Design and Development of Low P-Emission Substrate for the Protection of Urban Water Bodies Collecting Green Roof Runoff
Abstract
:1. Introduction
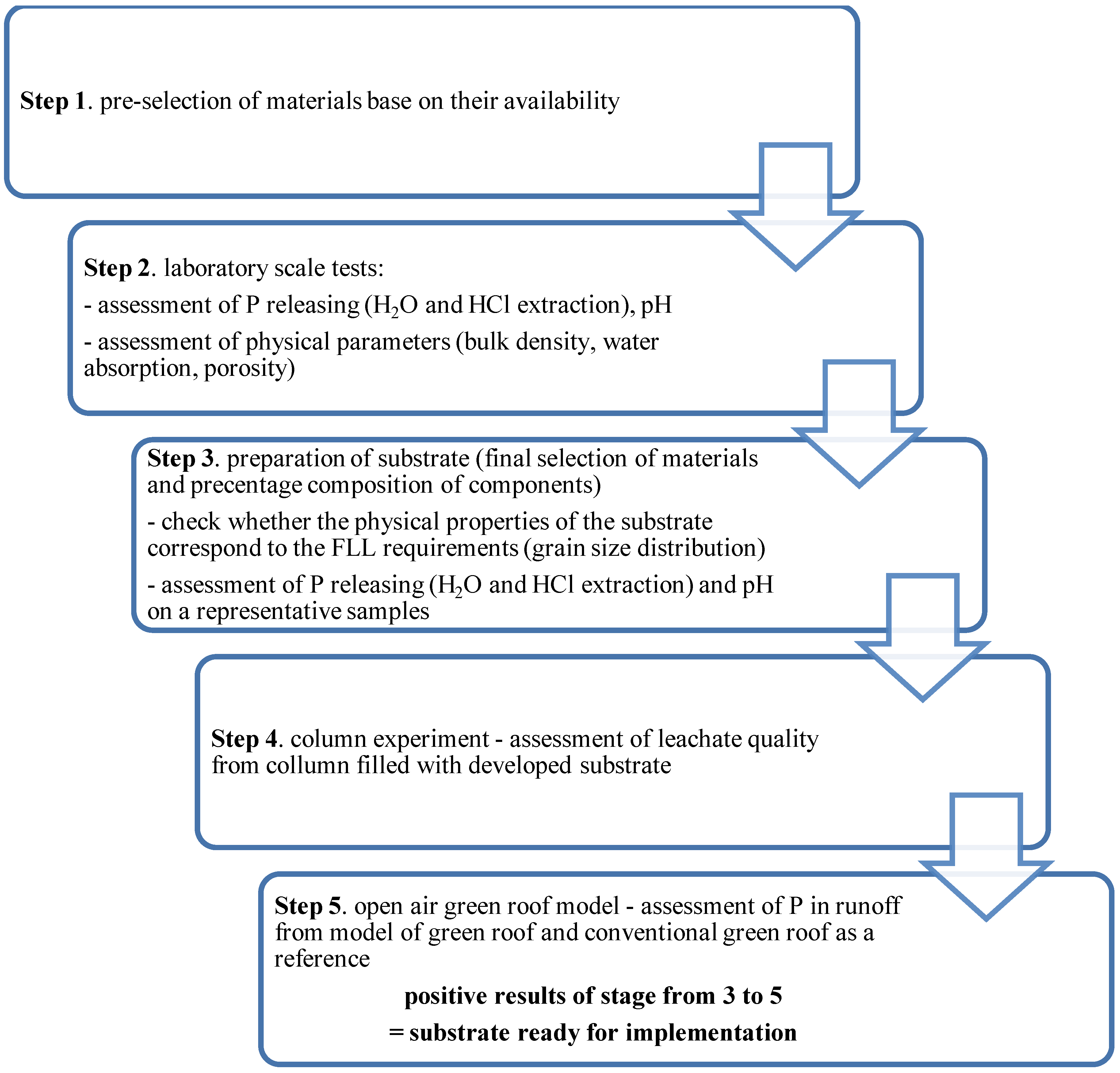
2. Materials and Methods
2.1. Materials
2.2. Laboratory Tests of Selected Materials
2.2.1. Physical Properties
2.2.2. Potential P Leaching
2.2.3. P Sorption Capacity of the Limestone
2.3. Column Experiment
2.4. Open Air Model Experiment
3. Results
3.1. Laboratory Tests of Selected Materials
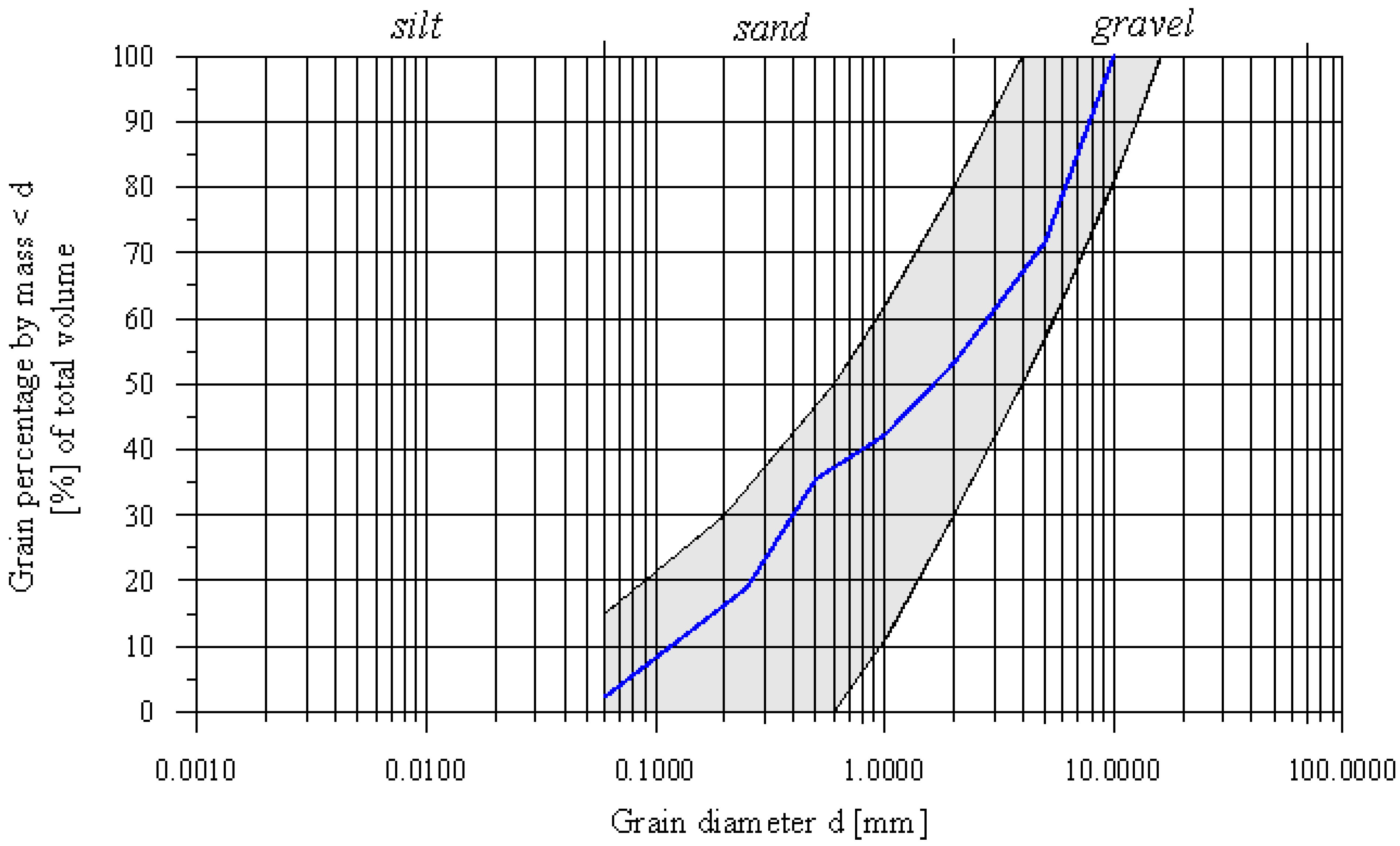
3.1.1. Physical Properties
3.1.2. Potential P Leaching
3.1.3. P Sorption Capacity of the Limestone
3.2. Preparation of the Substrate Mix
3.3. Column Experiment
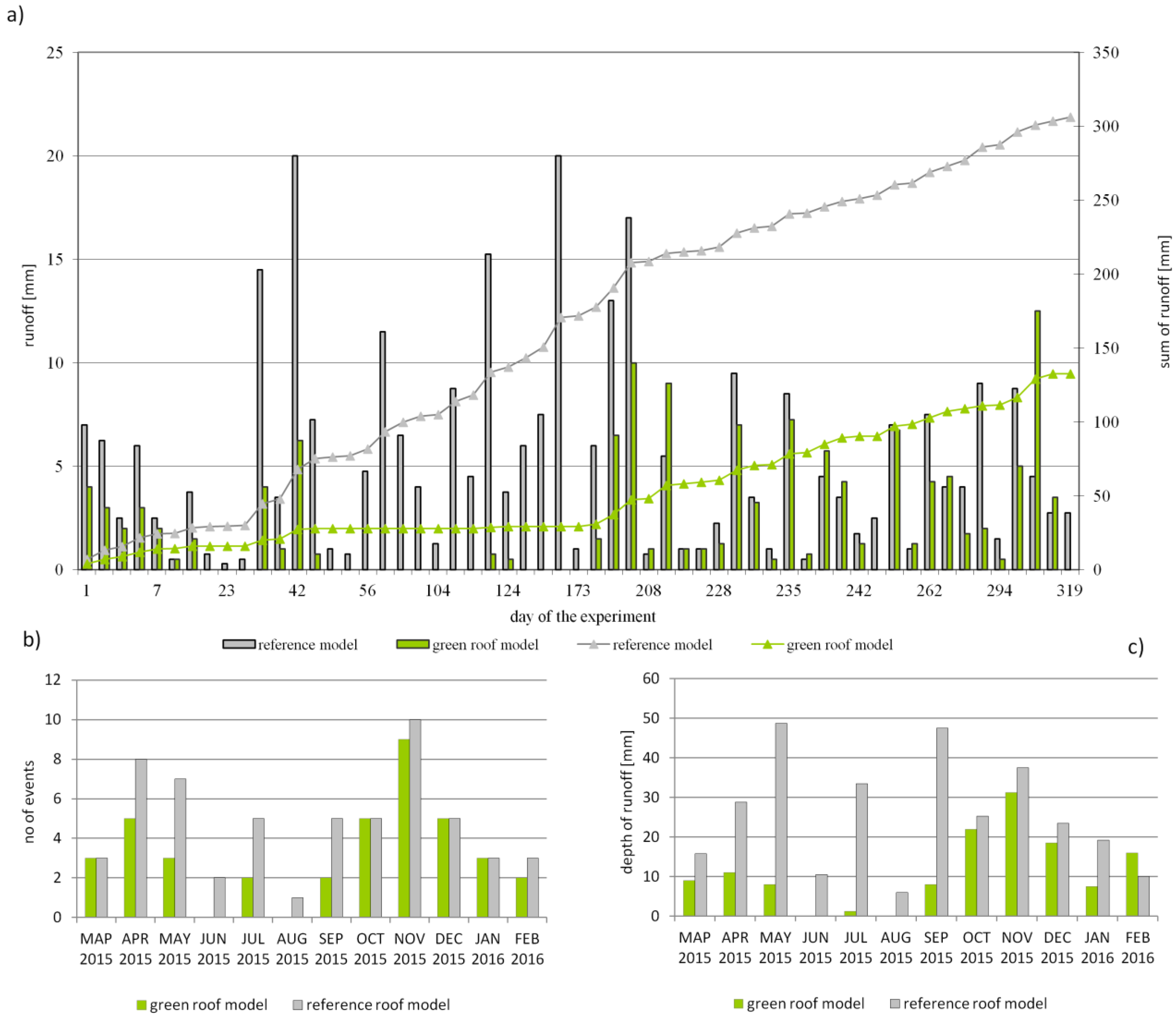
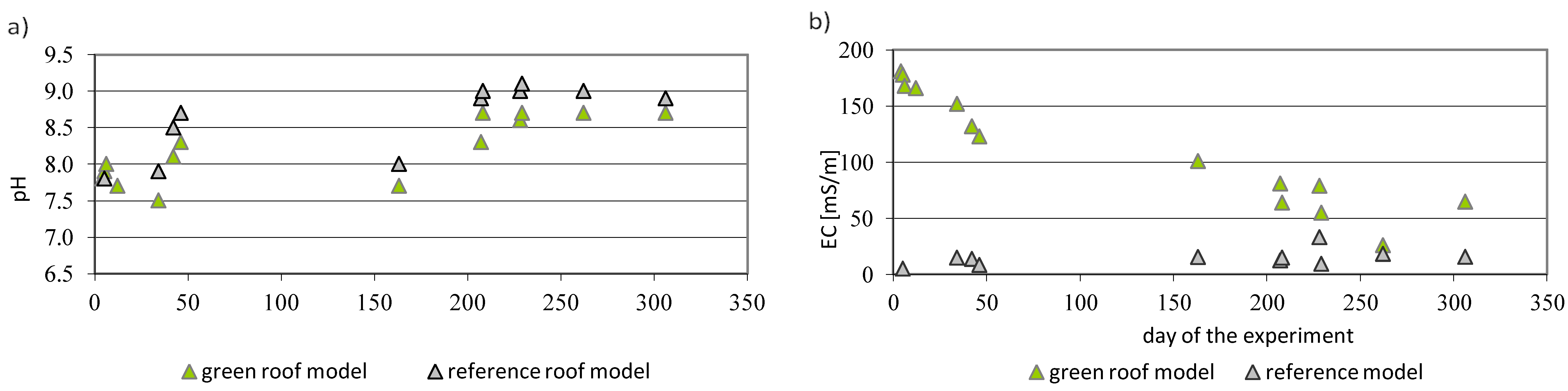
3.4. Open Air Model Experiment
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Song, K.; Xenopoulos, M.A.; Marsalek, J.; Frost, P.C. The fingerprints of urban nutrients: Dynamics of phosphorus speciation in water flowing through developed landscapes. Biogeochemistry 2015, 125, 1–10. [Google Scholar] [CrossRef]
- Paul, M.J.; Meyer, J.L. Streams in the urban landscape. Annu. Rev. Ecol. Syst. 2001, 32, 333–365. [Google Scholar] [CrossRef]
- Kaye, J.P.; Groffman, P.M.; Grimm, N.M.; Baker, L.A.; Pouyat, R.V. A distinct urban biogeochemistry? Trends Ecol. Evol. 2006, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Barałkiewicz, D.; Chudzińska, M.; Szpakowska, B.; Świerk, D.; Gołdyn, R.; Dondajewska, R. Storm water contamination and its effect on the quality of urban surface waters. Environ. Monit. Assess. 2014, 186, 6789–6803. [Google Scholar] [CrossRef] [PubMed]
- Jurik, L.; Húska, D.; Halászová, K.; Bandlerová, A. Small water reservoirs—Sources of water or problems? J. Ecol. Eng. 2015, 16, 22–28. [Google Scholar] [CrossRef]
