An Expert-Based Assessment Model for Evaluating Habitat Suitability of Pond-Breeding Amphibians
Abstract
:1. Introduction
2. Material and Methods
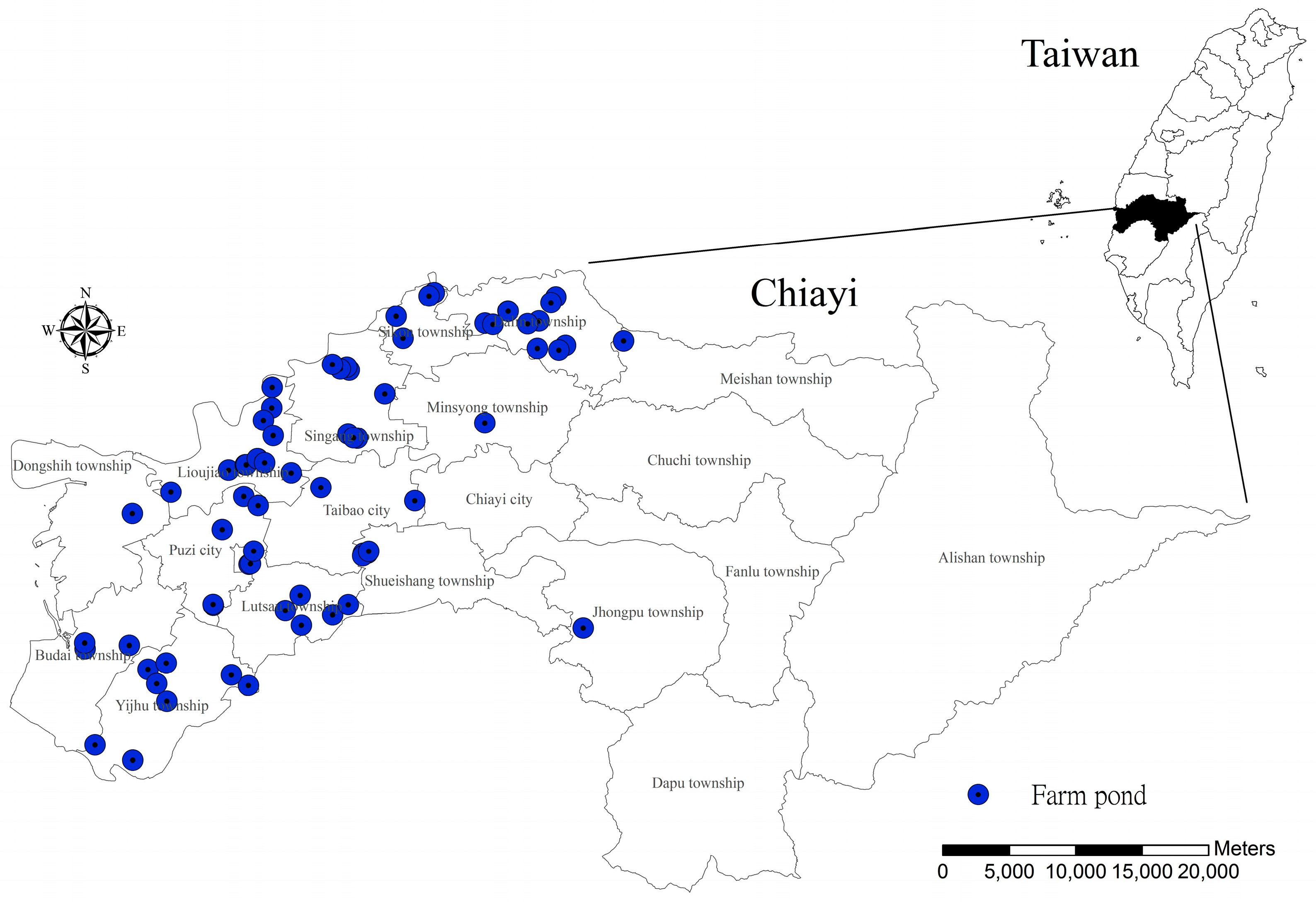
2.1. Study Area
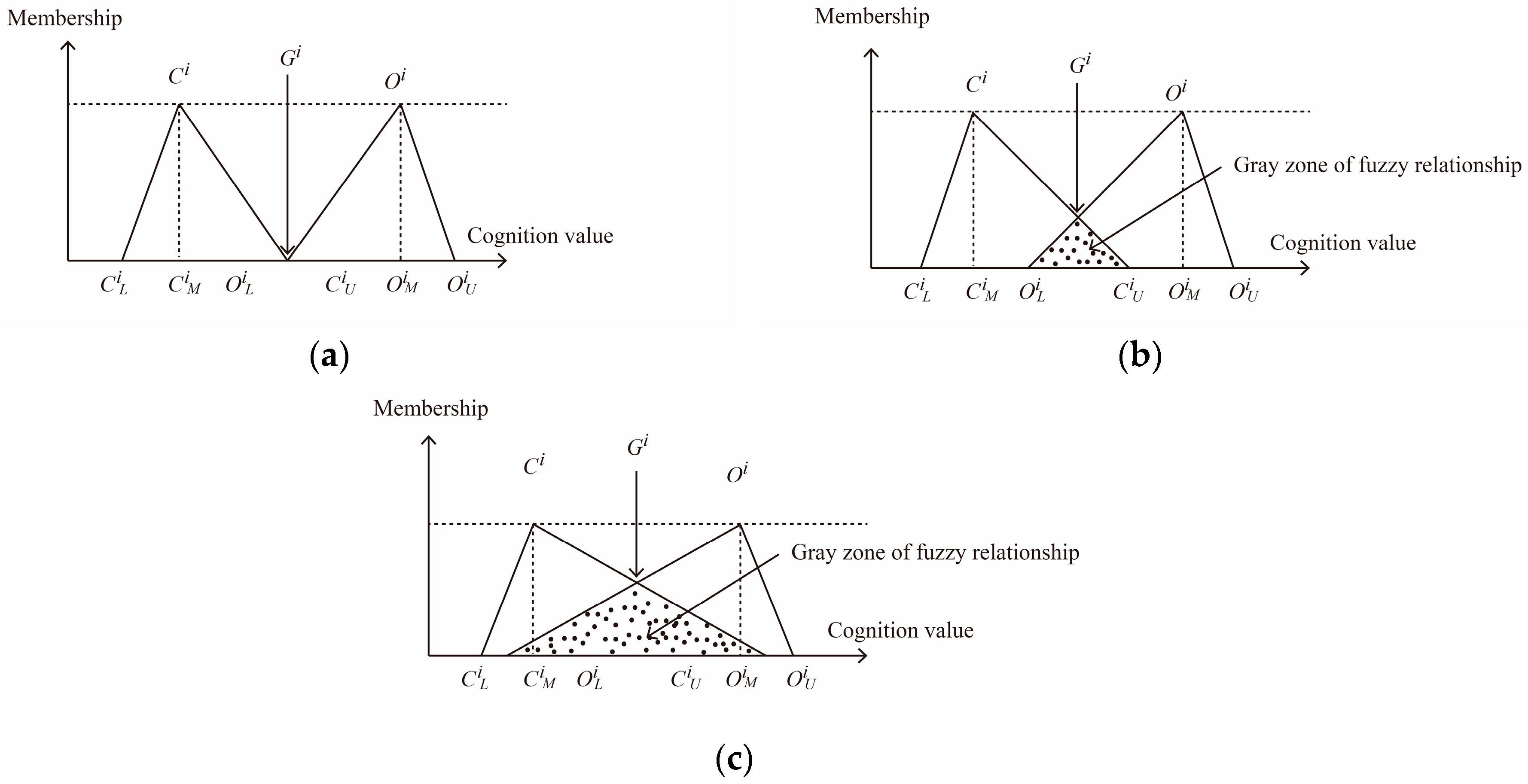
2.2. Fuzzy Delphi Method
2.3. Frog Census and Pond Environment Investigation
2.4. Questionnaire Design and Sampling Method for Resident Perspectives
2.5. Frog Habitat Suitability Assessment and Reliability Analysis
2.6. Kappa Analysis
3. Results
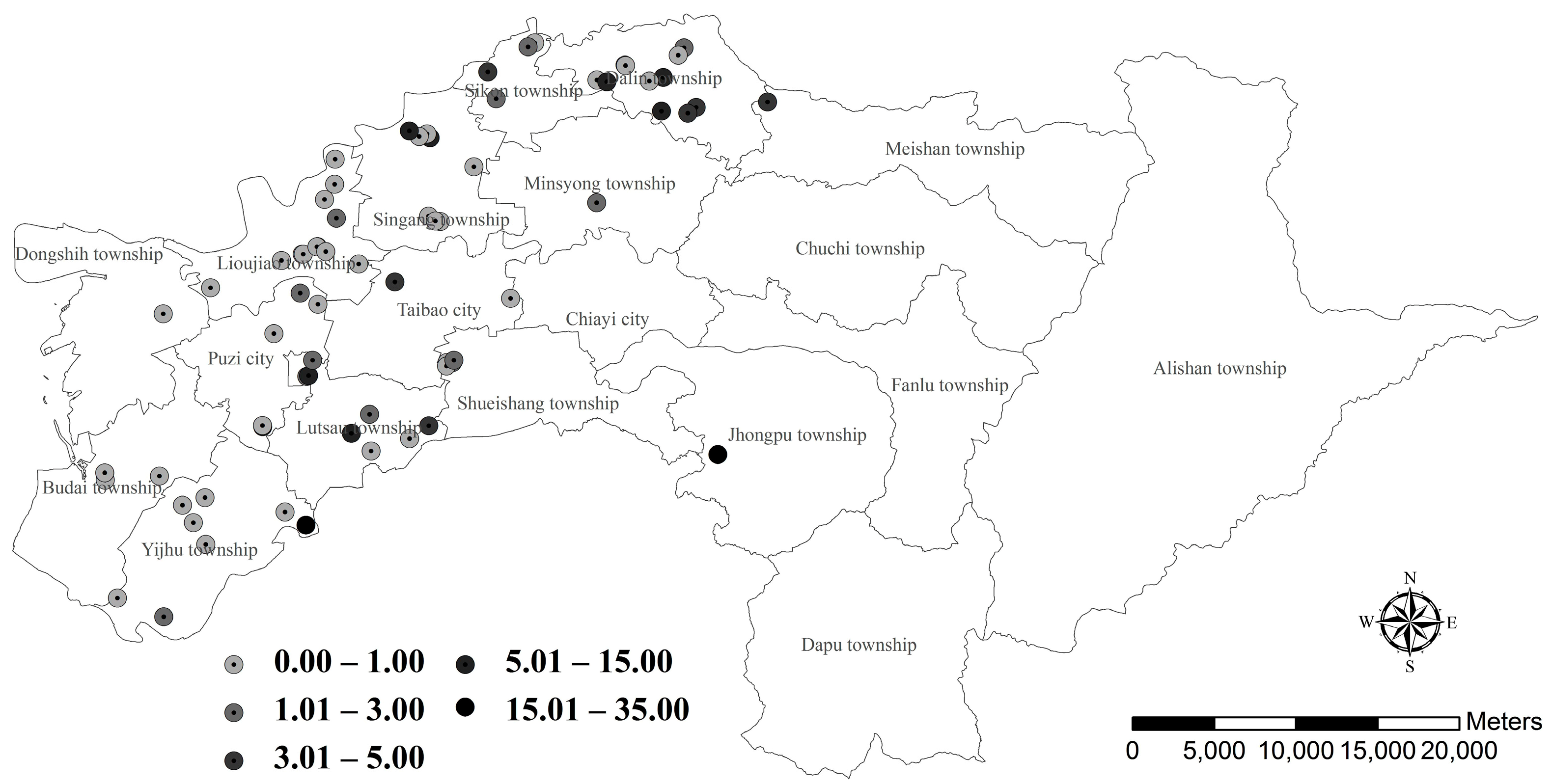
3.1. Günther’s Frog Population in Farm Ponds
3.2. Weight Coefficients of Günther’s Frog Habitat Suitability Evaluation for Farm Ponds
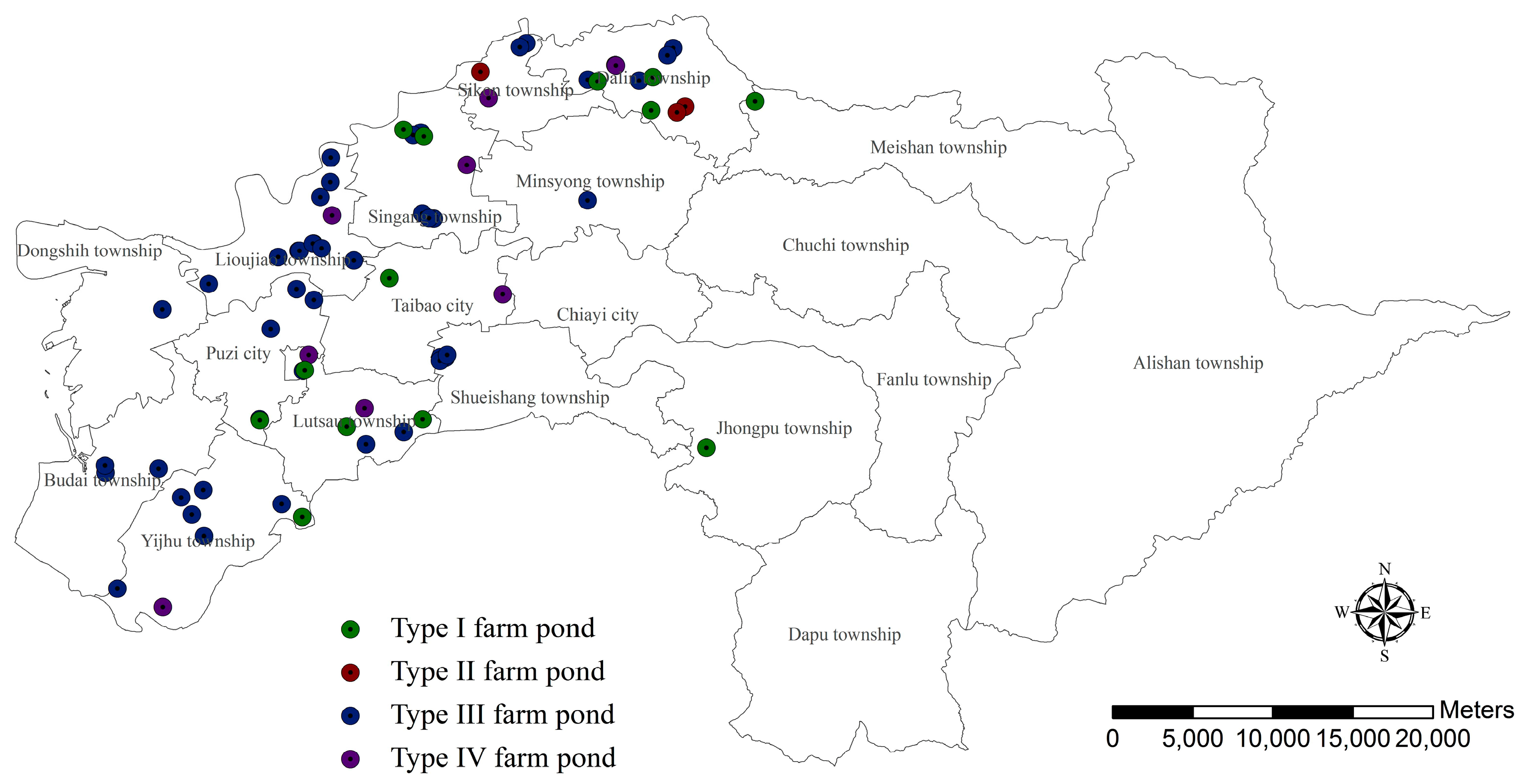
3.3. Günther’s Frog Habitat Suitability Assessment
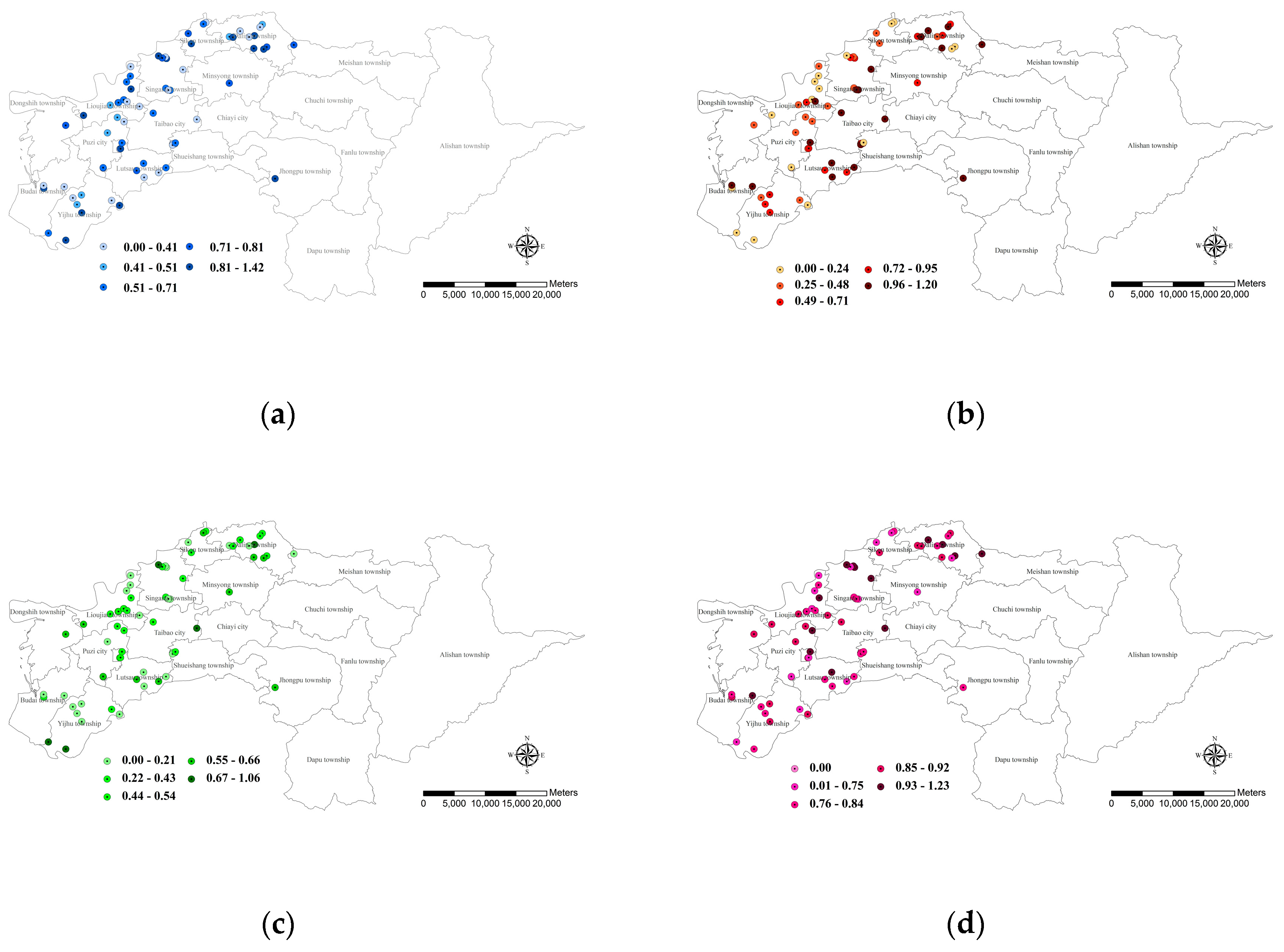
3.3.1. Farm Ponds with Aquatic Environment Value
3.3.2. Farm Ponds with Terrestrial Environment Value
3.3.3. Farm Ponds with Landscape Connectivity Value
3.3.4. Farm Ponds with Residents’ Conservation Perception Value
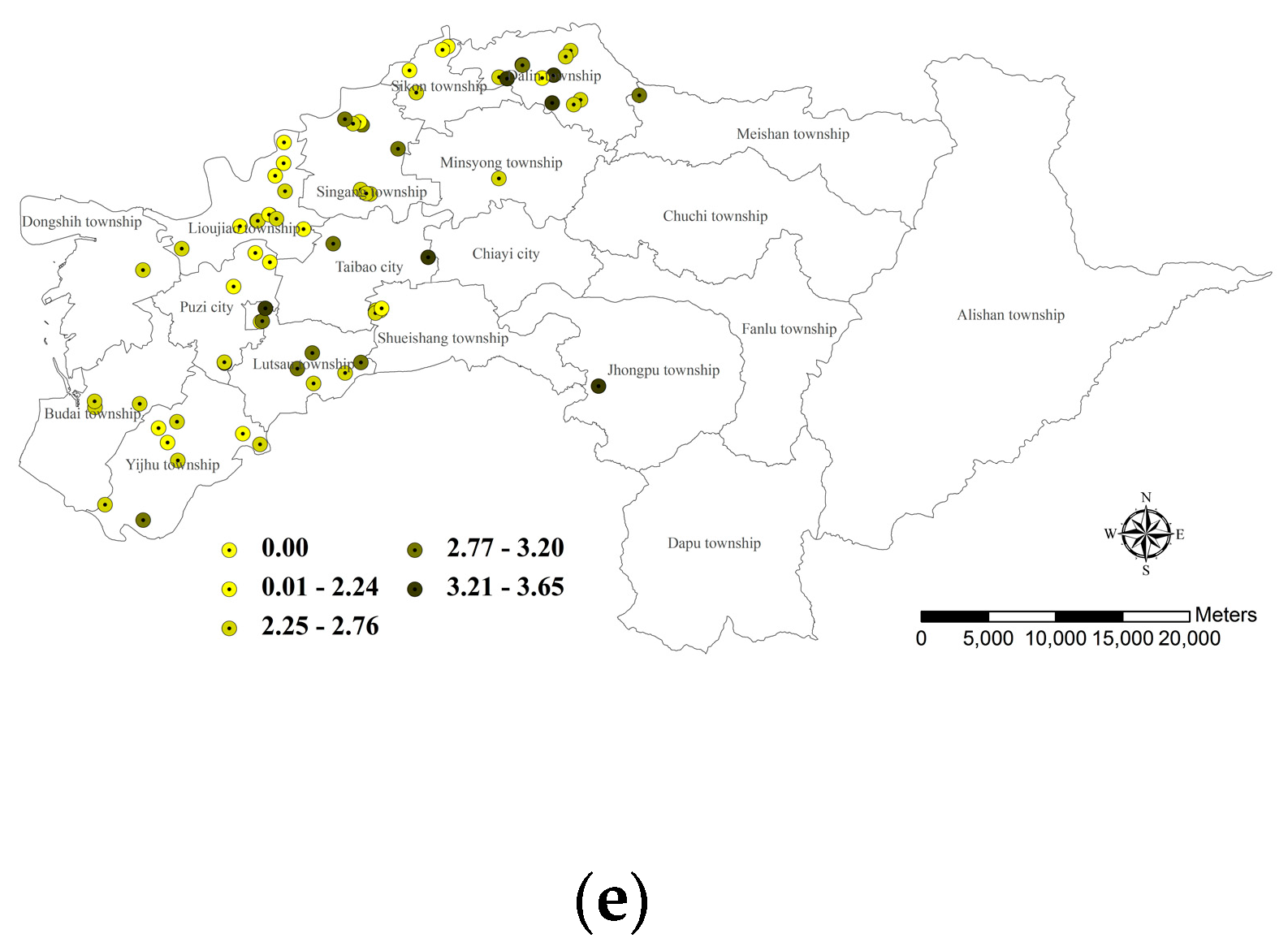
3.3.5. Farm Ponds with Overall Habitat Suitability Value
3.4. Reliability of Habitat Suitability Assessment
4. Discussion
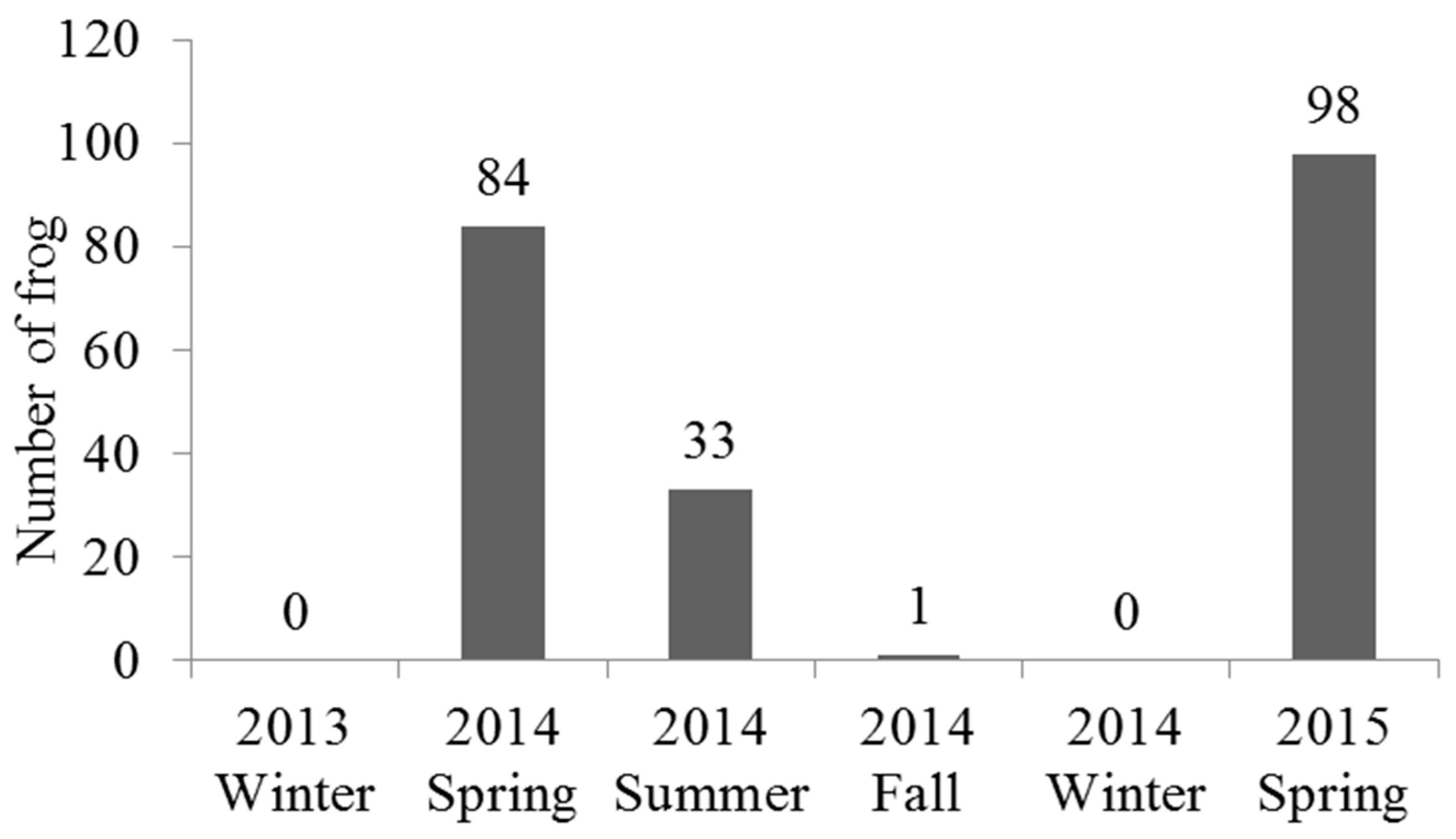
4.1. Seasonal Variation and Spatial Distribution of Günther’s Frog Population
4.2. Günther’s Frog Habitat Suitability Impact Factors
4.3. Farm Pond Habitat Management and Ecosystem Restoration for Günther’s Frog Preservation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Bank. The World Bank Annual Report 2013. Available online: https://openknowledge.worldbank.org/handle/10986/16091 (accessed on 13 January 2017).
