Stabilization of Organic Matter by Biochar Application in Compost-amended Soils with Contrasting pH Values and Textures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection and Biochar Preparation
2.2. Analytical Methods
2.3. Incubation Experiment
2.4. Calculations and Statistical Analysis
2.5. Soil Micromorphology
2.6. Statistical Methods
3. Results
3.1. Characteristics of Soils, Compost, and Biochars
| Properties | Soils | ||
|---|---|---|---|
| Laopi (Lo) | Shashuipu (Sp) | Choutseulun (Ct) | |
| pH | 4.43 | 6.90 | 8.25 |
| EC + (dS m−1) | 0.08 | 0.19 | 1.96 |
| Sand (%) | 14 | 17 | 95 |
| Silt (%) | 57 | 66 | 4.0 |
| Clay (%) | 29 | 17 | 1.0 |
| Texture # | SiCL | SiL | S |
| OC (%) | 1.78 | 1.38 | 0.12 |
| CaCO3 (g kg−1) | 0.12 | 0.27 | 5.08 |
| CEC (cmol(+) kg−1) ¶ | 15.0 | 14.6 | 4.00 |
| TN § (%) | 0.16 | 0.19 | 0.01 |
| NH4+-N (mg kg−1) | 2.84 | 11.2 | ND e |
| NO3--N (mg kg−1) | 14.2 | 47.8 | 8.33 |
| ROC (g kg−1) ǂ | 0.42 | 0.75 | ND |
| Compost | RHB-400 | RHB-700 | |
|---|---|---|---|
| pH (1:10 w/v) | 5.50 | 7.99 | 8.03 |
| TC (%) | 30.2 | 31.0 | 32.9 |
| TN (%) | 1.08 | 0.41 | 0.35 |
| C/N ratio | 28 | 76 | 94 |
