Hydraulic Properties of Porous Media Saturated with Nanoparticle-Stabilized Air-Water Foam
Abstract
:1. Introduction
2. Background—Literature Review
3. Experimental Details
3.1. Nanoparticles and Surfactant
3.2. Foam Generation and Stability
3.3. Viscosity Measurement and Calculation
3.4. Breakthrough Pressure Measurement—Microfluidic Chip
3.5. Breakthrough Pressure and Hydraulic Conductivity Experiment—Sand Column
4. Results and Analyses
4.1. Foam Stability
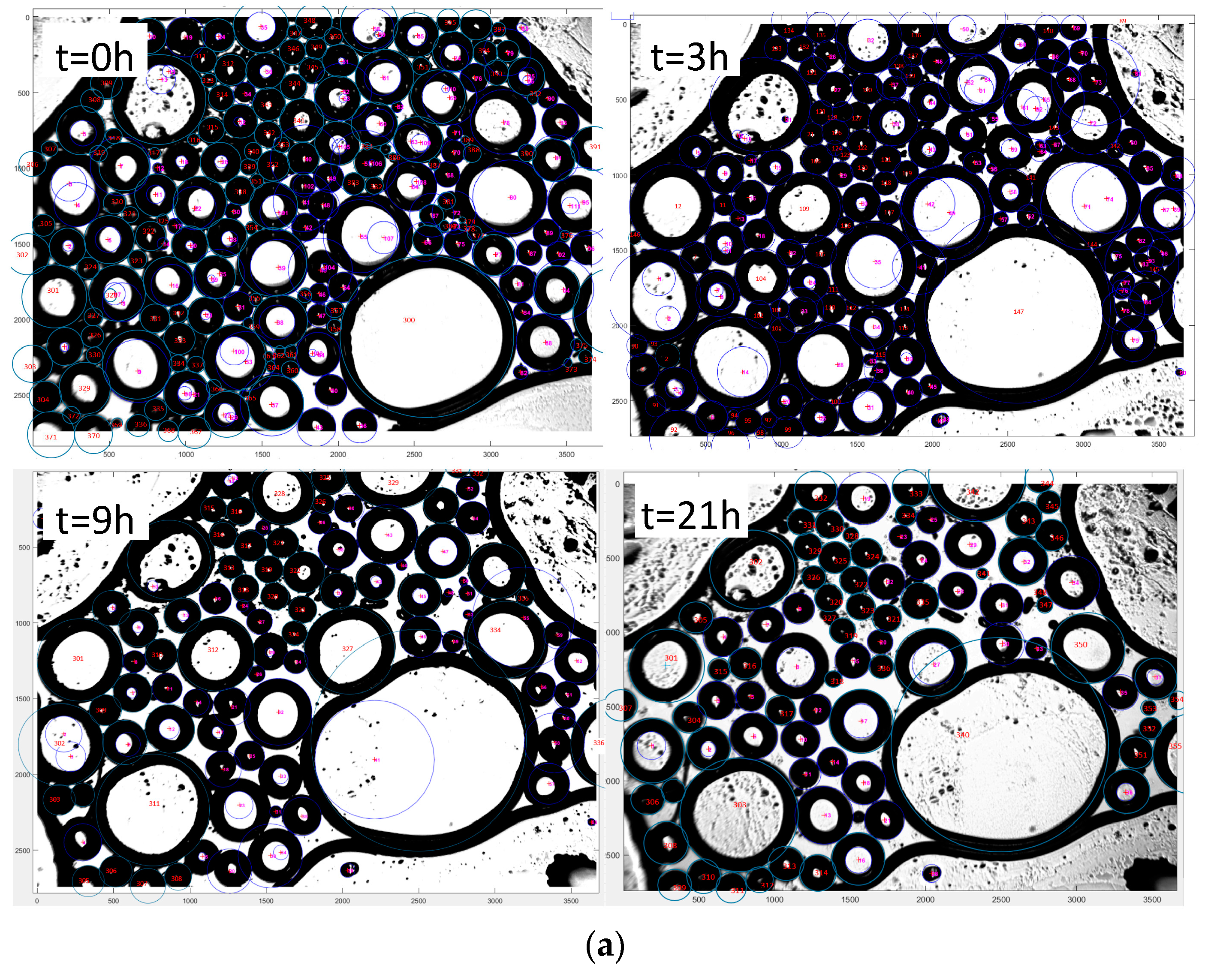
4.2. Bubble Size Distribution
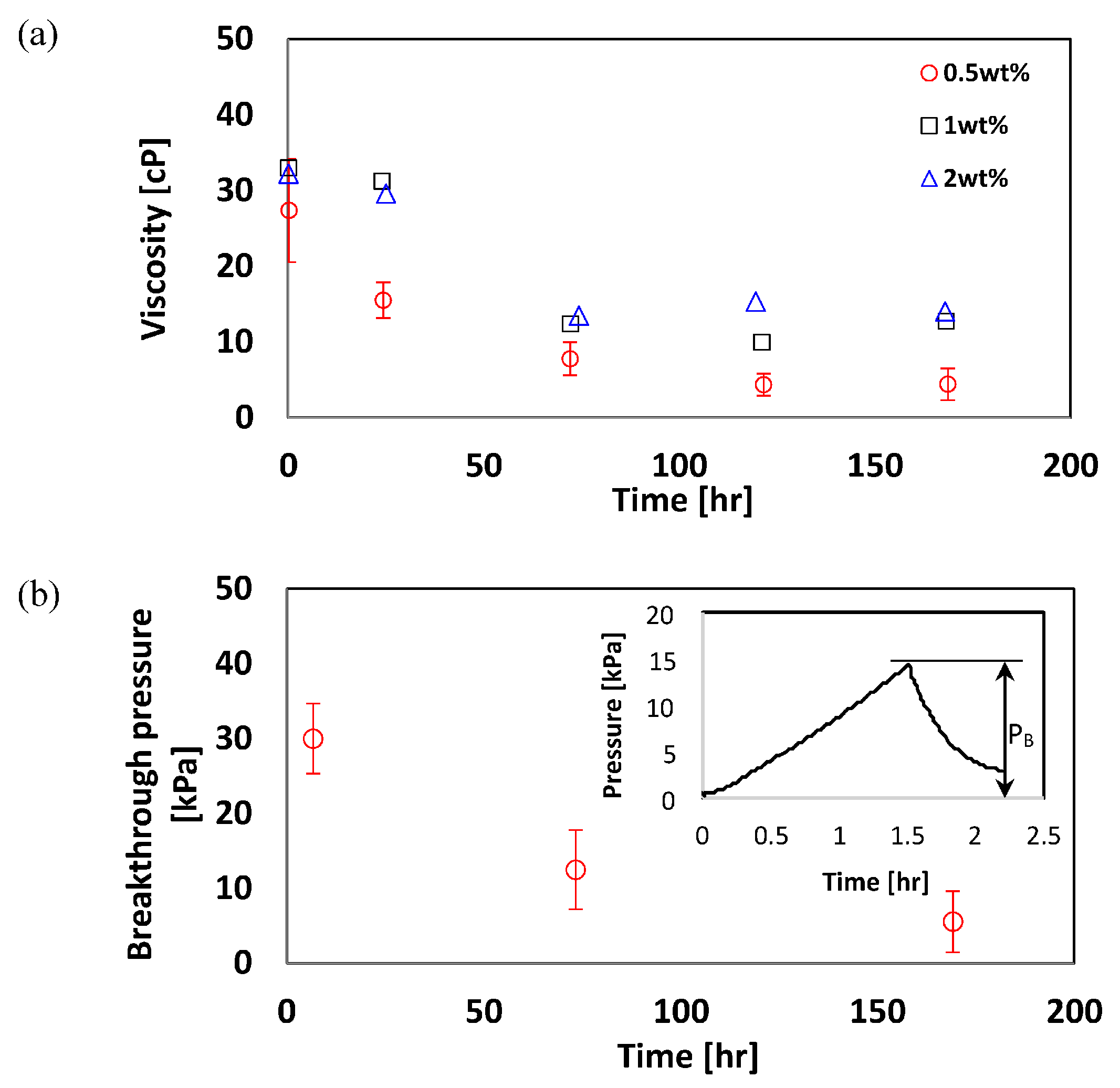
4.3. Effects of Time and Nanoparticle Concentration on Foam Viscosity
4.4. Breakthrough Pressure in a Microfluidic Chip
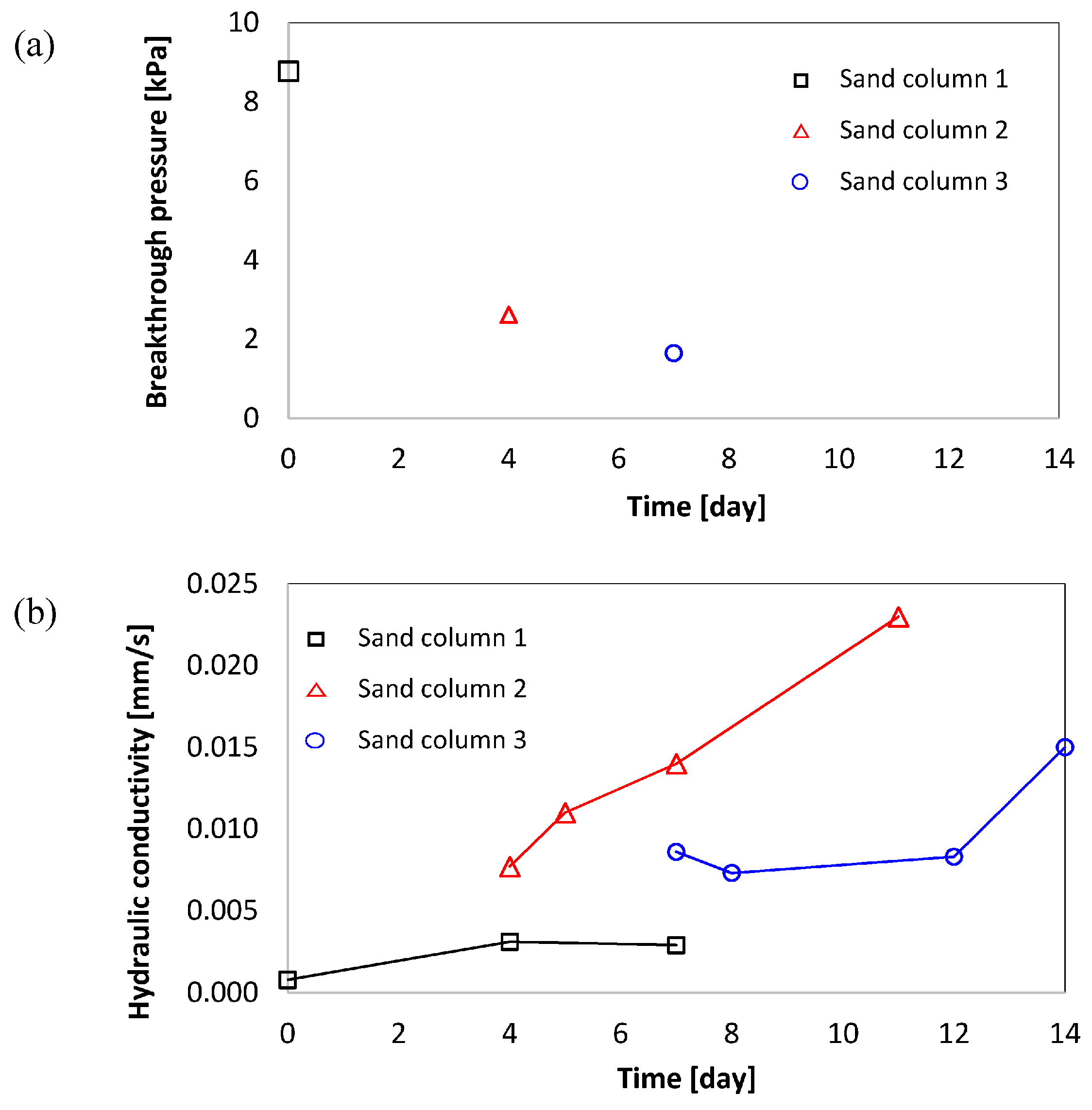
4.5. Breakthrough Pressure and Hydraulic Conductivity of Foam-Filled Sand Columns
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, S.Y.; Sun, D.J.; Dong, X.Q.; Li, C.F.; Xu, J. Aqueous foams stabilized with particles and nonionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2008, 324, 1–8. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous foams stabilized solely by silica nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef] [PubMed]
- Alargova, R.G.; Warhadpande, D.S.; Paunov, V.N.; Velev, O.D. Foam superstabilization by polymer microrods. Langmuir 2004, 20, 10371–10374. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.; Rumpel, A.; Walter, J.; Dombrowski, J.; Kulozik, U.; Braunschweig, B.; Peukert, W. Protein Adsorption at the Electrified Air-Water Interface: Implications on Foam Stability. Langmuir 2012, 28, 7780–7787. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, D.A.; Caldelas, F.M.; Johnston, K.P.; Bryant, S.L.; Huh, C. Nanoparticle-Stabilized Supercritical CO2 Foams for Potential Mobility Control Applications; Society of Petroleum Engineers: Dallas, TX, USA, 2010. [Google Scholar]