- Edvardsen, A.; Økland, R.H. Variation in plant species richness in and adjacent to 64 ponds in SE Norwegian agricultural landscapes. Aquat. Bot. 2006, 85, 79–91. [Google Scholar] [CrossRef]
- Fang, W.T.; Cheng, B.Y.; Shih, S.S.; Chou, J.Y.; Otte, M.L. Modelling driving forces of avian diversity in a spatial configuration surrounded by farm ponds. Paddy Water Environ. 2016, 14, 185–191. [Google Scholar] [CrossRef]
- Hazell, D.; Cunnningham, R.; Lindenmayer, D.; Mackey, B.; Osborne, W. Use of farm dams as frog habitat in an Australian agricultural landscape: factors affecting species richness and distribution. Biol. Conserv. 2001, 102, 155–169. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef] [PubMed]
- Nuno, C.; Hartel, T.; Arntzen, J.W. Amphibian pond loss as a function of landscape change—A case study over three decades in an agricultural area of northern France. Biol. Conserv. 2011, 144, 1610–1618. [Google Scholar]
- Scheffer, M.; Geest, G.J.; van Zimmer, K.; Jeppesen, E.; Sondergaard, M.; Butler, M.G.; Hanson, M.A.; Declerck, S.; de Meester, L. Small habitat size and isolation can promote species richness: Second-order effects on biodiversity in shallow lakes and pond. Oikos 2006, 112, 227–231. [Google Scholar] [CrossRef]
- Hazell, D.; Hero, J.-M.; Lindenmayer, D.; Cunningham, R. A comparison of constructed and natural habitat for frog conservation in an Australian agricultural landscape. Biol. Conserv. 2004, 119, 61–71. [Google Scholar] [CrossRef]