- Matzinger, A.; Schmidt, M.; Riechel, M.; Hein, A.; Bräcker, J.; Strehl, C.; Nickel, D.; Libbe, J.; Sieker, H.; Pallasch, M. Quantifying the effects of urban stormwater management—Towards a novel approach for integrated planning. In Proceedings of the 13th International Conference on Urban Drainage, Sarawak, Malaysia, 7–12 September 2014. [Google Scholar]
- Aitkenhead-Peterson, J.; Dvorak, B.D.; Volder, A.; Stanley, N.C. Chemistry of growth medium and leachate from green roof systems in south-central Texas. Urban Ecosyst. 2011, 14, 17–33. [Google Scholar] [CrossRef]
- Aloisio, J.M.; Tuininga, A.R.; Lewis, J.D. Crop species selection effects on stormwater runoff and edible biomass in an agricultural green roof microcosm. Ecol. Eng. 2016, 88, 20–27. [Google Scholar] [CrossRef]
- Beck, D.A.; Johnson, G.R.; Spolek, G.A. Amending greenroof soil with biochar to affect runoff water quantity and quality. Environ. Pollut. 2011, 159, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Buffam, I.; Mitchell, M.E.; Durtsche, R.D. Environmental drivers of seasonal variation in green roof water quality. Ecol. Eng. 2016, 91, 506–514. [Google Scholar] [CrossRef]
- Czemiel Berndtsson, J.; Emilsson, T.; Bengtsson, L. The influence of extensive vegetated roofs on runoff water quality. Sci. Total Environ. 2006, 355, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, B.G.; Clausen, J.C. Effect of modular extensive green roof on stormwater runoff and water quality. Ecol. Eng. 2011, 37, 963–969. [Google Scholar] [CrossRef]
- Seidl, M.; Gromaire, M.-C.; Saad, M.; De Gouvello, B. Effect of substrate depth and rain-event history on the pollutant abadement of green roofs. Environ. Pollut. 2013, 183, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Teemusk, A.; Mander, Ü. The Influence of Green Roofs on Runoff Water Quality: A Case Study from Estonia. Water Resour. Manag. 2011, 25, 3699–3713. [Google Scholar] [CrossRef]