- Dickson, J.L.; Binks, B.P.; Johnston, K.P. Stabilization of carbon dioxide-in-water emulsions with silica nanoparticles. Langmuir 2004, 20, 7976–7983. [Google Scholar] [CrossRef] [PubMed]
- Melle, S.; Lask, M.; Fuller, G.G. Pickering emulsions with controllable stability. Langmuir 2005, 21, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Roberts, M.R.; Yu, H.; Huh, C.; Bryant, S.L. Enhanced migration of surface-treated nanoparticles in sedimentary rocks. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009.
- Yu, H.; Yoon, K.Y.; Ingram, D.R.; Johnston, K.P.; Bryant, S.L.; Huh, C. Transport and retention of aqueous dispersions of paramagnetic nanoparticles in reservoir rocks. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010.
- Prodanović, M.A.; Ryoo, S.; Rahmani, A.R.; Kuranov, R.; Kotsmar, C.; Milner, T.E.; Johnston, K.P.; Bryant, S.L.; Huh, C. Effects of magnetic field on the motion of multiphase fluids containing paramagnetic particles in porous media. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010.
- Li, Y.Z.; DiCarlo, D.; Li, X.F.; Zang, J.L.; Li, Z.N. An experimental study on application of nanoparticles in unconventional gas reservoir CO2 fracturing. J. Pet. Sci. Eng. 2015, 133, 238–244. [Google Scholar] [CrossRef]
- Nguyen, P.; Fadaei, H.; Sinton, D. Pore-Scale Assessment of Nanoparticle-Stabilized CO2 Foam for Enhanced Oil Recovery. Energy Fuels 2014, 28, 6221–6227. [Google Scholar] [CrossRef]