- Chou, W.W.; Lee, S.H.; Wu, C.F. Evaluate the preservation value and location of farm ponds in Yunlin County. Int. J. Environ. Res. Public Health 2014, 11, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Céréghino, R.; Biggs, J.; Oerli, B.; Declerck, S. The ecology of European pond: defining the characteristics of a neglected freshwater habitat. Hydrobiologia 2008, 597, 1–6. [Google Scholar]
- Duellman, W.E.; Trueb, L. Biology of Amphibians, 6th ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Leong, T.M.; Lim, K.K.P. Occurrence of Günther’s frog, Hylarana Guentheri (Amphibia: Anura: Ranidae) in Singapore. Nat. Singap. 2011, 4, 135–141. [Google Scholar]
- Chan, S.K.F.; Cheung, K.S.; Ho, C.Y.; Lam, F.N.; Tang, W.S.M.; Lau, W.N.; Bogadek, A. A Field Guide to the Amphibians of Hong Kong; Friends of the Country Parks & Cosmos Books Limited: Hong Kong, China, 2005; p. 212. [Google Scholar]
- Carvalho, J.C.; Gomes, P. Habitat suitability model for European wild rabbit (Oryctolagus cuniculus) with implications for restocking. Game Wildl. Sci. 2003, 20, 287–301. [Google Scholar]
- Tian, B.; Zhou, Y.; Zhang, L.; Yuan, L. Analyzing the habitat suitability for migratory birds at the Chongming Dongtan Nature Reserve in Shanghai, China. Estuar. Coast. Shelf Sci. 2008, 80, 296–302. [Google Scholar] [CrossRef]
- Store, R.; Kangas, J. Integrating spatial multi-criteria evaluation and expert knowledge for GIS-based habitat suitability modelling. Landsc. Urban Plan. 2001, 55, 79–93. [Google Scholar] [CrossRef]
