A Comparative Life Cycle Assessment between a Metered Dose Inhaler and Electric Nebulizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goal and Scope Definition
2.2. Inventory Analysis: Metered Dose Inhaler
2.2.1. Active Substance
2.2.2. Propellant
2.2.3. Surfactant
2.2.4. Metering Valve
2.2.5. Actuator
2.2.6. Canister
2.2.7. Use Phase
2.3. Inventory Analysis: Electric Nebulizer
2.3.1. Active Substance: Albuterol Inhalation Solution
2.3.2. Exterior Shell
2.3.3. Stator: Core, Coils and Cover
2.3.4. Rotor: Squirrel Cage and Ball Bearings & Central Drive Shaft
2.3.5. Suction Chamber
2.3.6. Fan: Fan, Fan Housing, and Cross Brace
2.3.7. Medicine Delivery: Mouthpiece, Medical Tubing, and Medicine Chamber
2.3.8. Power Cord
2.3.9. Use Phase
2.4. Impact Assessment
2.5. Statistics
3. Results and Discussion
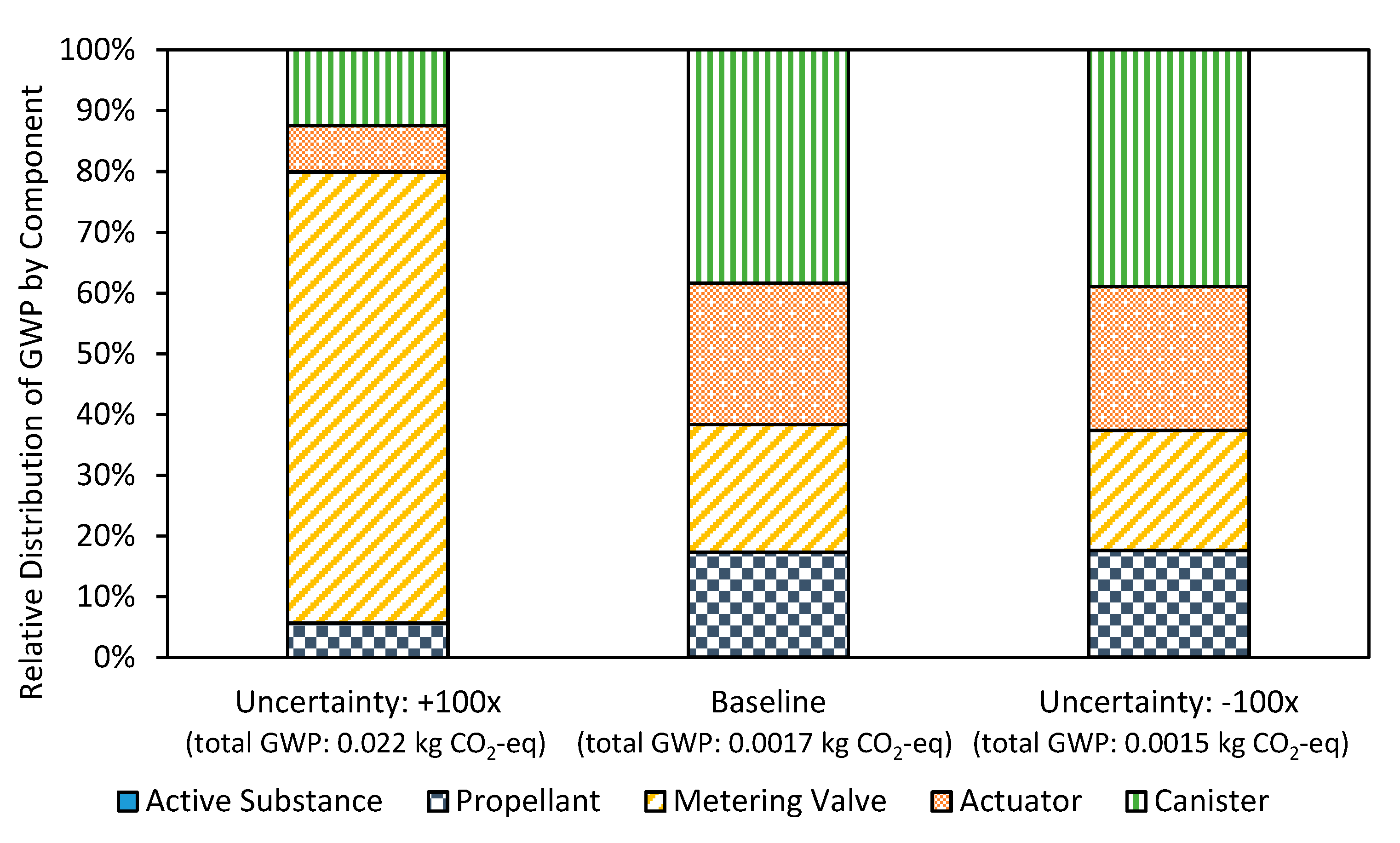
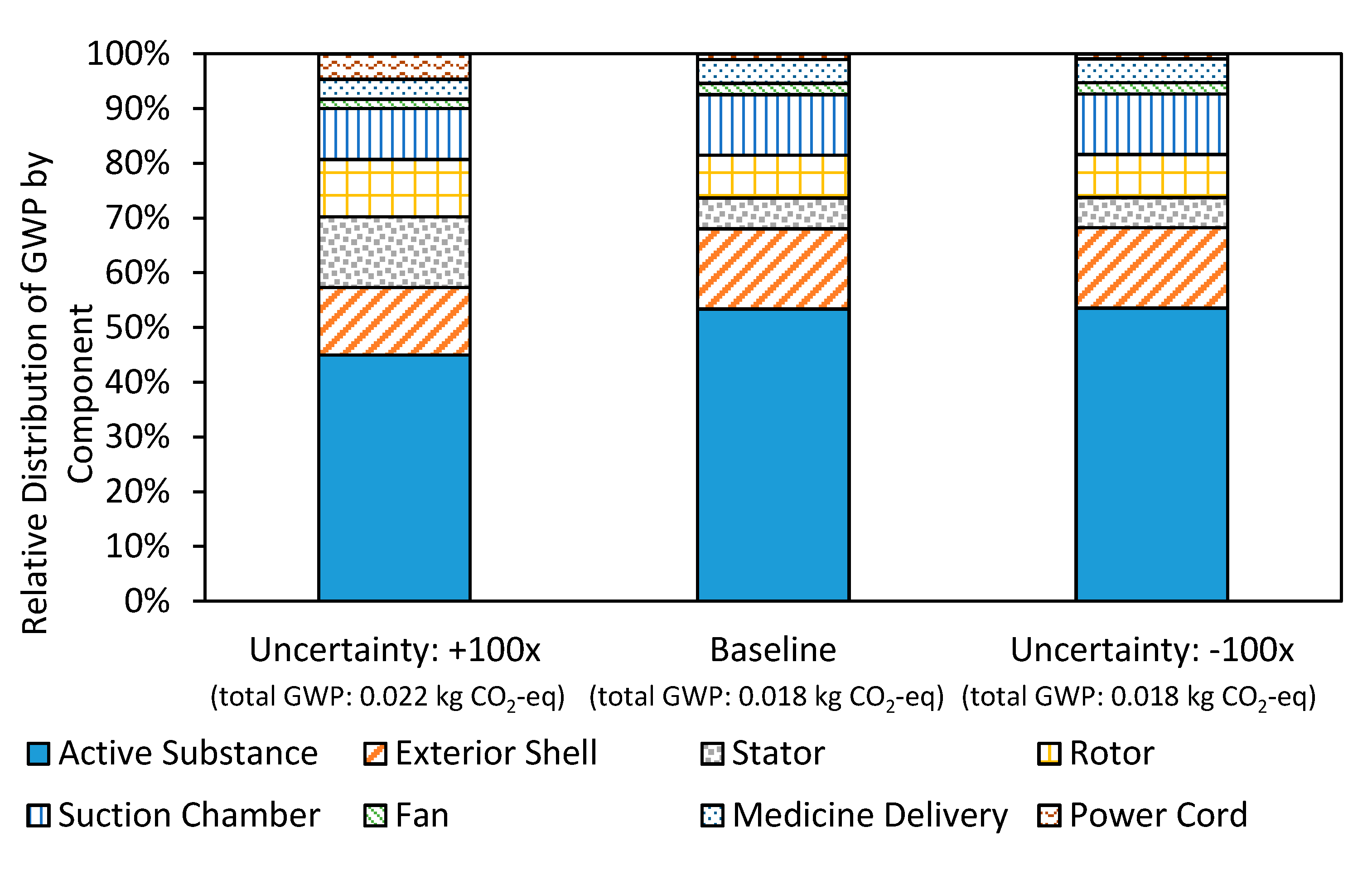
3.1. Uncertainty Analysis
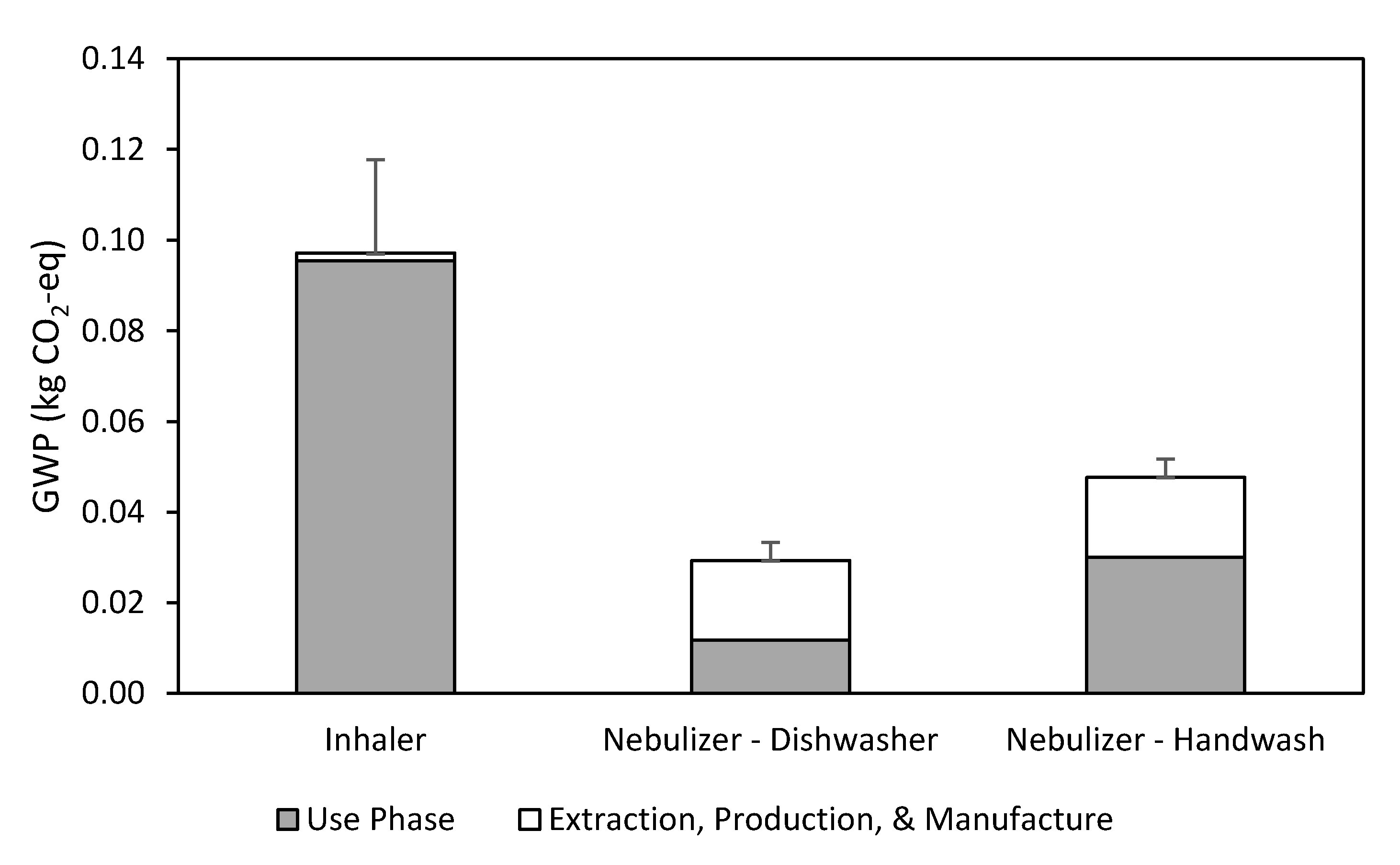
