Cover Crop Residue Amount and Quality Effects on Soil Organic Carbon Mineralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Treatments
2.2. Cover Crop Biomass Sampling and Analysis
2.3. Soil Sampling and the Establishment of the Laboratory Experiment
2.4. Soil Carbon Mineralization Kinetics
2.5. Statistical Analysis
3. Results
3.1. Cover Crop Biomass
3.2. Basic Soil Properties
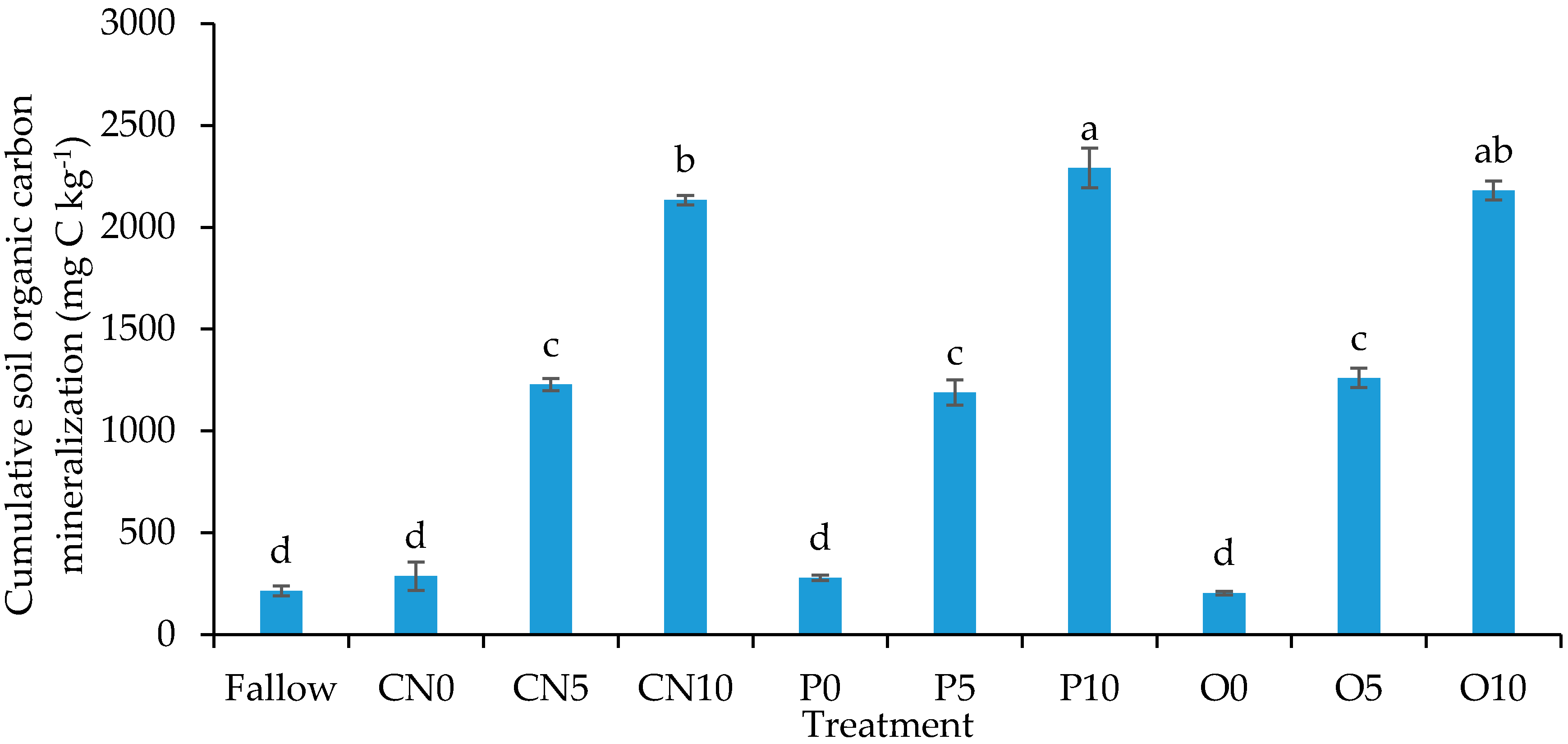
3.3. Cumulative Soil Organic Carbon Mineralization
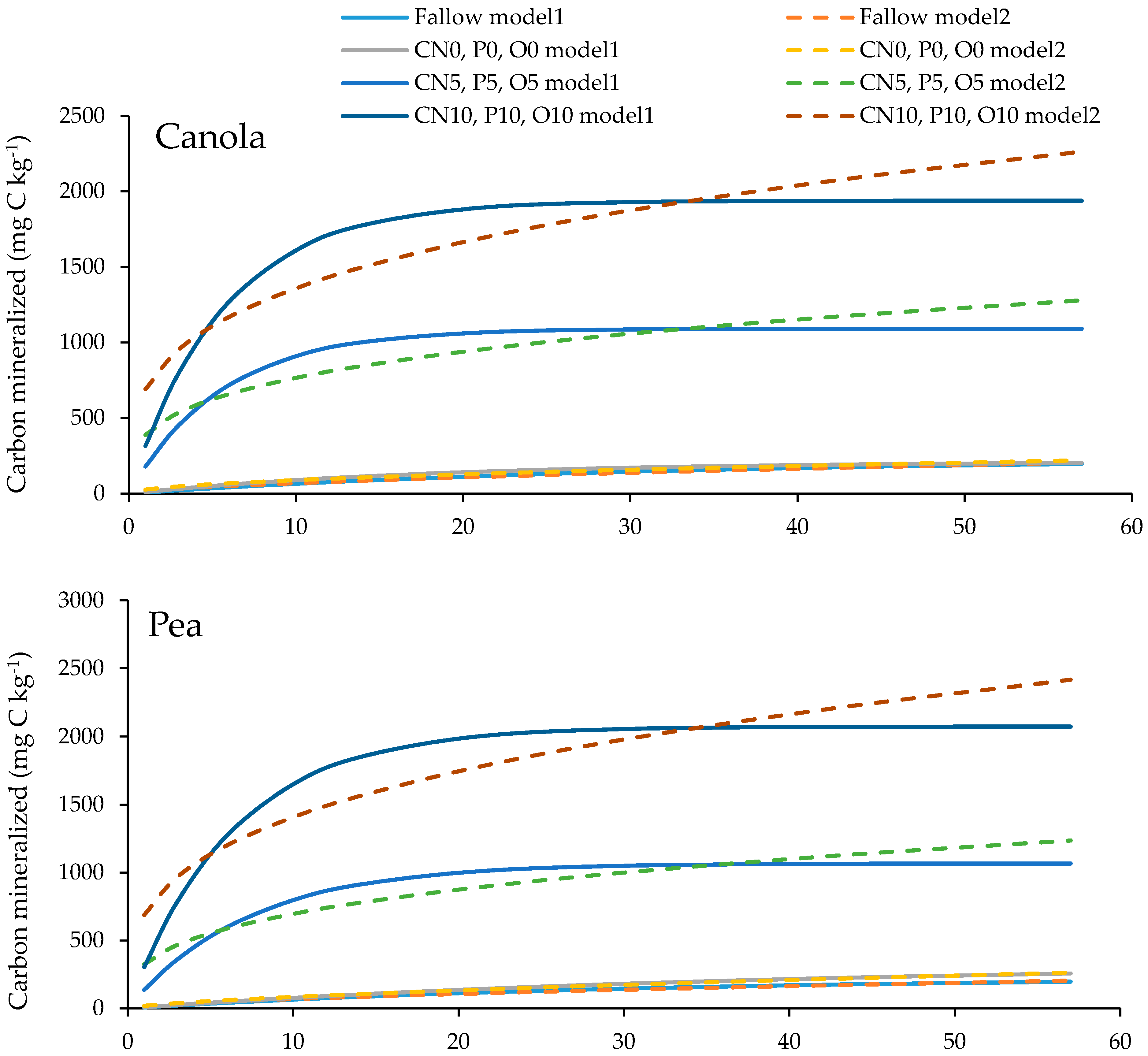
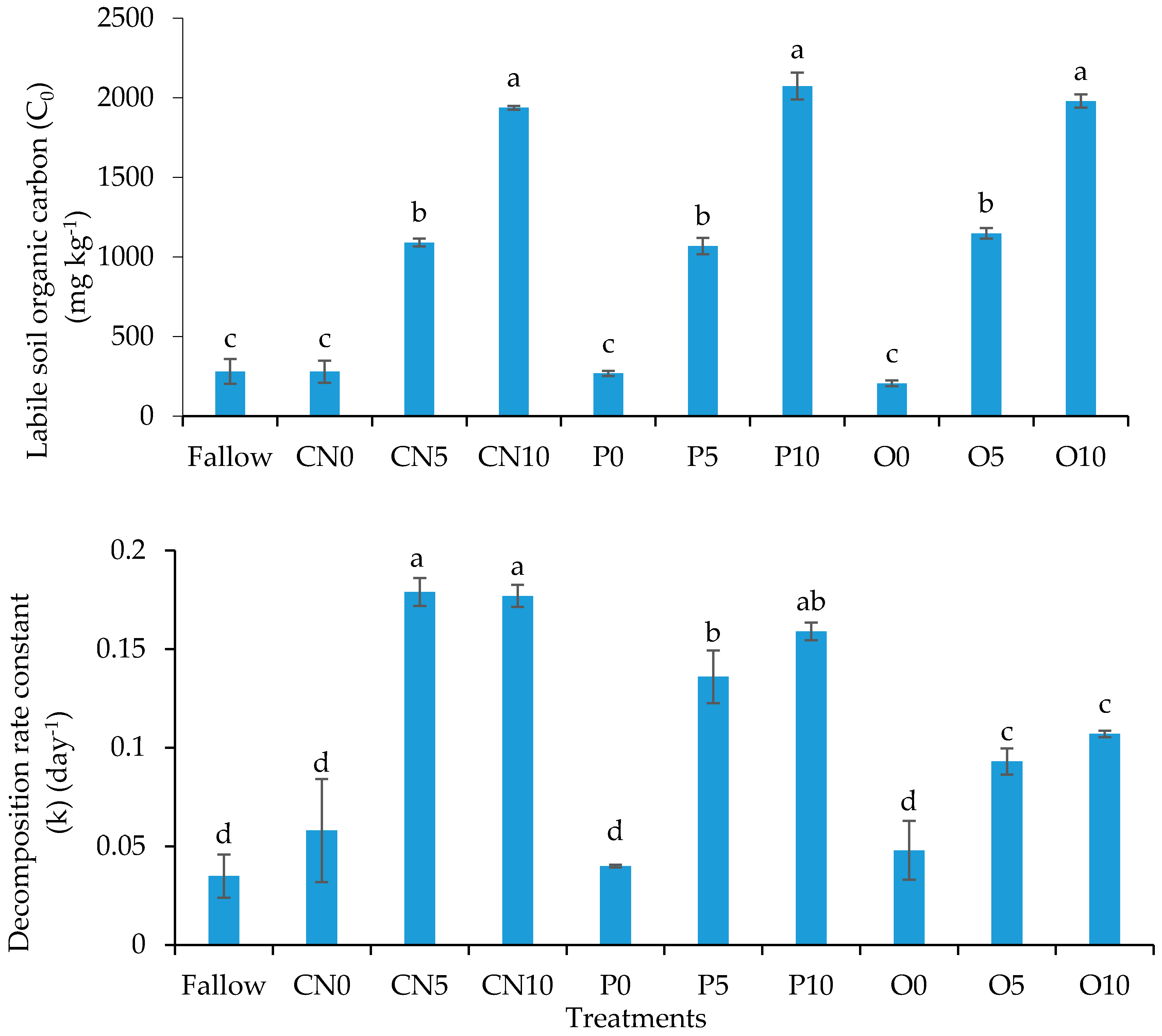
3.4. Soil Organic Carbon Mineralization Kinetics
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, X.; Herbert, S.J.; Hashemi, A.M.; Zhang, X.; Ding, G. Effects of agricultural management on soil organic matter and carbon transformation—A review. Plant Soil Environ. 2006, 52, 531–543. [Google Scholar]
- Follett, R.F. Soil management concepts and carbon sequestration zin cropland soils. Soil Tillage Res. 2001, 61, 77–92. [Google Scholar] [CrossRef]
- Nielsen, D.C.; Lyon, D.J.; Hergert, G.W.; Higgins, R.K.; Holman, J.D. Cover crop biomass production and water use in the central great plains. Agron. J. 2015, 107, 2047–2058. [Google Scholar] [CrossRef]