- Nekhay, O.; Arriaza, M.; Guzmán-Álvarez, R.J. Spatial analysis of the suitability of olive plantations for wildlife habitat restoration. Comput. Electron. Agric. 2009, 65, 49–64. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Z.; Liu, D.; Li, L.; Ren, C.; Tanga, X.; Jia, M.; Liu, C. Assessment of habitat suitability for waterbirds in the West Songnen Plain, China, using remote sensing and GIS. Ecol. Eng. 2013, 55, 94–100. [Google Scholar] [CrossRef]
- Luan, X.F.; Qu, Y.; Li, D.; Liu, S.; Wang, X.; Wu, B.; Zhu, C. Habitat evaluation of wild Amur tiger (Panthera tigris altaica) and conservation priority setting in north-eastern China. J. Environ. Manag. 2011, 92, 31–42. [Google Scholar]
- Jeng, T.B. Fuzzy Assessment Model for Maturity of Software Organization in Improving Its Staff’s Capability. Master’s Thesis, National Taiwan University of Science and Technology, Taipei, Taiwan, 2001; pp. 33–36. [Google Scholar]
- Ishikawa, A.; Amagasa, M.; Shiga, T.; Tomizawa, G.; Tatsuta, R.; Mieno, H. The Max-Min Delphi method and Fuzzy Delphi method via fuzzy integration. Fuzzy Sets Syst. 1993, 55, 241–253. [Google Scholar] [CrossRef]
- Yang, Y.J.; Shao, K.S.; Lee, C.E. The Field Investigation Guideline of Taiwan Anurans; Forestry Bureau, Council of Agriculture, Executive Yuan: Taipei, Taiwan, 2005. [Google Scholar]
- Yang, Y.J. Distribution and habitats of Taiwan Anurans. Anim. World 2010, 29, 46–49. [Google Scholar]
- Li, C.F.; Lue, K.Y. Altitudinal differences in temporal distribution, spatial preference and timing of breeding climax of frogs and toads in the central Taiwan. TW J. Biodivers. 2004, 6, 39–50. [Google Scholar]