3.2. Inhaler
3.3. Nebulizer
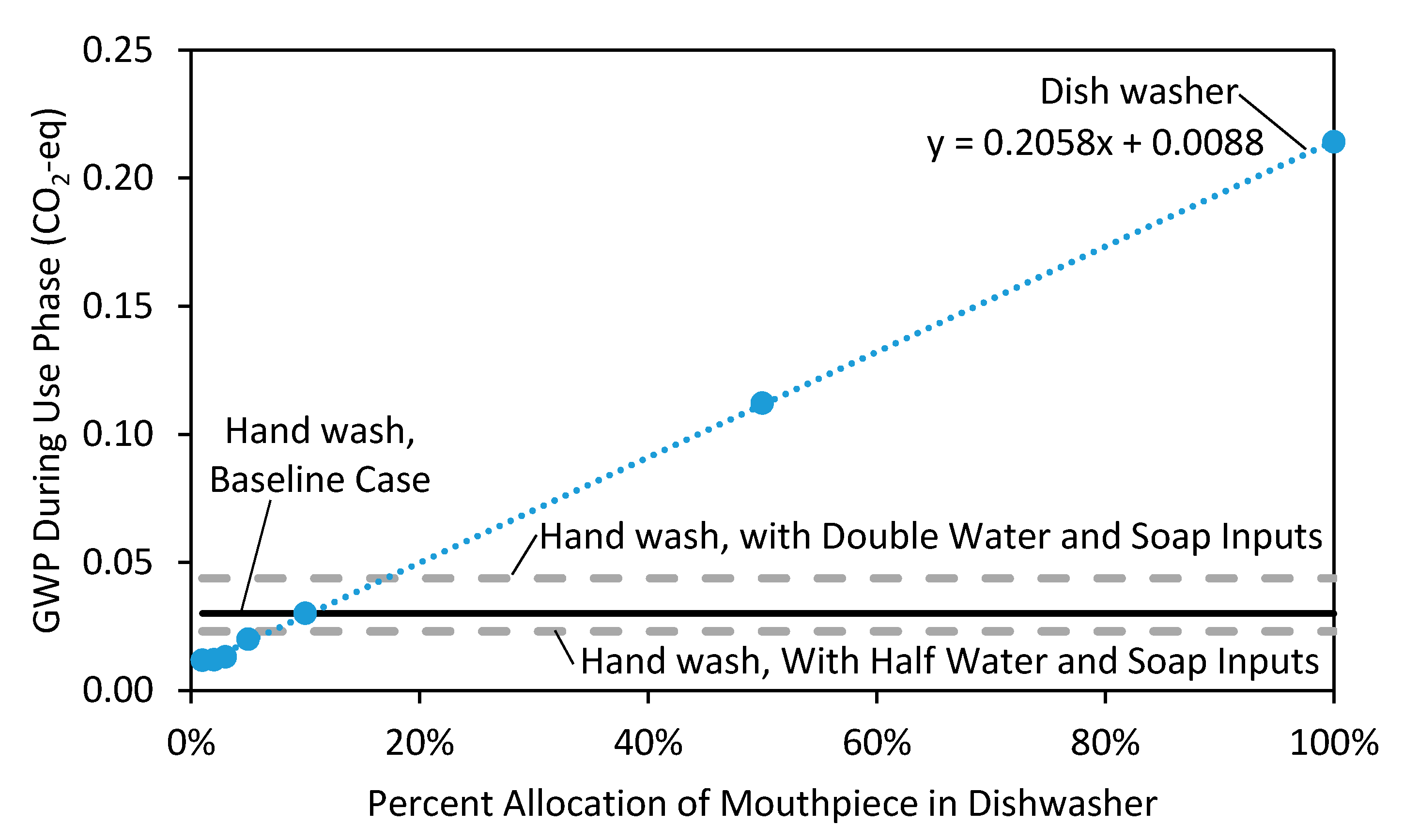
3.4. Breakeven Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. WHO Calls for Urgent Action to Protect Health from Climate Change. 2016. Available online: http://www.who.int/globalchange/global-campaign/cop21/en/ (accessed on 9 June 2017).