- Van Seters, T.; Rocha, L.; Smith, D.; MacMillan, G. Evaluation of green roofs for runoff retention, runoff quality, and leachability. Water Qual. Res. J. Can. 2009, 44, 33–47. [Google Scholar]
- Burszta-Adamiak, E. Zielone Dachy Jako Element Zrównoważonych Systemów Odwadniających na Terenach Zurbanizowanych Monografie CLXXV; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2014. [Google Scholar]
- Vijayaraghavan, K.; Joshi, U.M.; Balasubramanian, R. A field study to evaluate runoff quality from green roofs. Water Res. 2012, 46, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Babcook, R.W., Jr. Green roofs against pollution and climate change. A review. Agron. Sustain. Dev. 2014, 34, 695–705. [Google Scholar] [CrossRef]
- Harper, G.E.; Limmer, M.A.; Showalter, W.E.; Burken, J.G. Nine-month evaluation of runoff quality and quantity from an experimental green roof in Missouri, USA. Ecol. Eng. 2015, 78, 127–133. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Zhao, X. The influence of dual-substrate-layer extensive green roofs on rainwater runoff quantity and quality. Sci. Total Environ. 2017, 592, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Pęczkowski, G.; Orzepowski, W.; Pokładek, R.; Kowalczyk, T.; Żmuda, R.; Wójcik, R. Właściwości retencyjne zielonych dachów typu ekstensywnego na przykładzie badań modelowych. Acta Sci. Pol. Formatio Circumiectus 2016, 15, 113–120. [Google Scholar] [CrossRef]
- Buffam, I.; Mitchell, M.E. Nutrient cycling in green roof ecosystems. Chapter 5. In Green Roofs Ecosystems; Springer: Berlin, Germany, 2015; Chapter 5; pp. 107–137. [Google Scholar]