- Karlen, D.L.; Wollenhaupt, N.C.; Erbach, D.C.; Berry, E.C.; Swan, J.B.; Eash, N.S.; Jordahl, J.L. Crop residue effects on soil quality following 10-years of no-till corn. Soil Tillage Res. 1994, 31, 149–167. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Miyittah, M.; Inubushi, K. Decomposition and CO2-C evaluation of okara, sewage sludge, cow and poultry manure compost in soils. Soil Sci. Plant Nutr. 2003, 49, 61–68. [Google Scholar] [CrossRef]
- Ellert, B.H.; Janzen, H.H. Short-term influence of tillage on CO2 fluxes from a semi-arid soil on the Canadian Prairies. Soil Tillage Res. 1999, 50, 21–32. [Google Scholar] [CrossRef]
- Govaerts, B.; Verhulst, N.; Castellanos-Navarrete, A.; Sayre, K.; Dixon, J. Conservation agriculture and soil C sequestration: Between myth and farmer reality. Crit. Rev. Plant Sci. 2009, 28, 97–122. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop residues and management practices: Effects on soil quality, soil nitrogen dynamics, crop yield, and nitrogen recovery. Adv. Agron. 2000, 68, 197–319. [Google Scholar]
- Johnson, J.M.F.; Barbour, N.W.; Weyers, S.L. Chemical composition of crop biomass impacts its decomposition. Soil Sci. Soc. Am. J. 2007, 71, 155–162. [Google Scholar] [CrossRef]