- Yan, W.; Miller, C.A.; Hirasaki, G.J. Foam sweep in fractures for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 348–359. [Google Scholar] [CrossRef]
- Mas-Hernandez, E.; Grassia, P.; Shokri, N. Foam improved oil recovery: Foam front displacement in the presence of slumping. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 123–132. [Google Scholar] [CrossRef]
- Lee, S.; Lee, G.; Kam, S.I. Three-Phase Fractional Flow Analysis for Foam-Assisted Non-aqueous Phase Liquid (NAPL) Remediation. Transp. Porous Med. 2014, 101, 373–400. [Google Scholar] [CrossRef]
- Wang, X.W.; Chen, J.J.; Lv, C. Evaluation of foam surfactant for foam-flushing technique in remediation of DDT-contaminated soil using data envelopment analysis method. Environ. Sci. Pollut. Res. 2015, 22, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Wang, S.L. Remediation of a heavy metal-contaminated soil by a rhamnolipid foam. Eng. Geol. 2006, 85, 75–81. [Google Scholar] [CrossRef]
- Vitoonkijvanich, S.; AlSofi, A.M.; Blunt, M.J. Design of foam-assisted carbon dioxide storage in a North Sea aquifer using streamline-based simulation. Int. J. Greenh. Gas Control 2015, 33, 113–121. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.S.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.V.; Kuprin, D.S.; Abduragimov, I.M.; Kuprin, G.N.; Serebriyakov, E.; Vinogradov, V.V. Silica Foams for Fire Prevention and Firefighting. ACS Appl. Mater. Interfaces 2016, 8, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Hirasaki, G.J.; Miller, C.A.; Meinardus, H.; Dwarakanath, V. Application of foam for aquifer remediation. Abstr. Pap. Am. Chem. Soc. 2003, 225, U685. [Google Scholar]