- Chung, J.W.; Meltzer, D.O. Estimate of the carbon footprint of the US health care sector. J. Am. Med. Assoc. 2009, 302, 1970–1972. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.; Le, C.; Eckelman, M. Life cycle greenhouse gas emissions of anesthetic drugs. Anesth. Analg. 2012, 114, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Hertwich, E.G.; Peters, G.P. Carbon footprint of nations: A global, trade-linked analysis. Environ. Sci. Technol. 2009, 43, 6414–6420. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.L.; Eckelman, M.; Guido, R.; Huddleston, M.; Landis, A.E.; Sherman, J.; Shrake, S.O.; Copley-woods, N.; Bilec, M.M. Environmental impacts of surgical procedures: Life cycle assessment of hysterectomy in the United States. Environ. Sci. Technol. 2015, 49, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, M.; Mosher, M.; Sherman, J. Comparative life cycle assessment of disposable and reusable laryngeal mask airways. Anesth. Analg. 2012, 114, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- ISO. Environmental Mangement—Life Cycle Assessment—Principles and Framework, 2nd ed.; International Organization for Standardization: Genève, The Switzerland, 2006. [Google Scholar]
- Aït-Khaled, N.; Enarson, D.; Bousquet, J. Chronic respiratory diseases in developing countries: The burden and strategies for prevention and management. Bull. World Health Organ. 2001, 79, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Burney, P.; Jarvis, D.; Perez-Padilla, R. The global burden of chronic respiratory disease in adults. Int. J. Tuberc. Lung Dis. 2015, 19, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.G.; Nagar, S.P.; Dalal, A.A. Indirect costs in chronic obstructive pulmonary disease: A review of the economic burden on employers and individuals in the United States. Int. J. Chron. Obstr. Pulm. Dis. 2014, 9, 289–300. [Google Scholar] [CrossRef] [PubMed]
- DeVilbiss Healthcare. Pulmo-Aide(R) Compressor Nebulizer System. Available online: https://www.bpimedicalsupply.com/mm5/pdf/devilbiss-pulmoaide-compressor.pdf (accessed on 5 December 2016).
- Purewal, T.S.; Grant, D.J.W. Metered Dose Inhaler Technology, 1st ed.; Interpharm/CRC: Boca Raton, FL, USA, 1998. [Google Scholar]
- Corr, S.; Noakes, T.J. Composition Comprising Salbutamol Sulphate. Patent WO2,013,054,135, 18 April 2013. [Google Scholar]
- Mok, Y.S.; Lee, S.; Chang, M.S. Destruction of chlorodifluoromethane (CHF2Cl) by using dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2009, 37, 449–455. [Google Scholar]
- Barker, P.; Cary, R.; Dobson, S. Concise International Chemical Assessment Document 11: 1,1,1,2-Tetrafluoroethane; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Sivigny, M.B.; Hodson, P.D. Manufacture of Metered Dose Valve Components 2010. U.S. Patent US20100300437 A1, 2 December 2010. [Google Scholar]
- Low, D.; Li, L.; Bradley, L. Manufacture of Valve Stems 2005. U.S. Patent US20050248060 A1, 10 November 2005. [Google Scholar]
- Morrow, N.L. The industrial production and use of 1,3-butadiene. Environ. Health Perspect. 1990, 86, 7–8. [Google Scholar] [CrossRef] [PubMed]
- EPA. Locating and Estimating Air Emissions from Sources of Acrylonitrile; U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards: Washington, DC, USA, 1984.