- Li, L.J.; Han, X.Z.; You, M.Y.; Yuan, Y.R.; Ding, X.L.; Qiao, Y.F. Carbon and nitrogen mineralization patterns of two contrasting crop residues in a Mollisol: Effects of residue type and placement in soils. Eur. J. Soil Biol. 2013, 54, 1–6. [Google Scholar] [CrossRef]
- Martin, J.V.; de Imperial, R.M.; Calvo, R.; Garcia, M.C.; Leon-Cofreces, C.; Delgado, M.M. Carbon mineralization kinetics of poultry manure in two soils. Soil Res. 2012, 50, 222–228. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Liebman, M.; Cavigelli, M.; Wander, M. Influence of residue and nitrogen fertilizer additions on carbon mineralization in soils with different texture and cropping histories. PLoS ONE 2014, 9, e103720. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Nayak, A.K.; Kumar, A.; Tripathi, R.; Shahid, M.; Bhattacharyya, P.; Raja, R.; Panda, B.B. Carbon and nitrogen mineralization kinetics in soil of rice-rice system under long term application of chemical fertilizers and farmyard manure. Eur. J. Soil Biol. 2013, 58, 113–121. [Google Scholar] [CrossRef]
- Hatten, J.A.; Zabowski, D. Changes in soil organic matter pools and carbon mineralization as influenced by fire severity. Soil Sci. Soc. Am. J. 2009, 73, 262–273. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Jenkinson, D.S. Studies on decomposition of plant material in soil. Effect of rate of addition. J. Soil Sci. 1977, 28, 417–423. [Google Scholar] [CrossRef]
- Ladd, J.N.; Amato, M.; Jackson, R.B.; Butler, J.H.A. Utilization by wheat crops of nitrogen from legume residues decomposing in soils in the field. Soil Biol. Biochem. 1983, 15, 231–238. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Tabatabai, M.A. Decomposition of different organic materials in soils. Biol. Fertil. Soils 1994, 18, 175–182. [Google Scholar] [CrossRef]
- Hooker, T.D.; Stark, J.M. Soil C and N cycling in three semiarid vegetation types: Response to an in situ pulse of plant detritus. Soil Biol. Biochem. 2008, 40, 2678–2685. [Google Scholar] [CrossRef]
- Baumhardt, R.L.; Schwartz, R.; Howell, T.; Evett, S.R.; Colaizzi, P. Residue management effects on water use and yield of deficit irrigated corn. Agron. J. 2013, 105, 1035–1044. [Google Scholar] [CrossRef]
- Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov (accessed on 10 August 2017).