- Fang, H.H.; Lin, C.C.; Yang, Y.L.; Cheng, H.C.; Yang, Y.C. Suggestions on developing an investigation scheme for identifying terrestrial animal biodiversity hotspots of Taiwan with the wildlife database. TW J. Biodivers. 2010, 13, 51–68. [Google Scholar]
- Chiayi County Government. Report of Assessing Environmental Resource of Farm Ponds in Chiayi County; Chiayi County Government: Chiayi County, Taiwan, 2012.
- Wei, W.L.; Chang, W.C. A study on selecting optimal product design solution using fuzzy Delphi method and analytic. J. Des. 2007, 10, 59–76. [Google Scholar]
- Chou, W.W. A Study on Evaluation and Strategies for the Conservation Value of Farm Ponds in Yunlin County. Ph.D. Dissertation, National Cheng Kung University, Tainan, Taiwan, 2014. [Google Scholar]
- Kam, Y.C.; Wu, C.S.; Hsu, M.Y.; Lin, Y.H.; Chung, M.F. The survey method and technology for amphibians and reptiles. In Symposium of Integrated Biological Resource Survey and Manpower Training; The Plant Protection Society of Republic of China: Taichung, Taiwan, 2004; pp. 1–29. [Google Scholar]
- Pearman, P.B.; Velasco, A.M.; Lopez, A. Tropical amphibian monitoring: A comparison of methods for detecting inter-site variation in species composition. Herpetologica 1995, 51, 325–337. [Google Scholar]
- Parris, K.M. Amphibian surveys in forests and woodlands. Contemp. Herpetol. 1999, 1, 1–16. [Google Scholar]
- Parris, M.K.; Norton, W.T.; Cunningham, B.R. A comparison of techniques for sampling amphibians in the forests of south-east Queensland, Australia. Herpetologica 1999, 55, 271–283. [Google Scholar]
- Aichinger, M. Annual activity pattern of anurans in a seasonal neotropical environment. Oecologia 1987, 71, 583–592. [Google Scholar] [CrossRef]