- Zulhabri, I.; Haziq, A.A.; Nasyairi, M.N.; Mohd, Z.; Mohd, T. Comparative study on green roof mechanism in developed countries. In Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA), Bandung, Indonesia, 23–26 September 2012. [Google Scholar]
- Sam, C.; Hui, M. Development of technical guidelines for green roof systems in Hong Kong. In Proceedings of the Joint Symposium 2010 on Low Carbon High Performance Buildings, Hong Kong, China, 23 November 2010. [Google Scholar]
- Stowarzyszenie Wykonawców Dachów Płaskich i Fasad (DAFA). Dachy Zielone. Wytyczne do Projektowania, Wykonywania i Pielęgnacji Dachów Zielonych—Wytyczne Dla Dachów Zielonych; DAFA: Opole, Poland, 2015. [Google Scholar]
- Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau (FLL). Guidelines for the Planning, Construction and Maintenance of Green Roofing—Green Roofing Guideline; FLL: Bonn, Germany, 2008. [Google Scholar]
- Getter, K.L.; Rowe, D.B. The role of extensive green roofs in sustainable development. Hort. Sci. 2006, 41, 1276–1285. [Google Scholar]
- Emilsson, T. Vegetation development on extensive vegetated green roofs: Influence of substrate composition, establishment method and species mix. Ecol. Eng. 2008, 33, 265–277. [Google Scholar] [CrossRef]
- Stovin, V.; Poë, S.; De-Ville, S.; Berretta, C. The influence of substrate and vegetation configuration on green roof hydrological performance. Ecol. Eng. 2015, 85, 159–172. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.; Zhao, Z.; Li, Y.; Yu, S. Heavy metals in plants and substrate from simulated extensive green roofs. Ecol. Eng. 2013, 55, 29–34. [Google Scholar] [CrossRef]
- Buccola, N.; Spolek, G. A pilot-scale evaluation of green roof runoff retention, detention, and quality. Water Air Soil Pollut. 2011, 216, 83–92. [Google Scholar] [CrossRef]