- Gee, G.W.; Bauder, J.W. Particle-size analysis by hydrometer—Simplified method for routine textural analysis and a sensitivity test of measurement parameters. Soil Sci. Soc. Am. J. 1979, 43, 1004–1007. [Google Scholar] [CrossRef]
- Rayment, G.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press Pty Ltd.: Melbourne, Australia, 1992. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954.
- Stanford, G.; Smith, S. Nitrogen mineralization potentials of soils. Soil Sci. Soc. Am. J. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Broadbent, F. Empirical modeling of soil nitrogen mineralization. Soil Sci. 1986, 141, 208–213. [Google Scholar] [CrossRef]
- Marion, G.; Black, C. The effect of time and temperature on nitrogen mineralization in arctic tundra soils. Soil Sci. Soc. Am. J. 1987, 51, 1501–1508. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J.U.; Powlson, D.S.; McGill, W.B.; Arah, J.R.M.; Chertov, O.G.; Coleman, K.; Franko, U.; Frolking, S.; Jenkinson, D.S.; et al. A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 1997, 81, 153–225. [Google Scholar] [CrossRef]
- Janssen, P.H.M.; Heuberger, P.S.C. Calibration of process-oriented models. Ecol. Model. 1995, 83, 55–66. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.W.; Zheng, X.H.; Wang, Y.S.; Xu, X.K. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Abera, G.; Wolde-Meskel, E.; Bakken, L.R. Carbon and nitrogen mineralization dynamics in different soils of the tropics amended with legume residues and contrasting soil moisture contents. Biol. Fertil. Soils 2012, 48, 51–66. [Google Scholar] [CrossRef]
- Rochette, P.; van Bochove, E.; Prevost, D.; Angers, D.A.; Cote, D.; Bertrand, N. Soil carbon and nitrogen dynamics following application of pig slurry for the 19th consecutive year: II. Nitrous oxide fluxes and mineral nitrogen. Soil Sci. Soc. Am. J. 2000, 64, 1396–1403. [Google Scholar] [CrossRef]
- Trinsoutrot, I.; Recous, S.; Bentz, B.; Lineres, M.; Cheneby, D.; Nicolardot, B. Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci. Soc. Am. J. 2000, 64, 918–926. [Google Scholar] [CrossRef]
- Staaf, H.; Berg, B. Accumulation and release of plant nutrients in decomposing scots pine needle litter—Long-term decomposition in a scots pine forest. Can. J. Botany 1982, 60, 1561–1568. [Google Scholar] [CrossRef]