- Pickering, S.U. Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (Observation on surface-membraines, bubbles, emulsions, and mechanical coagulation)—Preliminary account. Proc. R. Soc. Lond. 1903, 72, 156–164. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Colloidal Particles at Liquid Interfaces; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Adkins, S.S.; Gohil, D.; Dickson, J.L.; Webber, S.E.; Johnston, K.P. Water-in-carbon dioxide emulsions stabilized with hydrophobic silica particles. Phys. Chem. Chem. Phys. 2007, 9, 6333–6343. [Google Scholar] [CrossRef] [PubMed]
- Golomb, D.; Barry, E.; Ryan, D.; Swett, P.; Duan, H. Macroemulsions of Liquid and Supercritical CO2-in-Water and Water-in-Liquid CO2Stabilized by Fine Particles. Ind. Eng. Chem. Res. 2006, 45, 2728–2733. [Google Scholar] [CrossRef]
- Golomb, D.; Barry, E.; Ryan, D.; Lawton, C.; Swett, P. Limestone-Particle-Stabilized Macroemulsion of Liquid and Supercritical Carbon Dioxide in Water for Ocean Sequestration. Environ. Sci. Technol. 2004, 38, 4445–4450. [Google Scholar] [CrossRef] [PubMed]
- Worthen, A.J.; Bryant, S.L.; Huh, C.; Johnston, K.P. Carbon dioxide-in-water foams stabilized with nanoparticle and surfactant acting in synergy. AIChE J. 2013, 59, 3490–3501. [Google Scholar] [CrossRef]
- Binks, B.P.; Kirkland, M.; Rodrigues, J.A. Origin of stabilisation of aqueous foams in nanoparticle-surfactant mixtures. Soft Matter 2008, 4, 2373–2382. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, Z.M.; Wang, P. Experimental Study of the Stabilization of CO2 Foam by Sodium Dodecyl Sulfate and Hydrophobic Nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 1243–1253. [Google Scholar] [CrossRef]
