Increased Production and Water Remediation by Land-Based Farm-Scale Sequentially Integrated Multi-Trophic Aquaculture Systems—An Example from Southern Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
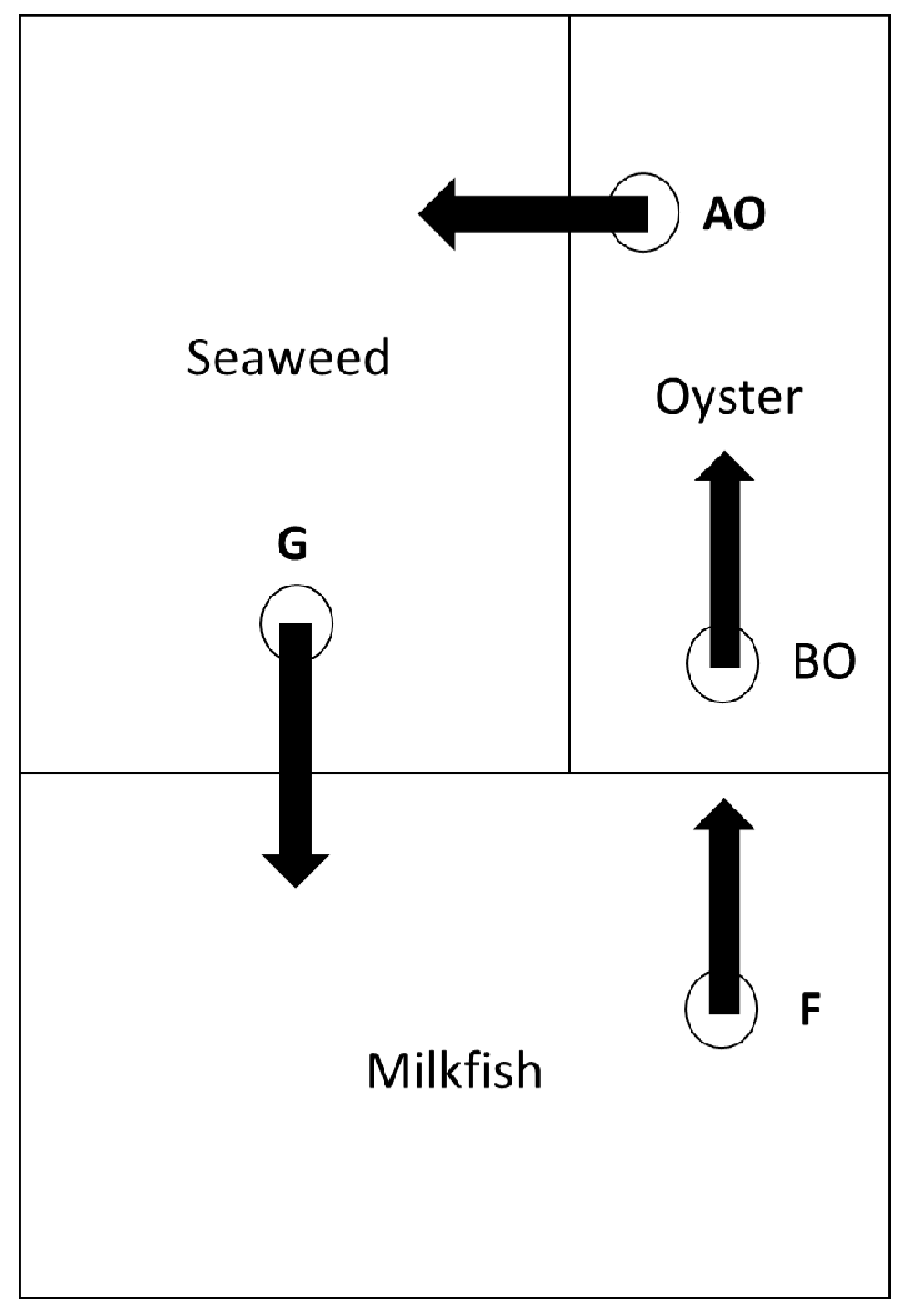
2.2. The Studied IMTA Systems
2.3. Water Sampling
2.4. Statistical Analysis
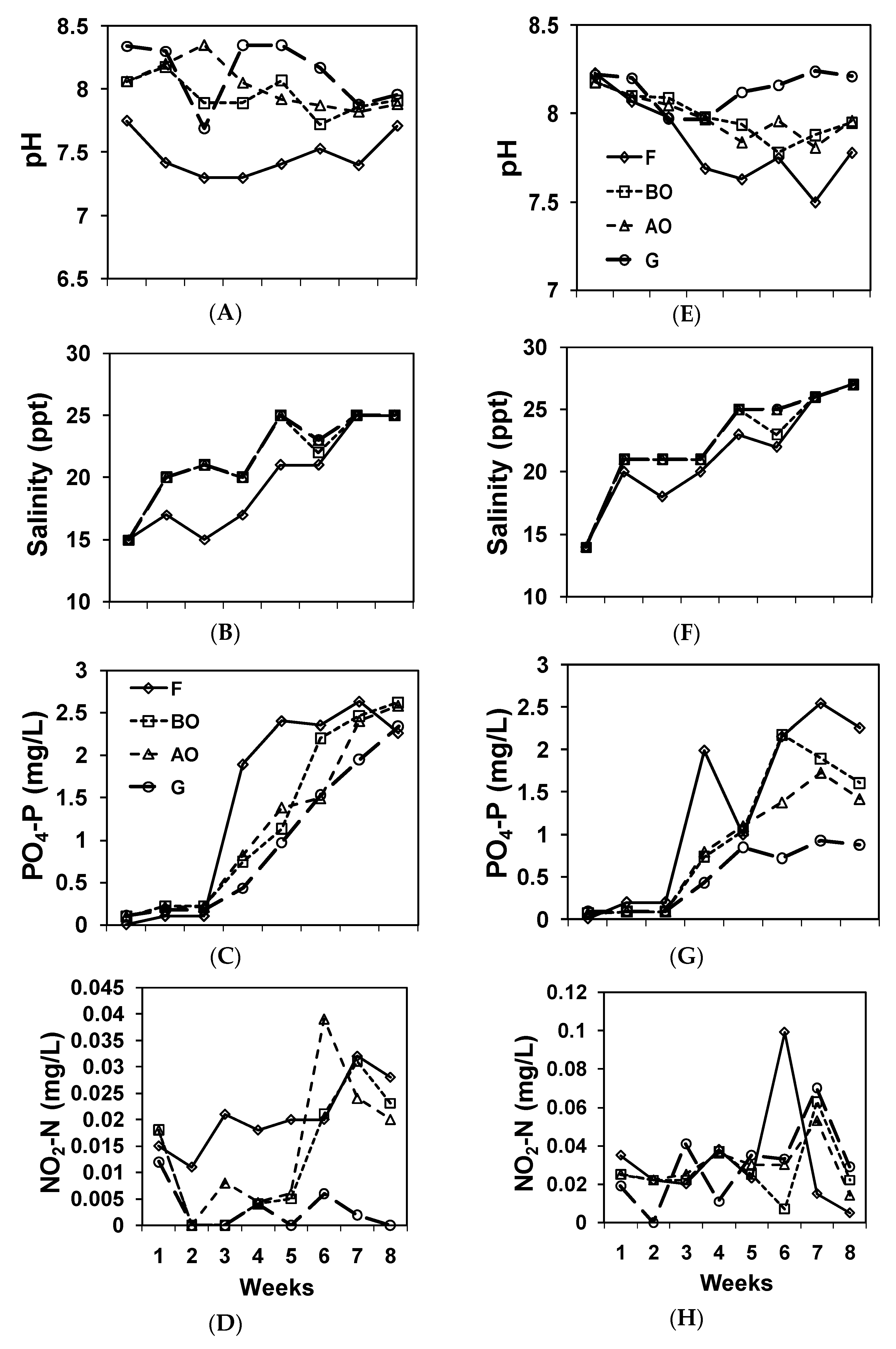
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- Pillay, T.V.R. Aquaculture and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Diana, J.S. Aquaculture Production and Biodiversity Conservation. BioScience 2009, 59, 27–38. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Martinez-Cordova, L.R. World Aquaculture: Environmental Impacts and Troubleshooting Alternatives. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oettinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Stephens, W.W.; Farris, J.L. Instream community assessment of aquaculture effluents. Aquaculture 2004, 231, 149–162. [Google Scholar] [CrossRef]
- Peterson, M.; Slack, W.T.; Woodley, C.M.; Springs, O. The occurrence of non-indigenous nile tilapia, Oreochromins niloticus (Linnaeus) in coastal Mississippi, USA: Ties to aquaculture and thermal effluents. Wetlands 2005, 25, 112–121. [Google Scholar] [CrossRef]
- Holmer, M.; Argyrou, M.; Dalsgaard, T.; Danovaro, R.; Diaz-Almela, E.; Duarte, C.M.; Frederiksen, M.; Grau, A.; Karakassis, I.; Marbà, N.; et al. Effects of fish farm waste on Posidonia oceanica meadows: Synthesis and provision of monitoring and management tools. Mar. Pollut. Bull. 2008, 56, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Y.; Courties, C.; El Helwe, Y.; Herbland, A.; Lemonnier, H. Spatial and temporal extension of eutrophication associated with shrimp farm wastewater discharges in the New Caledonia lagoon. Mar. Pollut. Bull. 2010, 61, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Ansah, Y.B.; Frimpong, E.A.; Amisah, S. Biological assessment of aquaculture effects on effluent-receiving streams in Ghana using structural and functional composition of fish and macroinvertebrate assemblages. Environ. Manag. 