1 ± 0.5a | 7.1 ± 1.4a | 6.6 ± 0.6a | 5.4 ± 0.4a | 6.0 ± 0.4a | 1.7 ± 0.2b | |
| 20–40 | 4.2 ± 0.4a | 3.5 ± 0.4a | 3.9 ± 0.3a | 1.5 ± 0.3b | 9.8 ± 0.8a | 8.6 ± 0.5ab | 9.2 ± 0.4ab | 6.7 ± 1.8b | 5.7 ± 0.4a | 5.8 ± 0.5a | 5.7 ± 0.4a | 1.6 ± 0.2b | |
| 40–60 | 3.3 ± 0.6a | 3.7 ± 0.3a | 3.5 ± 0.4a | 2.0 ± 0.6b | 9.6 ± 0.5a | 9.2 ± 0.4a | 9.4 ± 0.4a | 6.1 ± 1.6b | 5.3± 0.4aa | 5.8 ± 0.4a | 5.6 ± 0.4a | 2.2 ± 0.3b | |
| 60–100 | 3.6 ± 0.5a | 3.9 ± 0.5a | 3.7 ± 0.4a | 3.1 ± 0.4a | 10.8 ± 0.8a | 8.1 ± 0.8ab | 9.5 ± 0.7a | 5.3 ± 1.6bc | 5.1 ± 0.4a | 4.9 ± 0.3a | 5.0 ± 0.2a | 1.9 ± 0.3b | |
| 0–100 | 6.0 ± 0.9a | 4.8 ± 0.4a | 5.4 ± 0.5a | 4.3 ± 0.5a | 9.6 ± 0.3a | 8.9 ± 0.2a | 9.3 ± 0.3a | 6.5 ± 1.2b | 6.7 ± 0.5a | 6.0 ± 0.3a | 6.3 ± 0.4a | 2.0 ± 0.2b | |
| Silt + clay (<0.05 mm, %) | 0–10 | 15.8 ± 2.3a | 11.7 ± 1.7a | 13.7 ± 1.4a | 4.5 ± 0.9b | 6.5 ± 0.9a | 3.4±0.6bc | 4.9 ± 0.4ab | 2.5 ± 0.5c | 16.5 ± 1.7a | 14.5 ± 1.2a | 15.5 ± 1.0a | 2.4 ± 0.3b |
| 10–20 | 6.9 ± 2.1a | 3.8 ± 0.8ab | 5.4 ± 1.1a | 1.3 ± 0.3b | 4.4 ± 0.9a | 2.1±0.7b | 3.3 ± 0.5ab | 2.1 ± 0.4b | 7.2 ± 0.8a | 4.4 ± 0.6b | 5.8 ± 0.5ab | 1.5 ± 0.3c | |
| 20–40 | 1.9 ± 0.3a | 2.0 ± 0.2a | 2.0 ± 0.1a | 0.8 ± 0.2b | 2.6 ± 0.6a | 2.0 ± 0.2ab | 2.3 ± 0.3ab | 1.4±0.4b | 5.6 ± 0.5a | 3.9 ± 0.5b | 4.8 ± 0.4ab | 1.5 ± 0.2c | |
| 40–60 | 1.4 ± 0.2a | 1.0 ± 0.2ab | 1.2 ± 0.1ab | 0.8 ± 0.3b | 2.2 ± 0.2a | 1.5 ± 0.1a | 1.9 ± 0.1a | 1.7 ± 0.7a | 3.6 ± 0.5a | 3.7 ± 0.5a | 3.6 ± 0.5a | 1.5 ± 0.2b | |
| 60–100 | 1.1 ± 0.3a | 1.6 ± 0.2a | 1.4 ± 0.2a | 1.6 ± 0.6a | 1.8 ± 0.4a | 1.7 ± 0.2a | 1.8 ± 0.2a | 1.0 ± 0.3a | 3.4 ± 0.4a | 3.8 ± 0.5a | 3.6 ± 0.3a | 1.5 ± 0.3b | |
| 0–100 | 5.4 ± 0.9a | 4.0 ± 0.4a | 4.7 ± 0.5a | 1.8 ± 0.4b | 3.5 ± 0.4a | 2.1 ± 0.2bc | 2.8 ± 0.1ab | 1.7 ± 0.3c | 7.2 ± 0.6a | 6.1 ± 0.4a | 6.7 ± 0.4a | 1.7 ± 0.2b | |
| Bulk density (g·cm−3) | 0–10 | 1.46 ± 0.03a | 1.49 ± 0.03a | 1.47 ± 0.03a | 1.50 ± 0.01a | 1.47 ± 0.03a | 1.56 ± 0.01bc | 1.51 ± 0.01ab | 1.60 ± 0.03c | 1.41 ± 0.05a | 1.53 ± 0.04bc | 1.47 ± 0.01ab | 1.62 ± 0.02c |
| 10–20 | 1.51 ± 0.03a | 1.52 ± 0.03a | 1.51 ± 0.03a | 1.54 ± 0.02a | 1.49 ± 0.01a | 1.56 ± 0.02b | 1.52 ± 0.01ab | 1.55 ± 0.02b | 1.53 ± 0.04a | 1.53 ± 0.02a | 1.53 ± 0.01a | 1.61 ± 0.02a | |