- Mohammed, A.; Naab, J.; Nartey, E.; Adiku, S. Carbon mineralization from plant residue-amended soils under varying moisture conditions. J. Exp. Biol. Agric. Sci. 2014, 1, 492–498. [Google Scholar]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Passioura, J.B.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Nutrient availability limits carbon sequestration in arable soils. Soil Biol. Biochem. 2014, 68, 402–409. [Google Scholar] [CrossRef]
- Bingeman, C.W.; Varner, J.E.; Martin, W.P. The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci. Soc. Am. Proc. 1953, 17, 34–38. [Google Scholar] [CrossRef]
- Lou, Y.S.; Ren, L.X.; Li, Z.P.; Zhang, T.L.; Inubushi, K. Effect of rice residues on carbon dioxide and nitrous oxide emissions from a paddy soil of subtropical China. Water Air Soil Pollut. 2007, 178, 157–168. [Google Scholar] [CrossRef]
- Kallanbach, C.M.; Frey, S.D.; Grandy, S.A. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.; Norton, J.B.; Stahl, P.D.; Norton, U. Soil microbial substrate properties and microbial community responses under irrigated organic and reduced-tillage crop and forage production systems. PLoS ONE 2014, 9, e103901. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.E.; Wickings, K.; McDaniel, M.D.; Robertson, G.P.; Grandy, A.S. Cover crop root contributions to soil C in a no-till corn bioenergy cropping system. GCB Bioenergy 2017, 9, 1252–1263. [Google Scholar] [CrossRef]
- Quemada, M.; Cabrera, M.L. Carbon and nitrogen mineralized from leaves and stems of 4 cover crops. Soil Sci. Soc. Am. J. 1995, 59, 471–477. [Google Scholar] [CrossRef]
- Vestgarden, L.S. Carbon and nitrogen turnover in the early stage of Scots pine (Pinus sylvestris L.) needle litter decomposition: Effects of internal and external nitrogen. Soil Biol. Biochem. 2001, 33, 465–474. [Google Scholar] [CrossRef]
- Green, C.J.; Blackmer, A.M. Residue decomposition effects on nitrogen availability to corn following corn or soybean. Soil Sci. Soc. Am. J. 1995, 59, 1065–1070. [Google Scholar] [CrossRef]

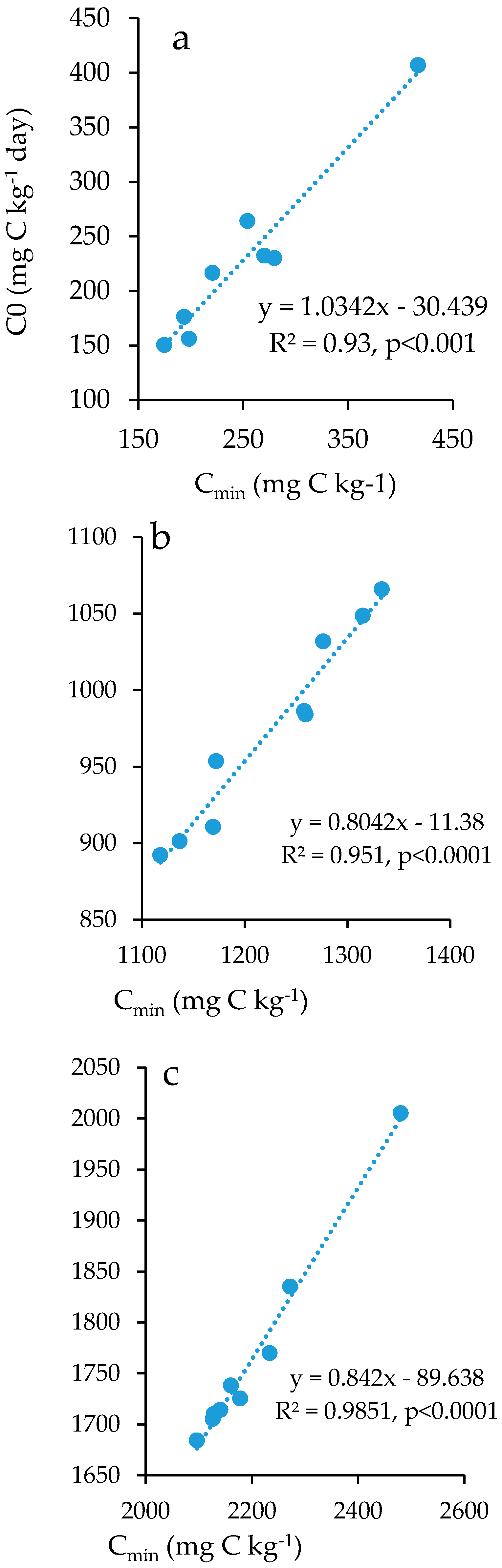
| Cover Crop | Biomass Carbon (%) | Biomass Nitrogen (%) | Carbon to Nitrogen Ratio | Lignin (%) |
|---|---|---|---|---|
| Pea | 46.3 | 4.49 | 10.3 | 5.16 |
| Oat | 46.1 | 2.53 | 18.2 | 2.85 |
| Canola | 41.2 | 5.28 | 7.8 | 3.30 |
| Treatment | Soil Water Content (%) | Available Nitrogen (kg ha−1) | Phosphorous (kg ha−1) |