2012, 50, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, M.; Neori, A.; Popper, D.M.; Gordin, H. A proposed model for “environmentally clean” land-based culture of fish, bivalves and seaweeds. Aquaculture 1993, 117, 115–128. [Google Scholar] [CrossRef]
- Shpigel, M.; Neori, A. The integrated culture of seaweed, abalone, fish and clams in modular intensive land-based systems: I. Proportions of size and projected revenues. Aquac. Eng. 1996, 15, 313–326. [Google Scholar] [CrossRef]
- Neori, A.; Ragg, N.; Shpigel, M. The integrated culture of seaweed, abalone, fish and clams in modular intensive land-based systems: II. Performance and nitrogen partitioning within an abalone (Haliotis tuberculata) and macroalgae culture system. Aquac. Eng. 1998, 17, 215–239. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M.; Ben-Ezra, D. A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture 2000, 186, 279–291. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Martínez-Córdova, L.R.; Porchas-Cornejo, M.A.; López-Elías, J.A. Shrimp polyculture: A potentially profitable, sustainable, but uncommon aquacultural practice. Rev. Aquac. 2010, 2, 73–85. [Google Scholar] [CrossRef]
- Sri-uam, P.; Donnuea, S.; Powtongsook, S.; Pavasant, P. Integrated multi-trophic recirculating aquaculture system for Nile tilapia (Oreochromis niloticus). Sustainability 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-gonzález, J.; Yarish, C.; Neefus, C. Integrating seaweeds into marine aquaculture systems: A key towards sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Ridler, N.; Wowchuk, M.; Robinson, B.; Barrington, K.; Chopin, T.; Robinson, S.; Page, F.; Reid, G.; Szemerda, M.; Sewuster, J. Integrated Multi-Trophic Aquaculture (IMTA): A Potential Strategic Choice for Farmers. Aquac. Econ. Manag. 2007, 11, 99–110. [Google Scholar] [CrossRef]