- Grimm, D.C. Method for the Production of Nitrile Rubber 1998. U.S. Patent US5708132 A, 13 January 1998. [Google Scholar]
- Stein, S.W.; Sheth, P.; Hodson, P.D.; Myrdal, P.B. Advances in metered dose inhaler technology: Hardware development. AAPS PharmSciTech 2014, 15, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Finley, W.R.; Hodowanec, M.M. Selection of copper versus aluminum rotors for induction motors. IEEE Trans. Ind. Appl. 2001, 37, 1563–1573. [Google Scholar] [CrossRef]
- Mechler, G.C. Manufacturing and Cost Analysis for Aluminum and Copper die Cast Induction Motors for GM’s Powertrain and R&D Divisions; Massachusetts Institute of Technology: Cambridge, MA, USA, 2010. [Google Scholar]
| Major Components of the MDI | Mass (g) | Composition | GaBi Model Manufacturing Process |
|---|---|---|---|
| Active substance | 0.02 | Albuterol Sulfate | N/A 1 |
| Propellant | 6.68 | HFA-134a | N/A 1 |
| Surfactant | 0.002 | Oleic acid and ethanol | N/A 2 |
| Metering Valve | |||
| Ferrule | 1.10 | Aluminum | Stamping and Bending |
| Ferrule Gasket | 0.13 | High Density Polyethylene (HDPE) | Plastic Injection Molding |
| Valve Stem | 0.30 | Stainless Steel | Deep Drawing |
| Compression Spring | 0.09 | Steel Wire | Auto Coiler |
| Tank | 0.26 | Stainless Steel | Stamping and Bending |
| Bottle Emptier | 0.39 | Stainless Steel | Stamping and Bending |
| Diaphragm | 0.07 | Nitrile Rubber | Plastic Injection Molding |
| Tank Seal | <0.01 | Nitrile Rubber | N/A 2 |
| Actuator | |||
| Actuator | 8.62 | Polypropylene | Plastic Injection Molding |
| Actuator Cap | 1.20 | Polypropylene | Plastic Injection Molding |
| Canister | 4.56 | Aluminum | Deep Drawing |
| Major Components of the EN | Mass (g) | Composition | GaBi Model Manufacturing Process |
|---|---|---|---|
| Active Substance | 0.003 2 | Albuterol Sulfate | N/A 1 |
| Exterior Shell | 1102 | Acrylonitrile Butadiene Styrene (ABS) | Plastic Injection Molding |
| Stator | |||
| Core | 539 | Steel | Casting |
| Coils | 135 | Copper | Wire Drawing |
| Cover | 182 | Steel | Stamping and Bending |
| Rotor | |||
| Squirrel Cage | 288 | Aluminum | Casting |
| Ball Bearings & | 124 | Stainless Steel | Casting |
| Central Shaft | |||
| Suction Chamber | 510 | Aluminum | Casting |
| Fan | |||
| Fan | 16 | Polyvinyl Chloride (PVC) | Plastic Injection Molding |
| Fan Housing | 148 | PVC | Plastic Injection Molding |
| Cross Brace | 37 | Steel | Stamping and Bending |
| Medicine Delivery | |||
| Mouthpiece | 19 | Polypropylene | Plastic Injection Molding |
| Medical Tubing | 41 | PVC | Extrusion |
| Medicine Chamber | 12 | Polymethylmethacrylate (PMMA) | Plastic Injection Molding |
| Power Cord | 112 | 70% Copper / 30% PVC | Wire Drawing/Extrusion |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulet, B.; Olson, L.; Mayer, B.K. A Comparative Life Cycle Assessment between a Metered Dose Inhaler and Electric Nebulizer. Sustainability 2017, 9, 1725. https://doi.org/10.3390/su9101725
Goulet B, Olson L, Mayer BK. A Comparative Life Cycle Assessment between a Metered Dose Inhaler and Electric Nebulizer. Sustainability. 2017; 9(10):1725. https://doi.org/10.3390/su9101725
Chicago/Turabian StyleGoulet, Brandon, Lars Olson, and Brooke K. Mayer. 2017. "A Comparative Life Cycle Assessment between a Metered Dose Inhaler and Electric Nebulizer" Sustainability 9, no. 10: 1725. https://doi.org/10.3390/su9101725
APA StyleGoulet, B., Olson, L., & Mayer, B. K. (2017). A Comparative Life Cycle Assessment between a Metered Dose Inhaler and Electric Nebulizer. Sustainability, 9(10), 1725. https://doi.org/10.3390/su9101725