- Saito, H.; McKenna, S.A.; Goovaerts, P. Accounting for geophysical information in geostatistical characterization of unexploded ordnance (UXO) sites. Environ. Ecol. Stat. 2005, 12, 7–25. [Google Scholar] [CrossRef]
- Lin, Y.P.; Chu, H.J.; Wu, C.F.; Verburg, P.H. Predictive ability of logistic regression, auto-logistic regression and neural network models in empirical land-use change modeling—A case study. Int. J. Geogr. Inform. Sci. 2011, 25, 65–87. [Google Scholar] [CrossRef]
- Chuang, M.H. Survey Report of Amphibians in Guantian Pheasant-Tailed Jacana Conservation Area; Wetlands Taiwan: Taichung, Taiwan, 2003; Volume 45, pp. 24–31. [Google Scholar]
- Yang, Y.C. Frog-Watching Map, 1st ed.; Morning Star: Taichung, Taiwan, 2009. [Google Scholar]
- Chiang, H.J. Study on Food Habits of Six Frog Species Community in a Still Water Pond Located in the Beipu Area, Hsinchu. Master’s Thesis, National Taipei University of Education, Taipei, Taiwan, 2013. [Google Scholar]
- Tang, L.C. Identification guidelines for quarantine pest larva of Coleoptera, Lipedoptera and Diptera. In Special Issue or the Symposium on Diagnosis of Plant Pests and Case Study; Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture: Taipei, Taiwan, 2006; Volume 6, pp. 73–103. [Google Scholar]
- Wu, C.F.; Li, M.H.; Cheng, S.H.; Chou, W.W. Assessing the Ecological Value and Residents’ Perception of Farm Pond Habitat Conservation. In Proceedings of the Council of Educators in Landscape Architecture Conferences, Baltimore, MD, USA, 26–29 March 2014.