- Troell, M. Integrated marine and brackishwater aquaculture in tropical regions. In Integrated Mariculture—A Global Review; FAO Fisheries and Aquaculture Technical. Paper No. 529; Soto, D., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; pp. 47–132. [Google Scholar]
- Nobre, A.M.; Robertson-Andersson, D.; Neori, A.; Sankar, K. Ecological-economic assessment of aquaculture options: Comparison between abalone monoculture and integrated multi-trophic aquaculture of abalone and seaweeds. Aquaculture 2010, 306, 116–126. [Google Scholar] [CrossRef]
- Bartol, I.K.; Mann, R.; Luckenbach, M. Growth and mortality of oysters (Crassostrea virginica) on constructed intertidal reefs: Effects of tidal height and substrate level. J. Exp. Mar. Biol. Ecol. 1999, 237, 157–184. [Google Scholar] [CrossRef]
- La Peyre, M.K.; Eberline, B.S.; Soniat, T.M.; La Peyre, J.F. Differences in extreme low salinity timing and duration differentially affect eastern oyster (Crassostrea virginica) size class growth and mortality in Breton Sound, LA. Estuar. Coast. Shelf Sci. 2013, 135, 146–157. [Google Scholar] [CrossRef]
- Munroe, D.; Tabatabai, A.; Burt, I.; Bushek, D.; Powell, E.N.; Wilkin, J. Oyster mortality in Delaware Bay: Impacts and recovery from Hurricane Irene and Tropical Storm Lee. Estuar. Coast. Shelf Sci. 2013, 135, 209–219. [Google Scholar] [CrossRef]
- Solís, D.; Perruso, L.; del Corral, J.; Stoffle, B.; Letson, D. Measuring the initial economic effects of hurricanes on commercial fish production: The US Gulf of Mexico grouper (Serranidae) fishery. Nat. Hazards 2013, 66, 271–289. [Google Scholar] [CrossRef]
- Spalding, M.D.; Ruffo, S.; Lacambra, C.; Meliane, I.; Hale, L.Z.; Shepard, C.C.; Beck, M.W. The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean Coast. Manag. 2014, 90, 50–57. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Horner, R.A.; Caron, D.A.; Garcia-Mendoza, E.; Hickey, B.M.; Hunter, M.; Huppert, D.D.; Kudela, R.M.; Langlois, G.W.; Largier, J.L. Harmful algal blooms along the North American west coast region: History, trends, causes, and impacts. Harmful Algae 2012, 19, 133–159. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Cordova, L.R.; Martinez-Porchas, M. Polyculture of Pacific white shrimp, Litopenaeus vannamei, giant oyster, Crassostrea gigas and black clam, Chione fluctifraga in ponds in Sonora, Mexico. Aquaculture 2006, 258, 321–326. [Google Scholar] [CrossRef]
- Neori, A.; Krom, M.D.; Ellner, S.P.; Boyd, C.E.; Popper, D.; Rabinovitch, R.; Davison, P.J.; Dvir, O.; Zuber, D.; Ucko, M.; et al. Seaweed biofilters as regulators of water quality in integrated fish-seaweed culture units. Aquaculture 1996, 141, 183–199. [Google Scholar] [CrossRef]
- Schuenhoff, A.; Shpigel, M.; Lupatsch, I.; Ashkenazi, A.; Msuya, F.E.; Neori, A. A semi-recirculating, integrated system for the culture of fish and seaweed. Aquaculture 2003, 221, 167–181. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Samocha, T.M.; Fricker, J.; Ali, A.M.; Shpigel, M.; Neori, A. Growth and nutrient uptake of the macroalga Gracilaria tikvahiae cultured with the shrimp Litopenaeus vannamei in an Integrated Multi-Trophic Aquaculture (IMTA) system. Aquaculture 2015, 446, 263–271. [Google Scholar] [CrossRef]
- Granéli, E.; Pavia, H. Allelopathy in marine ecosystems. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M.J., Pedrol, N., González, L., Eds.; Springer: New York, NY, USA, 2006; pp. 415–431. [Google Scholar]
- Tang, Y.Z.; Gobler, C.J. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 2011, 10, 480–488. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, Z.R.; Xia, M.S. The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fish. Sci. 2005, 71, 1036–1041. [Google Scholar] [CrossRef]
- Luo, W.; Deng, X.; Zeng, W.; Zheng, D. Treatment of wastewater from shrimp farms using a combination of fish, photosynthetic bacteria, and vegetation. Desalin. Water Treat. 2012, 47, 221–227. [Google Scholar] [CrossRef]
- Fisheries Agency. Fisheries Statistical Yearbook of Taiwan, Kinmen, and Matsu Areas; Fisheries Agency, Council of Agriculture: Taipei City, Taiwan, 2011.