| 20–40 | 1.52 ± 0.03a | 1.53 ± 0.03a | 1.52 ± 0.03a | 1.56 ± 0.01a | 1.52 ± 0.01a | 1.54 ± 0.01a | 1.53 ± 0.01a | 1.54 ± 0.03a | 1.52 ± 0.03a | 1.54 ± 0.03a | 1.53 ± 0.03a | 1.60 ± 0.01a | |
| 40–60 | 1.52 ± 0.03a | 1.57 ± 0.05a | 1.54 ± 0.04a | 1.56 ± 0.02a | 1.53 ± 0.03a | 1.55 ± 0.02a | 1.54 ± 0.02a | 1.56 ± 0.02a | 1.50 ± 0.04a | 1.55 ± 0.02a | 1.53 ± 0.03a | 1.58 ± 0.01a | |
| 60–100 | 1.52 ± 0.03a | 1.54 ± 0.03a | 1.53 ± 0.03a | 1.58 ± 0.01a | 1.56 ± 0.01a | 1.55 ± 0.01a | 1.56 ± 0.01a | 1.55 ± 0.01a | 1.53 ± 0.06a | 1.55 ± 0.02a | 1.54 ± 0.03a | 1.57 ± 0.01a | |
| 0–100 | 1.50 ± 0.03a | 1.53 ± 0.03a | 1.52 ± 0.03a | 1.55 ± 0.01a | 1.51 ± 0.01a | 1.55 ± 0.01ab | 1.53 ± 0.01ab | 1.56 ± 0.02b | 1.50 ± 0.04a | 1.54 ± 0.03ab | 1.52 ± 0.02ab | 1.60 ± 0.01b | |
| LFOC (g·kg−1 LF) | 0–100 | 182.4 ± 2.8a | 155.3 ± 5.8bd | 168.9 ± 3.6c | 150.4 ± 3.9d | 204.8 ± 11.3a | 183.3 ± 13.3a | 194.0 ± 11.8a | 149.4 ± 4.0b | 184.9 ± 7.5a | 172.7 ± 6.8a | 178.8 ± 5.5a | 149.7 ± 2.4b |
| LFN (g·kg−1 LF ) | 0–100 | 13.3 ± 0.3a | 10.0 ± 0.5b | 11.7 ± 0.3c | 7.2 ± 0.2d | 12.7 ± 0.5a | 11.7 ± 0.7a | 12.2 ± 0.6a | 6.4 ± 0.4b | 12.2 ± 0.8a | 11.7 ± 0.3a | 12.0 ± 0.4a | 6.8 ± 0.5b |
| Layer (cm) | 9-Year | 15-Year | 31-Year | |||
|---|---|---|---|---|---|---|
| Plantation | Fixed Dunes | Plantation | Active Dunes | Plantation | Active Dunes | |
| SOC storage (kg·ha−1) | ||||||
| 0–10 | 8406 ± 1068a | 2279 ± 183b | 3000 ± 179a | 966 ± 102b | 4908 ± 391a | 1017 ± 133b |
| 10–20 | 3291 ± 478a | 1240 ± 77b | 1920 ± 191a | 936 ± 60b | 2651 ± 283a | 948 ± 164b |
| 20–40 | 2849 ± 338a | 2004 ± 128b | 2378 ± 247a | 1636 ± 102b | 4243 ± 202a | 1720 ± 273b |
| 40–60 | 1753 ±146a | 1786 ± 67a | 1837 ± 120a | 1137 ± 175b | 2386 ± 182a | 1461 ± 308b |
| 60–100 | 2961 ± 182a | 2918 ± 210a | 3039 ± 169a | 2314 ± 313b | 4665 ± 443a | 2559 ± 115b |
| Subtotal | 19,260 ± 1867a | 10,227 ± 357b | 12,174 ± 643a | 6989 ± 413b | 18,853 ± 908a | 7705 ± 896b |
| TN storage (kg·ha−1) | ||||||
| 0–10 | 925 ± 111a | 274 ± 22b | 321 ± 11a | 119 ± 9b | 590 ± 41a | 104 ± 12b |
| 10–20 | 364 ± 40a | 142 ± 17b | 219 ± 28a | 90 ± 7b | 325 ± 30a | 102 ± 13b |