- Hanspach, J.; Kühn, I.; Pompe, S.; Klotz, S. Predictive performance of plant species distribution models depends on species traits. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 219–225. [Google Scholar] [CrossRef]
- Bennetsen, E.; Gobeyn, S.; Goethals, P.L.M. Species distribution models grounded in ecological theory for decision support in river management. Ecol. Model. 2016, 325, 1–12. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L. Feasibility of an Ecological Corridor Establishment Using the Least-Cost-Analysis—A Case Study between Taroko and Shei-Pa National Parks. Master’s Thesis, National Dong Hwa University, Hualien County, Taiwan, 2003. [Google Scholar]
| Family | Common/Species Name | 2013 | 2014 | 2014 | 2014 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Fall | Winter | Spring | |||
| Ranidae | Günther’s frog Hylarana guentheri | 0 | 84 | 33 | 1 | 0 | 98 | 216 |
| Kuhl’s creek frog Limnonectes kuhlii | 0 | 4 | 0 | 0 | 0 | 0 | 4 | |
| Latouchti’s frog Hylarana latouchii | 0 | 8 | 2 | 2 | 0 | 7 | 19 | |
| Rhacophoridae | Farmland tree frog Rhacophorus arvalis | 0 | 89 | 60 | 3 | 0 | 12 | 164 |
| Brauer’s tree frog Polypedates braueri | 0 | 3 | 0 | 0 | 0 | 0 | 3 | |
| Spot-legged tree frog Polypedates megacephalus | 0 | 0 | 0 | 0 | 0 | 3 | 3 | |
| Microhylidae | Ornate narrow-mouthed toad Microhyla fissipes | 0 | 110 | 139 | 0 | 0 | 63 | 312 |
| Heymons’ narrow-mouthed toad Microhyla heymonsi | 0 | 3 | 0 | 0 | 0 | 0 | 3 | |
| Stejneger’s narrow-mouthed toad Micryletta steinegeri | 0 | 0 | 0 | 0 | 0 | 80 | 80 | |
| Dicroglossinae | Rice field frog Fejervarya limnocharis | 0 | 258 | 73 | 0 | 0 | 65 | 396 |
| Chinese bull frog Hoplobatrachus rugulosus | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Bufonidae | Spectacled toad Duttaphrynus melanosticus | 0 | 321 | 13 | 10 | 0 | 87 | 431 |
| Hylidae | Common Chinese tree toad Hyla chinensis | 0 | 6 | 0 | 0 | 0 | 0 | 6 |
| Total | 0 | 886 | 320 | 16 | 0 | 416 | 1638 |
| First-Layer Evaluation Criteria | Second-Layer Evaluation Criteria | Final Weight Coefficient (A × B) | Ranking | Ref. | ||
|---|---|---|---|---|---|---|
| Main Category | Relative Weight Coefficient (A) | Sub-Category: Specific Indicators | Relative Weight Coefficient (B) | |||
| Aquatic environments of farm pond | 0.305 | Water depth of farm pond | 0.345 | 0.105 | 4 | [12,36] |
| Vegetation coverage on pond banks | 0.3338 | 0.102 | 5 | [12,37] | ||
| Area ratio and distribution of pond water surfaces covered by aquatic plants | 0.3211 | 0.098 | 7 | [8,37] | ||
| Terrestrial environments around pond | 0.238 | Terrestrial plant diversity (# of species) within 100-m radius around pond | 0.532 | 0.127 | 1 | [38,39] |
| Terrestrial plant abundance (# of plants) within 100-m radius around pond | 0.468 | 0.112 | 3 | [38,39] | ||
| Landscape connectivity | 0.211 | Farm pond connected with trenches/creeks | 0.471 | 0.100 | 6 | [36,37] |
| Distance to nearby farm pond | 0.530 | 0.112 | 2 | [36,37] | ||
| Residents’ conservation perception | 0.247 | Residents understand that farm pond are frog habitats | 0.342 | 0.084 | 9 | [40] |
| Residents realize frog population decrease or/and habitat degradation | 0.304 | 0.075 | 10 | [40] | ||
| Residents are willing to participate frog conservation | 0.355 | 0.087 | 8 | [40] | ||
| Evaluation Criteria | Score = 1 | Score = 2 | Score = 3 | Score = 4 | Score = 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Explanation | n | Explanation | n | Explanation | n | Explanation | n | Explanation | n | ||
| Aquatic Environments of farm pond | Water depth of farm pond | Water depth ≥80 cm | 53 (76.82%) | 80 cm > Water depth ≥60 cm | 12 (17.39%) | 60 cm > Water depth ≥40 cm | 3 (4.35%) | 40 cm > Water depth ≥20 cm | 1 (1.45%) | Water depth <20 cm | 0 (0%) |
| Vegetation coverage on pond banks | Vegetation coverage <20% | 19 (27.54%) | 20%≤ Vegetation coverage <40% | 13 (18.84%) | 40%≤ Vegetation coverage <60% | 10 (14.49%) | 60%≤ Vegetation coverage <80% | 8 (11.59%) | Vegetation coverage ≥80% | 19 (27.54%) | |
| Area ratio and distribution of pond water surfaces covered by aquatic plants | Area ratio of water surface covered by aquatic plants <25%, but <50% aquatic plants growing along pond banks | 31 (44.93%) | Area ratio of water surface covered by aquatic plants <25%, and ≥50 plants growing along pond banks | 22 (31.88%) | 25%≤ Area ratio of water surface covered by aquatic plants <50%, and ≥50% plants growing along pond banks | 12 (17.39%) | 50%≤ Area ratio of water surface covered by aquatic plants < 75%, and ≥50% plants growing along pond banks | 1 (1.45%) | 75%≤ Area ratio of water surface covered by aquatic plants, and ≥50% plants growing along pond banks | 3 (7.25%) | |