- Chen, C.F. Performance evaluation and development strategies for green roofs in Taiwan: A review. Ecol. Eng. 2013, 52, 51–58. [Google Scholar] [CrossRef]
- Farrell, C.; Mitchell, R.E.; Szota, C.; Rayner, J.P.; Williams, N.S.G. Green roofs for hot and dry climates: Interacting effect of plant water use, succulence and substrate. Ecol. Eng. 2012, 40, 270–276. [Google Scholar] [CrossRef]
- Hathaway, A.M.; Hunt, W.F.; Jennings, G.D. A field study of green roof hydrologic and water quality performance. Trans. ASABE 2008, 51, 37–44. [Google Scholar] [CrossRef]
- Gromaire, M.C.; Ramier, D.; Seidl, M.; Berthier, E.; Saad, M.; de Gouvello, B. Impact of extensive green roofs on the quantity and the quality of runoff—First results of a test bench in the Paris region. NOVATECH 2013, 1–10. Available online: http://hdl.handle.net/2042/51380 (accessed on 30 September 2017).
- Retzlaff, W.; Ebbs, S.; Aslup, S.; Morgan, S.; Woods, E.; Jost, V.; Luckett, K. What is that running off my green roof? In Proceedings of the Sixth Annual Greening Rooftops for Sustainable Communities Conference, Awards and Trade Show, Baltimore, MD, USA, 30 April–2 May 2008. [Google Scholar]
- Molineux, C.J.; Fentiman, C.H.; Gange, A.C. Characterising alternative recycled waste materials for use as green roof growing media in the U.K. Ecol. Eng. 2009, 35, 1507–1513. [Google Scholar] [CrossRef]
- Nagase, A.; Dunnett, N. Amount of water runoff from different vegetation types on extensive green roofs: Effects of plant species, diversity and plant structure. Landsc. Urban Plan. 2012, 104, 356–363. [Google Scholar] [CrossRef]