- Dong, X.Q.; Xu, J.A.; Cao, C.B.; Sun, D.J.; Jiang, X.R. Aqueous foam stabilized by hydrophobically modified silica particles and liquid paraffin droplets. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 181–188. [Google Scholar] [CrossRef]
- Subramaniam, A.B.; Mejean, C.; Abkarian, M.; Stone, H.A. Microstructure, morphology, and lifetime of armored bubbles exposed to surfactants. Langmuir 2006, 22, 5986–5990. [Google Scholar] [CrossRef] [PubMed]
- Pal, R. Effect of droplet size on the rheology of emulsions. AIChE J. 1996, 42, 3181–3190. [Google Scholar] [CrossRef]
- Kim, J.U.; Park, B.H.; Lee, M.H. Critical parameters to determine mean bubble size of generated foams from a foam generator. J. Appl. Polym. Sci. 2013, 130, 2062–2067. [Google Scholar] [CrossRef]
- Schramm, L.L.; Wassmuth, F. Foams: Basic Principles, in Foams: Fundamentals and Applications in the Petroleum Industry; American Chemical Society: Washington, DC, USA, 1994; pp. 3–45. [Google Scholar]
- Kim, I.; Worthen, A.J.; Johnston, K.P.; DiCarlo, D.A.; Huh, C. Size-dependent properties of silica nanoparticles for Pickering stabilization of emulsions and foams. J. Nanopart. Res. 2016, 18. [Google Scholar] [CrossRef]
- Gao, W.C.; Zhang, Y.X.; Zhang, X.Y.; Duan, Z.L.; Wang, Y.H.; Qin, C.; Hu, X.; Wang, H.; Chang, S. Permeable reactive barrier of coarse sand-supported zero valent iron for the removal of 2,4-dichlorophenol in groundwater. Environ. Sci. Pollut. Res. 2015, 22, 16889–16896. [Google Scholar] [CrossRef] [PubMed]
- Thiruverikatachari, R.; Vigneswaran, S.; Naidu, R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Stevenson, P. (Ed.) Foam Engineering Fundamentals and Applications; Wiley: Chichester, UK, 2012; p. 548.