| N/A | 25%≤ Area ratio of water surface covered by aquatic plants <50%, but <50% aquatic plants growing along pond banks | 50%≤ Area ratio of water surface covered by aquatic plants <75%, but <50% aquatic plants growing along pond banks | 75%≤ Area ratio of water surface covered by aquatic plants, but <50% aquatic plants growing along pond banks | 75%≤ Area ratio of water surface covered by aquatic plants, and ≥50% plants growing along pond banks | |||||||
| Terrestrial environments around pond | Terrestrial plant diversity (# of species) within 100-m radius around pond | Number of terrestrial plant species <50 | 18 (26.97%) | 50≤ Number of terrestrial plant species <60 | 16 (23.19%) | 60≤ Number of terrestrial plant species <70 | 10 (14.49%) | 70≤ Number of terrestrial plant species <80 | 6 (8.7%) | Number of terrestrial plant species ≥80 | 19 (27.54%) |
| Terrestrial plant abundance (# of plants) within 100-m radius around pond | Number of terrestrial plants <170 | 27 (39.13%) | 170≤ Number of terrestrial plants <190 | 12 (17.39%) | 190≤ Number of terrestrial plants <210 | 5 (7.25%) | 210≤ Number of terrestrial plants <230 | 4 (5.80%) | Number of terrestrial plants ≥230 | 21 (30.43%) | |
| Landscape connectivity | Farm pond connected with trenches/creeks | No trench/creek connected | 36 (52.17%) | 1 dry trench/creek connected | 9 (13.04%) | 1 with-water trench/creek connected | 11 (15.94%) | 1 dry trench/creek and 1 with-water trench/creek connected | 6 (8.7%) | ≥3 trenches/creeks connected, but half of them with water | 7 (10.14%) |
| No trench/creek connected | 1 dry trench/creek connected | 2 dry trenches /creeks connected | ≥3 trenches/Creeks connected, but half of them without water | 2 trenches/creeks connected and both with water | |||||||
| Distances to nearby farm pond | No nearby farm pond | 44 (63.77%) | Distances <15 m, but separated by roads | 9 (13.04%) | 11 m ≤ Distances ≤15 m, but separated by roads | 4 (5.80%) | 11 m ≤ Distances ≤15 m, and not separated by roads | 9 (13.04%) | Distances <10 m, and not separated by roads | 3 (7.25%) | |
| Blocked by buildings | Distances <15 m, but separated by roads | Distances >15 m, and not separated by roads | Distances <10m, but separated by roads | More than 2 farm ponds within 15 m, and not separated by roads | |||||||
| Residents’ conservation perception | Residents understand that farm pond are frog habitat. | x ≤3 | 4 (5.80%) | 3< x ≤4 | 13 (18.84%) | 4< x ≤5 | 23 (33.33%) | 5< x ≤6 | 25 (36.23%) | 6< x ≤7 | 4 (5.80%) |
| Residents realize the issue of frog population decrease or/and habitat degradation | x ≤3 | 3 (7.25%) | 3< x ≤4 | 4 (5.80%) | 4< x ≤5 | 25 (26.23%) | 5< x ≤6 | 35 (50.72%) | 6< x ≤7 | 2 (2.90%) | |
| Residents are willing to participate frog conservation | x ≤3 | 0 (0%) | 3< x ≤4 | 2 (2.9%) | 4< x ≤5 | 8 (11.59%) | 5< x ≤6 | 53 (76.82%) | 6< x ≤7 | 6 | |
| Items | Threshold of Habitat Suitability: 2.645 | Total (%) | ||
|---|---|---|---|---|
| Higher | Lower | |||
| Presence of Günther’s frog | Yes | 20 | 10 | 30 |
| (28.99%) | (14.49%) | (43.48%) | ||
| No | 3 | 36 | 39 | |
| (4.35%) | (52.17%) | (56.52%) | ||
| Total | 23 | 46 | 69 | |
| (33.33%) | (66.67%) | (100%) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, S.-R.; Chen, S.-H.; Wu, C.-F. An Expert-Based Assessment Model for Evaluating Habitat Suitability of Pond-Breeding Amphibians. Sustainability 2017, 9, 278. https://doi.org/10.3390/su9020278
Juang S-R, Chen S-H, Wu C-F. An Expert-Based Assessment Model for Evaluating Habitat Suitability of Pond-Breeding Amphibians. Sustainability. 2017; 9(2):278. https://doi.org/10.3390/su9020278
Chicago/Turabian StyleJuang, Shin-Ruoh, Szu-Hung Chen, and Chen-Fa Wu. 2017. "An Expert-Based Assessment Model for Evaluating Habitat Suitability of Pond-Breeding Amphibians" Sustainability 9, no. 2: 278. https://doi.org/10.3390/su9020278
APA StyleJuang, S.-R., Chen, S.-H., & Wu, C.-F. (2017). An Expert-Based Assessment Model for Evaluating Habitat Suitability of Pond-Breeding Amphibians. Sustainability, 9(2), 278. https://doi.org/10.3390/su9020278