- Hwang, D. Research on marine toxins in Taiwan. A festschrift in honor of Professor Anthony T. Tu. J. Toxicol. Toxin Rev. 2003, 22, 663–678. [Google Scholar] [CrossRef]
- Hung, J.J.; Hung, C.S.; Su, H.M. Biogeochemical responses to the removal of maricultural structures from an eutrophic lagoon (Tapong Bay) in Taiwan. Mar. Environ. Res. 2008, 65, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.S.; Meng, P.J.; Lee, H.J.; Ye, Y.X.; Kuo, J.; Fang, L.S.; Chou, W.R. Dynamics of phytoplankton and picoplankton over a tidal cycle in a subtropical lagoon. Chin. Sci. Bull. 2010, 55, 2522–2528. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M. An Integrated System for Farming Fish, Seaweed and Abalone; CAB International Aquaculture Compendium: Wallingford, UK, 2006. [Google Scholar]
- Abreu, M.H.; Varela, D.A.; Henríquez, L.; Villarroel, A.; Yarish, C.; Sousa-Pinto, I.; Buschmann, A.H. Traditional vs. Integrated Multi-Trophic Aquaculture of Gracilaria chilensis C.J. Bird, J. McLachlan & E.C. Oliveira: Productivity and physiological performance. Aquaculture 2009, 293, 211–220. [Google Scholar]
- Beutler, M.; Wiltshire, K.H.; Meyer, B.; Moldaenke, C.; Lüning, C.; Meyerhöfer, M.; Hansen, U.P.; Dau, H. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 2002, 72, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Chen, P.Y.; Dahms, H.U.; Yeh, S.L.; Chiu, Y.J. Comparing methods for measuring phytoplankton biomass in aquaculture ponds. Aquac. Environ. Interact. 2016, 8, 665–673. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS; SAGE Publications: Thousand Oaks, CA, USA, 2005; p. 816. [Google Scholar]
- Chang, T.J.; Wu, Y.T.; Hsu, H.Y.; Liao, C.M.; Chu, C.R. Assessment of wind characteristics and wind turbine characteristics in Taiwan. Renew. Energy 2003, 28, 851–871. [Google Scholar] [CrossRef]
- Lai, C.; Lin, T. Technical assessment of the use of a small-scale wind power system to meet the demand for electricity in a land aquafarm in Taiwan. Renew. Energy 2006, 31, 877–892. [Google Scholar] [CrossRef]
- Jones, A.B.; Dennison, W.C.; Preston, N.P. Integrated treatment of shrimp effluent by sedimentation, oyster filtration and macroalgal absorption: A laboratory scale study. Aquaculture 2001, 193, 155–178. [Google Scholar] [CrossRef]
- Jones, A.B.; Preston, N.P.; Dennison, W.C. The efficiency and condition of oysters and macroalgae used as biological filters of shrimp pond effluent. Aquac. Res. 2002, 33, 1–19. [Google Scholar] [CrossRef]