- Adkins, S.S.; Chen, X.; Chan, I.; Torino, E.; Nguyen, Q.P.; Sanders, A.W.; Johnston, K.P. Morphology and stability of CO2-in-water foams with nonionic hydrocarbon surfactants. Langmuir 2010, 26, 5335–5348. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.J. A study on the permeability and flow behavior of surfactant foam in unconsolidated media. Environ. Earth Sci. 2013, 68, 567–576. [Google Scholar] [CrossRef]
- Degussa. The Use of Hydrophobic AEROSIL in the Coatings Industry. In Basic Characteristics and Applications of AEROSIL; Ferch, H., Ed.; Degussa AG: Darmstadt, Germany, 1993. [Google Scholar]
- Xue, Z.; Worthen, A.; Qajar, A.; Robert, I.; Bryant, S.L.; Huh, C.; Prodanovic, M.; Johnston, K.P. Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes. J. Colloid Interface Sci. 2016, 461, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Khalil, M.; Liu, N.; Lee, R. Effect of particle hydrophobicity on CO2 foam generation and foam flow behavior in porous media. Fuel 2014, 126, 104–108. [Google Scholar] [CrossRef]
- Géraud, B.; Jones, S.A.; Cantat, I.; Dollet, B.; Méheust, Y. The flow of a foam in a two-dimensional porous medium. Water Resour. Res. 2016, 52, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, T.; Honorez, C.; Jumeau, M.; Elias, F.; Drenckhan, W. A simple technique for the automation of bubble size measurements. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 68–74. [Google Scholar] [CrossRef]