|---|---|---|---|
| Fallow | 13.1 ± 0.67 a,† | 20.1 ± 2.45 a | 21.4 ± 2.96 a |
| Pea | 10.5 ± 0.40 b | 11.9 ± 0.81 b | 15.9 ± 2.35 a,b |
| Oat | 11.3 ± 0.26 b | 11.3 ± 0.86 b | 17.7 ± 5.36 a,b |
| Canola | 11.9 ± 0.10 a,b | 10.5 ± 0.90 b | 14.7 ± 3.51 b |
| Models | Parameter | Fallow (F) | Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CN0 | CN5 | CN10 | P0 | P5 | P10 | O0 | O5 | O10 | |||
| Model 1 | C0 | 233 | 277 | 1091 | 1938 | 340 | 1067 | 2074 | 193 | 1147 | 1979 |
| k | 0.03 | 0.05 | 0.18 | 0.18 | 0.02 | 0.14 | 0.16 | 0.05 | 0.09 | 0.11 | |
| r | 0.960 | 0.78 | 0.970 | 0.98 | 0.95 | 0.94 | 0.97 | 0.95 | 0.97 | 0.97 | |
| (C = C0 (1 − e−kt) | MSE | 372 | 678 | 6064 | 12,984 | 774 | 13,249 | 25,773 | 368 | 9490 | 19,798 |
| RMSE | 19.29 | 26 | 77.9 | 114 | 27.8 | 115 | 160 | 19.2 | 97.4 | 141 | |
| NRMSE | 22.9 | 19.3 | 10.4 | 8.64 | 25.4 | 16.6 | 11.6 | 22.1 | 14.5 | 11.7 | |
| Model 2 | C0 | 14.2 | 27.4 | 389 | 691 | 18.9 | 325 | 688 | 21.6 | 257 | 495 |
| k | 0.62 | 0.51 | 0.29 | 0.29 | 0.65 | 0.33 | 0.31 | 0.54 | 0.39 | 0.38 | |
| r | 0.96 | 0.78 | 0.94 | 0.94 | 0.96 | 0.93 | 0.94 | 0.96 | 0.97 | 0.97 | |
| (C = C0 × tk) | MSE | 290 | 603 | 11,545 | 38,955 | 595 | 14,401 | 47,699 | 239 | 8070 | 21,796 |
| RMSE | 17 | 24.6 | 107 | 197 | 24.4 | 120 | 218 | 15.5 | 90 | 148 | |
| NRMSE | 20.3 | 18.2 | 14.4 | 14.9 | 22.3 | 17.3 | 15.7 | 17.8 | 13.4 | 12.3 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, B.; Ghimire, R.; VanLeeuwen, D.; Mesbah, A. Cover Crop Residue Amount and Quality Effects on Soil Organic Carbon Mineralization. Sustainability 2017, 9, 2316. https://doi.org/10.3390/su9122316
Ghimire B, Ghimire R, VanLeeuwen D, Mesbah A. Cover Crop Residue Amount and Quality Effects on Soil Organic Carbon Mineralization. Sustainability. 2017; 9(12):2316. https://doi.org/10.3390/su9122316
Chicago/Turabian StyleGhimire, Binod, Rajan Ghimire, Dawn VanLeeuwen, and Abdel Mesbah. 2017. "Cover Crop Residue Amount and Quality Effects on Soil Organic Carbon Mineralization" Sustainability 9, no. 12: 2316. https://doi.org/10.3390/su9122316
APA StyleGhimire, B., Ghimire, R., VanLeeuwen, D., & Mesbah, A. (2017). Cover Crop Residue Amount and Quality Effects on Soil Organic Carbon Mineralization. Sustainability, 9(12), 2316. https://doi.org/10.3390/su9122316