- Ramos, R.; Vinatea, L.; Seiffert, W.; Beltrame, E.; Silva, J.S.; da Costa, R.H.R. Treatment of shrimp effluent by sedimentation and oyster filtration using Crassostrea gigas and C. rhizophorae. Braz. Arch. Biol. Technol. 2009, 52, 775–783. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Mao, Y.; Li, X.; Liu, Y.; Zhang, F. Growth characters and photosynthetic capacity of Gracilaria lemaneiformis as a biofilter in a shellfish farming area in Sanggou Bay, China. J. Appl. Phycol. 2005, 17, 199–206. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ishihi, Y. Bioindicator and biofilter function of Ulva spp. (Chlorophyta) for dissolved inorganic nitrogen discharged from a coastal fish farm—Potential role in integrated multi-trophic aquaculture. Aquaculture 2010, 310, 74–83. [Google Scholar] [CrossRef]
- Wang, Y.; He, Z. Effect of probiotics on alkaline phosphatase activity and nutrient level in sediment of shrimp, Penaeus vannamei, ponds. Aquaculture 2009, 287, 94–97. [Google Scholar] [CrossRef]
- McIntosh, D.; Samocha, T.M.; Jones, E.R.; Lawrence, A.L.; McKee, D.A.; Horowitz, S.; Horowitz, A. The effect of a commercial bacterial supplement on the high-density culturing of Litopenaeus vannamei with a low-protein diet in an outdoor tank system and no water exchange. Aquac. Eng. 2000, 21, 215–227. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Li, W. Effect of probiotics on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 2009, 287, 349–353. [Google Scholar] [CrossRef]

| Water Quality Variables | Mean | SD | T | p |
|---|---|---|---|---|
| PO4−3-P (ppm) | −0.28 | 0.80 | −1.00 | 0.353 |
| NO2−-N (ppm) | −0.01 | 0.09 | −0.29 | 0.778 |
| NH4+-N (ppm) | 0.04 | 0.36 | 0.34 | 0.742 |
| BOD5 (ppm) | 5.38 | 13.23 | 1.15 | 0.288 |
| Turbidity (NTU) | 21.08 | 21.45 | 2.78 | 0.027 |
| Phytoplankton (μg/L) | 28.96 | 62.71 | 1.31 | 0.233 |
| Green (μg/L) | 62.33 | 74.41 | 2.37 | 0.050 |
| Cyanobacteria (μg/L) | −7.58 | 9.65 | −2.22 | 0.062 |
| Brown (μg/L) | −9.73 | 21.84 | −1.26 | 0.248 |
| PE-rich (μg/L) | −0.38 | 2.53 | −0.42 | 0.687 |
| Water Variables | North | Sampling Points | South | Sampling Points | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | BO | AO | G | F | p | F | BO | AO | G | |
| pH | 19.15 | <0.001 | 7.48 ± 0.17 (b) | 7.95 ± 0.15 (a) | 8.02 ± 0.18 (a) | 8.13 ± 0.26 (a) | 8.62 | 0.008 | 7.83 ± 0.24 (c) | 7.99 ± 0.13 (a,b) | 7.98 ± 0.12 (b) | 8.14 ± 0.11 (a) |
| Salinity (‰) | 7.93 | 0.024 | 19.50 ± 1.45 (b) | 21.63 ± 1.22 (a) | 21.75 ± 1.24 (a) | 21.75 ± 1.24 (a) | 6.26 | 0.023 | 21.25 ± 1.50 (b) | 22.25 ± 1.45 (a) | 22.50 ± 1.49 (a) | 22.50 ± 1.49 (a) |
| DO (ppm) | 0.09 | 0.876 | 4.72 ± 0.42 | 4.88 ± 0.30 | 4.76 ± 0.28 | 4.78 ± 0.52 | 1.63 | 0.238 | 4.58 ± 0.39 | 4.70 ± 0.38 | 4.60 ± 0.29 | 4.23 ± 0.37 |
| Water temp. (°C) | 1.5 | 0.262 | 26.13 ± 1.39 | 25.85 ± 1.24 | 25.87 ± 1.22 | 26.28 ± 1.42 | 0.93 | 0.411 | 26.18 ± 1.35 | 26.01 ± 1.25 | 25.96 ± 1.23 | 26.02 ± 1.29 |
| PO4−3-P (ppm) | 3.09 | 0.104 | 1.47 ± 0.42 | 1.21 ± 0.38 | 1.15 ± 0.35 | 0.96 ± 0.31 | 6.57 | 0.019 | 1.30 ± 0.38 (a) | 0.97 ± 0.30 (a,b) | 0.84 ± 0.24 (b) | 0.51 ± 0.13 (c) |