- Tcholakova, S.; Mitrinova, Z.; Golemanov, K.; Denkov, N.D.; Vethamuthu, M.; Ananthapadmanabhan, K.P. Control of Ostwald ripening by using surfactants with high surface modulus. Langmuir 2011, 27, 14807–14819. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Canceliere, C.; McClements, D.J. Mass transport phenomena inoil-in-water emulsions containing surfactant micelles: Ostwald ripening. Langmuir 2000, 16, 6833–6838. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S. An experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Fuel 2016, 186, 430–442. [Google Scholar] [CrossRef]
- Street, R.L.; Watters, G.Z.; Vennard, J.K. Elementary Fluid Mechanics, 7th ed.; John Wiley&Sons: Etobicoke, ON, Canada, 1996. [Google Scholar]
- Cantat, I. Liquid meniscus friction on a wet plate: Bubbles, lamellae, and foams. Phys. Fluids 2013, 25, 031303. [Google Scholar] [CrossRef] [Green Version]

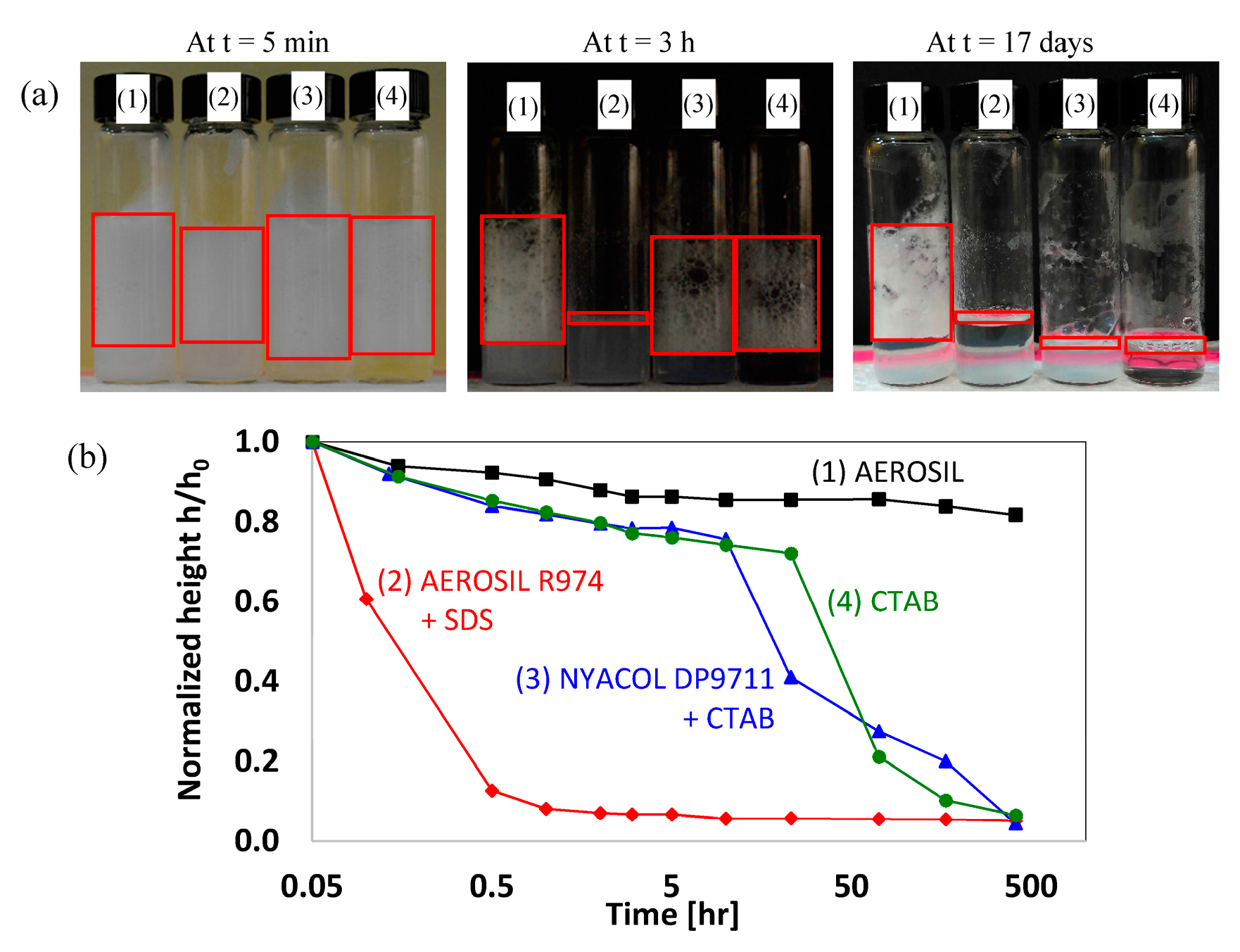

| Nanofluid |
| AEROSIL R974 (1 wt % and 0.5%) + ethanol (2 wt %) |
| SDS (0.1 wt %) + AEROSIL R974 (1 wt %) + ethanol (2 wt %) |
| CTAB (0.1 wt %) |
| CTAB (0.1 wt %) + NYACOL DP9711 (1 wt %) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Jang, J. Hydraulic Properties of Porous Media Saturated with Nanoparticle-Stabilized Air-Water Foam. Sustainability 2016, 8, 1317. https://doi.org/10.3390/su8121317
Zheng X, Jang J. Hydraulic Properties of Porous Media Saturated with Nanoparticle-Stabilized Air-Water Foam. Sustainability. 2016; 8(12):1317. https://doi.org/10.3390/su8121317
Chicago/Turabian StyleZheng, Xianglei, and Jaewon Jang. 2016. "Hydraulic Properties of Porous Media Saturated with Nanoparticle-Stabilized Air-Water Foam" Sustainability 8, no. 12: 1317. https://doi.org/10.3390/su8121317