- Razzaghmanesh, M.; Beecham, S.; Brien, C.J. Developing resilient green roofs in a dry climate. Sci. Total Environ. 2014, 490, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Rumble, H.; Gange, A.C. Soil microarthropod community dynamics in extensive green roofs. Ecol. Eng. 2013, 57, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Aslup, S.E.; Ebbs, S.D.; Battaglia, L.L.; Retzlaff, W.A. Heavy metals in leachate from simulated green roof systems. Ecol. Eng. 2011, 37, 1709–1717. [Google Scholar]
- Solano, L.; Ristvey, A.G.; Lea-Cox, J.D.; Cohan, S.M. Sequestring zinc from recycled crumb rubber in extensive green roof media. Ecol. Eng. 2012, 47, 284–290. [Google Scholar] [CrossRef]
- Gnecco, I.; Palla, A.; Lanza, L.G.; La Barbera, P. The role of green roofs as a source/sink of pollutants in storm water outflows. Water Resour. Manag. 2013, 27, 4715–4730. [Google Scholar] [CrossRef]
- Savi, T.; Andri, S.; Nardini, A. Impact of different green roof layering on plant water status and drought survival. Ecol. Eng. 2013, 57, 188–196. [Google Scholar] [CrossRef]
- Bianchini, F.; Hewage, K. How “green” are green roofs? Lifecycle analysis of green roof materials. Build. Environ. 2012, 48, 57–65. [Google Scholar] [CrossRef]
- Zurauskiene, R.; Valentukeviciene, M.; Boussouga, Y.A. Filter medias from recycled concrete, properties investigated for sorption processes. In Proceedings of the Environmental Engineering 10th International Conference, Vilnius Gediminas Technical University (VGTU), Vilnius, Lithuania, 27–28 April 2017; p. 6. [Google Scholar]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A.; Baryła, A. Wybór materiału reaktywnego do usuwania fosforu z wód i ścieków na przykładzie kruszywa popiołoporytowego Pollytag®. Inżynieria Ekologiczna 2014, 39, 33–41. [Google Scholar]
- PN EN 933–1:2012 equivalent to EN 933–1:2012 Tests for geometrical properties of aggregates. Determination of particle size distribution. Sieving method.