| NO2−-N (ppm) | 8.48 | 0.002 | 0.02 ± 0.00 (a) | 0.01 ± 0.00 (b) | 0.02 ± 0.01 (a,b) | 0.00 ± 0.00 (c) | 0.65 | 0.483 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.01 |
| NH4+-N (ppm) | 3.28 | 0.059 | 0.30 ± 0.08 | 0.43 ± 0.08 | 0.32 ± 0.07 | 0.27 ± 0.05 | 0.42 | 0.601 | 0.51 ± 0.12 | 0.58 ± 0.11 | 0.59 ± 0.11 | 0.52 ± 0.10 |
| BOD5 (ppm) | 6.58 | 0.029 | 18.25 ± 3.59 (a) | 15.00 ± 2.88 (b) | 13.25 ± 2.43 (b) | 7.38 ± 0.73 (b) | 2.15 | 0.164 | 21.50 ± 2.42 | 16.63 ± 2.82 | 15.75 ± 1.46 | 16.00 ± 3.34 |
| Turbidity (NTU) | 5.22 | 0.020 | 20.16 ± 3.61 (a) | 15.30 ± 3.05 (a) | 13.17 ± 2.73 (b) | 5.35 ± 1.19 (c) | 2.19 | 0.146 | 22.08 ± 3.05 | 31.47 ± 3.73 | 31.88 ± 3.26 | 32.96 ± 3.50 |
| Phytoplankton (μg/L) | 9.08 | 0.007 | 97.99 ± 26.98 (a) | 64.11 ± 19.64 (a,b) | 63.78 ± 19.92 (b) | 18.02 ± 5.25 (c) | 5.13 | 0.029 | 102.05 ± 13.52 (a) | 79.26 ± 12.24 (a,b) | 84.78 ± 4.97 (a) | 51.04 ± 6.50 (b) |
| Green (μg/L) | 7.12 | 0.013 | 88.09 ± 28.17 (a) | 50.17 ± 21.25 (b) | 47.94 ± 20.80 (b) | 10.59 ± 3.87 (b) | 1.18 | 0.322 | 46.66 ± 10.65 | 35.45 ± 5.58 | 42.40 ± 3.15 | 31.50 ± 4.17 |
| Cyanobacteria (μg/L) | 3.22 | 0.057 | 6.57 ± 1.63 | 10.73 ± 2.64 | 8.18 ± 2.09 | 3.42 ± 1.26 | 7.35 | 0.016 | 35.97 ± 5.12 (a) | 18.79 ± 3.68 (b,c) | 20.93 ± 2.65 (a,b) | 14.38 ± 1.53 (c) |
| Brown (μg/L) | 0.26 | 0.782 | 3.09 ± 1.35 | 2.75 ± 0.50 | 2.23 ± 0.48 | 3.08 ± 1.11 | 1.14 | 0.344 | 19.07 ± 8.66 | 20.17 ± 6.33 | 19.81 ± 5.94 | 9.32 ± 1.66 |
| Production Parameters | Northern System | Southern System |
|---|---|---|
| Harvest weight (g) (N = 30) | 140.34 ± 24.58 | 96.72 ± 18.15 |
| Harvest length (cm) (N = 30) | 24.72 ± 1.79 | 20.57 ± 1.63 |
| Growth (g/per capita) | 118.21 | 74.59 |
| Survival rate (%) | 93.1 | 94.62 |
| Total yield (kg) | 1629.3 | 1111.8 |
| Feed conversion ratio | 1.96 | 2.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, S.-L.; Dahms, H.-U.; Chiu, Y.-J.; Chang, S.-J.; Wang, Y.-K. Increased Production and Water Remediation by Land-Based Farm-Scale Sequentially Integrated Multi-Trophic Aquaculture Systems—An Example from Southern Taiwan. Sustainability 2017, 9, 2173. https://doi.org/10.3390/su9122173
Yeh S-L, Dahms H-U, Chiu Y-J, Chang S-J, Wang Y-K. Increased Production and Water Remediation by Land-Based Farm-Scale Sequentially Integrated Multi-Trophic Aquaculture Systems—An Example from Southern Taiwan. Sustainability. 2017; 9(12):2173. https://doi.org/10.3390/su9122173
Chicago/Turabian StyleYeh, Shinn-Lih, Hans-Uwe Dahms, Ying-Jer Chiu, Su-Jung Chang, and Yi-Kuang Wang. 2017. "Increased Production and Water Remediation by Land-Based Farm-Scale Sequentially Integrated Multi-Trophic Aquaculture Systems—An Example from Southern Taiwan" Sustainability 9, no. 12: 2173. https://doi.org/10.3390/su9122173