| 20–40 | 339 ± 18a | 248 ± 12b | 266 ± 37a | 160 ± 19b | 465 ± 15a | 172 ± 24b |
| 40–60 | 186 ± 5a | 211 ± 14a | 190 ± 26a | 141 ± 13a | 274 ± 20a | 139 ± 25b |
| 60–100 | 319 ± 18a | 392 ± 21a | 319 ± 23a | 309 ± 39a | 556 ± 50a | 231 ± 31b |
| Subtotal | 2133 ± 141a | 1267 ± 45b | 1315 ± 97a | 819 ± 66b | 2210 ± 105a | 748 ± 90b |
| LFOC storage (kg·ha−1) | ||||||
| 0–10 | 1734 ± 343a | 673 ± 77b | 633 ± 85a | 199 ± 37b | 930 ± 58a | 181 ± 65b |
| 10–20 | 686 ± 116a | 342 ± 59b | 463 ± 72a | 147 ± 46b | 653 ± 70a | 165 ± 49b |
| 20–40 | 610 ± 47a | 395 ± 62b | 738 ± 126a | 256 ± 66b | 982 ± 78a | 321 ± 115b |
| 40–60 | 342 ± 30a | 206 ± 34b | 573 ± 74a | 164 ± 51b | 507 ± 73a | 193 ± 69b |
| 60–100 | 663 ± 76a | 311 ± 65b | 1139 ± 130a | 419 ± 156b | 885 ± 67a | 449 ± 185b |
| Subtotal | 4035 ± 446a | 1927 ± 246b | 3546 ± 300a | 1185 ± 223b | 3957 ± 148a | 1309 ± 432b |
| LFN storage (kg·ha−1) | ||||||
| 0–10 | 120 ± 24a | 32 ± 4b | 40 ± 5a | 9 ± 2b | 62 ± 4a | 8 ± 3b |
| 10–20 | 47 ± 8a | 16 ± 3b | 29 ± 5a | 6 ± 2b | 44± 5a | 8 ± 2b |
| 20–40 | 42 ± 3a | 19 ± 3b | 46 ± 8a | 11 ± 3b | 66 ± 5a | 15 ± 5b |
| 40–60 | 24 ± 2a | 10 ± 2b | 36 ± 5a | 7 ± 2b | 34 ± 5a | 9 ± 3b |
| 60–100 | 46 ± 5a | 15 ± 3b | 72 ± 8a | 18 ± 7b | 59 ± 5a | 20 ± 8b |
| Subtotal | 279 ± 31a | 92 ± 12b | 223 ± 19a | 51 ± 10b | 265 ± 10a | 60 ± 20b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, Y.; Wang, X.; Niu, Y.; Lian, J. Improvements in Soil Carbon and Nitrogen Capacities after Shrub Planting to Stabilize Sand Dunes in China’s Horqin Sandy Land. Sustainability 2017, 9, 662. https://doi.org/10.3390/su9040662
Li Y, Chen Y, Wang X, Niu Y, Lian J. Improvements in Soil Carbon and Nitrogen Capacities after Shrub Planting to Stabilize Sand Dunes in China’s Horqin Sandy Land. Sustainability. 2017; 9(4):662. https://doi.org/10.3390/su9040662
Chicago/Turabian StyleLi, Yuqiang, Yinping Chen, Xuyang Wang, Yayi Niu, and Jie Lian. 2017. "Improvements in Soil Carbon and Nitrogen Capacities after Shrub Planting to Stabilize Sand Dunes in China’s Horqin Sandy Land" Sustainability 9, no. 4: 662. https://doi.org/10.3390/su9040662