- International Organization for Standardization (ISO). PN-ISO 11277:2005 Equivalent to ISO 11277:2009 Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- PN EN 1097–3:2000 equivalent to EN 1097–3:1998 Tests for mechanical and physical properties of aggregates. Determination of loose bulk density and voids.
- PN-EN 1936:2010 equivalent to EN 1936:2006 Natural stone test methods. Determination of real density and apparent density, and of total and open porosity.
- International Organization for Standardization (ISO). PN-ISO 10390:1997 Equivalent to ISO 10390:1994 Soil Quality—Determination of pH; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Upmeier, M. Ermittlung einer geeigneten Methode zur Bestimmung der Gehalte von Phosphor in Substraten für Schwimmteiche unter besonderer Berücksichtigung der biologischen Verfügbarkeit (Pflanzen, Algen). Report of research funding by FLL and DGfnB 2011/2012. 2014. Available online: http://www.fll.de/leistungsprofil/forschung/phosphor-in-substraten-fuer-schwimmteiche.html (accessed on 3 September 2017).
- Al Duri, B. Adsorption modeling and mass transfer. Chapter 7. In Use of Adsorbents for the Removal of Pollutants from Wastewaters; McKay, G., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 133–173. [Google Scholar]
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://www.eea.europa.eu/policy-documents/council-directive-98-83-ec (accessed on 30 September 2017).
- Valentukevičienė, M.; ˇurauskienė, R.; Satkūnas, J. The main microelements and phosphorus content of sediments formed in a drinking water supply system. Estonian J. Earth Sci. 2016, 65, 248–257. [Google Scholar] [CrossRef]
- Available online: www.xeroflor.pl (accessed on 3 September 2017).
- Karczmarczyk, A.; Bus, A. Testing of reactive materials for phosphorus removal from water and wastewater. Ann. Wars. Univ. Life Sci. 2014, 46, 57–67. [Google Scholar]
- Karczmarczyk, A.; Baryła, A.; Bus, A. Effect of P-reactive drainage aggregates on green roof runoff quality. Water 2014, 6, 2575–2589. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A.; Baryła, A. The use of reactive material for limiting P-leaching from green roof substrate. Water Sci. Tech. 2016, 73, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Cucarella, V.; Renman, G. Phosphorus sorption capacity of filter materials used for on-site wastewater treatment determined in batch experiments—A comparative study. J. Environ. Qual. 2009, 38, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Monitoring Chemizmu Opadów Atmosferycznych i Ocena Depozycji Zanieczyszczeń do Podłoża. Available online: www.gios.gov.pl/chemizm2010/index.html (accessed on 3 September 2017).
- Saljnikov, E.; Cakmak, D. Phosphorus: Chemism and Interactions, Principles, Application and Assessment in Soil Science. InTech 2011. [Google Scholar] [CrossRef]
- Graceson, A.; Hare, M.; Monaghan, J.; Hall, N. The water retention capabilities of growing media for green roofs. Ecol. Eng. 2013, 61, 328–334. [Google Scholar] [CrossRef]
- Villarreal, E.L.; Dixon, A. Analysis of rainwater collection system for domestic water supply in Ringansen, Norrköping, Sweden. Build. Environ. 2005, 40, 1174–1184. [Google Scholar] [CrossRef]
- Sazakli, E.; Alexopoulos, A.; Leotsinidis, M. Rainwater harvesting, quality assessment and utilization in Kefalonia Island, Greece. Water Res. 2007, 41, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Schriewer, A.; Horn, H.; Helmreich, B. Time focused measurements of roof runoff quality. Corros. Sci. 2008, 50, 384–391. [Google Scholar] [CrossRef]
- Vialle, C.; Sablayrolles, C.; Lovera, M.; Jacob, S.; Huau, M.C.; Montrejaud-Vignoles, M. Monitoring of water quality from roof runoff: Interpretation using multivariate analysis. Water Res. 2011, 45, 3765–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayaraghavan, K.; Joshi, U.M. Application of seaweed as substrate additive in green roofs: Enhancement of water retention and sorption capacity. Lansc. Urban Plan. 2015, 143, 25–32. [Google Scholar] [CrossRef]
- Dunnett, N.; Nagase, A.; Booth, R.; Grime, P. Influence of vegetation composition on runoff in two simulated green roof experiments. Urban Ecosyst. 2008, 11, 385–398. [Google Scholar] [CrossRef]




| Material | Origin | Availability | Weight [kg/m2] | Cost [Euro/Mg] * [Euro/m3] ** | Main Advantage |
|---|---|---|---|---|---|
| crushed red brick | recycled waste | common | 92 | 5.4–5.8 * | price/availability |
| gravel | natural | common | 150 | 8.4–19.1 * | price/availability |
| sand | natural | common | 150 | 4.0–8.4 * | price/availability |
| crushed limestone | natural | common | 115 | 9.8–25.6 * | P-sorption capacity/availability |
| volcanic rock | natural/recycled waste | limited | 75 | 232.6–418.6 ** | free in this study |
| expanded clay | product | common | 60 | 48.8–81.4 * | weight/availability |
| pollytag | product/recycled waste | common | 670 | 46.5 * | weight/availability |
| Material | Grain Size [mm] | Porosity [%] | Bulk Density [g/cm3] | Bulk Density at max WHC [g/cm3] |
|---|---|---|---|---|
| crushed red brick | 1–10 | 60 | 0.95 | 1.30 |
| gravel | 4–25 | 39 | 1.50 | 1.57 |
| sand | 0.02–2 | 32 | 1.50 | 1.71 |
| crushed limestone | 5–10 | 55 | 1.20 | 1.27 |
| volcanic rock | 4–16 | 53 | 0.75 | 9.25 |
| expanded clay | 6–18 | 50 | 0.67 | 0.69 |
| pollytag | 6–14 | 54 | 0.77 | 0.81 |
| Material | P-PO4 in H2O Extract [mg/kg] | P-PO4 in HCl Extract [mg/kg] | pH |
|---|---|---|---|
| crushed red brick | 8.8 ± 0.5 | 65.0 ± 1.0 | 9.27 |
| gravel | n.d. | 45.2 ± 11.3 | 7.44 |
| sand | n.d. | 18.8 ± 6.0 | 7.84 |
| crushed limestone | n.d. | n.d. | 9.68 |
| volcanic rock | n.d. | 5.3 ± 2.8 | 6.08 |
| expanded clay | n.d. | 38.4 ± 1.8 | 8.10 |
| pollytag | 2.9 ± 0.5 | 130.0 ± 1.2 | 8.59 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczmarczyk, A.; Baryła, A.; Kożuchowski, P. Design and Development of Low P-Emission Substrate for the Protection of Urban Water Bodies Collecting Green Roof Runoff. Sustainability 2017, 9, 1795. https://doi.org/10.3390/su9101795
Karczmarczyk A, Baryła A, Kożuchowski P. Design and Development of Low P-Emission Substrate for the Protection of Urban Water Bodies Collecting Green Roof Runoff. Sustainability. 2017; 9(10):1795. https://doi.org/10.3390/su9101795
Chicago/Turabian StyleKarczmarczyk, Agnieszka, Anna Baryła, and Paweł Kożuchowski. 2017. "Design and Development of Low P-Emission Substrate for the Protection of Urban Water Bodies Collecting Green Roof Runoff" Sustainability 9, no. 10: 1795. https://doi.org/10.3390/su9101795