| CEC (cmol(+) kg−1) | 82.5 | 26.1 | 35.6 |
| Exchangeable K (g kg−1) | 6.94 | 7.01 | 7.02 |
| Exchangeable Na (g kg−1) | 0.44 | 0.28 | 0.24 |
| Exchangeable Ca (g kg−1) | 4.61 | 0.47 | 0.54 |
| Exchangeable Mg (g kg−1) | 2.12 | 0.22 | 0.23 |
| Carbonate (g kg−1) | ND | 1.52 | 1.88 |
| ROC (g kg−1) | 36.0 | 3.22 | 2.10 |
3.2. Carbon Dioxide Emissions from Soils
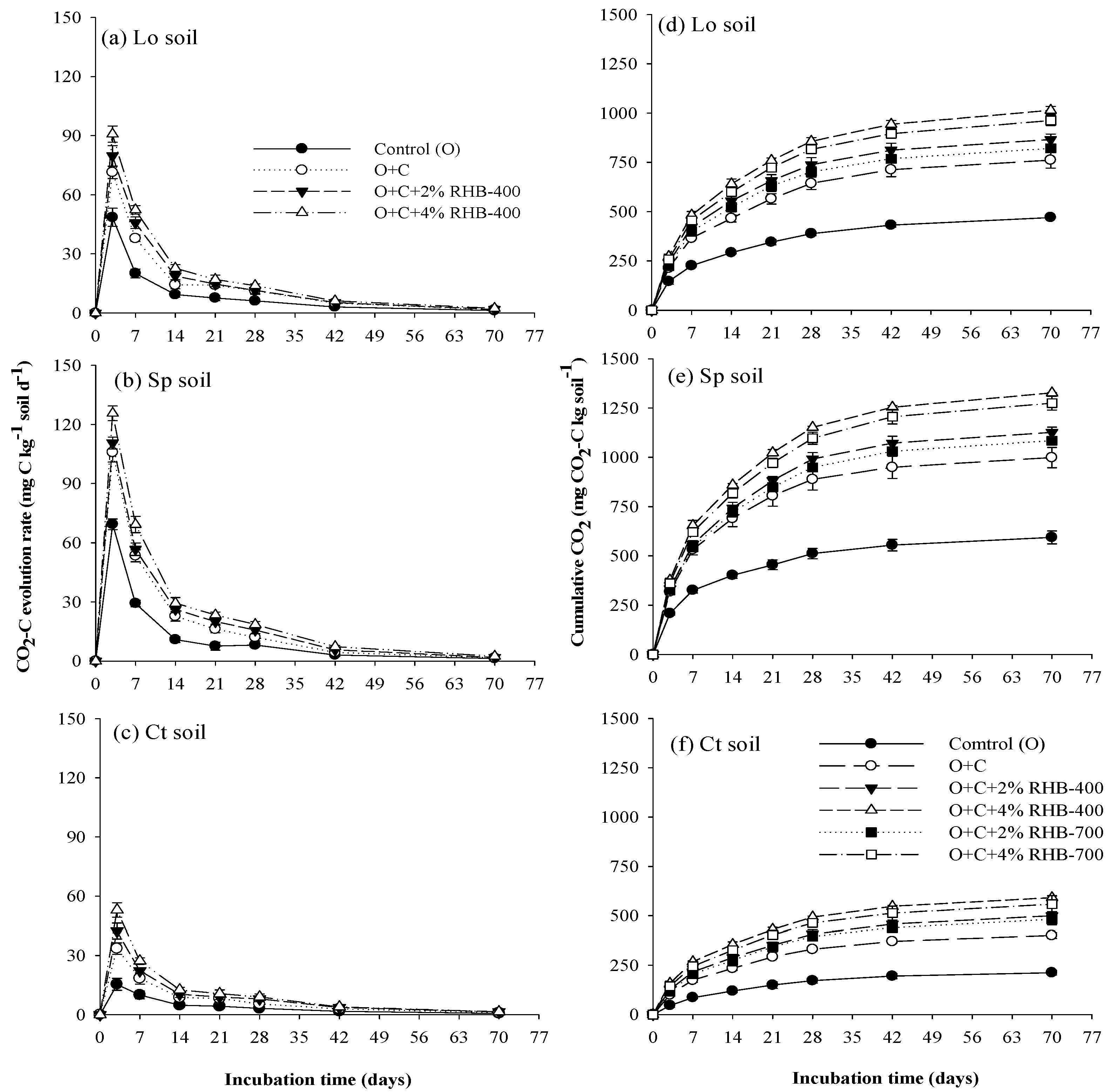
| Control | O + C | O + C + 2% RHB-400 | O + C + 4% RHB-400 | O + C + 2% RHB-700 | O + C + 4% RHB-700 | |
|---|---|---|---|---|---|---|
| Lo | 470 ± 4.00 a | 761 ± 40.6 a | 865 ± 27.5 a | 1014 ± 20.2 a | 821 ± 19.9 a | 963 ± 25.7 a |
| Sp | 594 ± 33.1 b | 999 ± 51.7 b | 1127 ± 26.4 b | 1327 ± 2.12 b | 1085 ± 11.8 b | 1274 ± 34.9 b |
| Ct | 212 ± 11.3 c | 400 ± 15.0 c | 499 ± 21.2 c | 591 ± 8.09 c | 482 ± 27.9 c | 558 ± 43.9 c |
| Parameter | mg C kg-1 soil | ||
|---|---|---|---|
| Freedom degree | F-value | Significance | |
| Soil type (S) | 2 | 2626.6 | < 0.001 |
| Biochar type (B) | 1 | 0.26 | 0.6094 |
| Application rate (R) | 2 | 243.2 | < 0.001 |
| S × R | 4 | 9.02 | < 0.001 |
| S × B | 2 | 0.75 | 0.4802 |
| B × R | 2 | 0.20 | 0.8166 |
| S × B × R | 4 | 0.04 | 0.9960 |
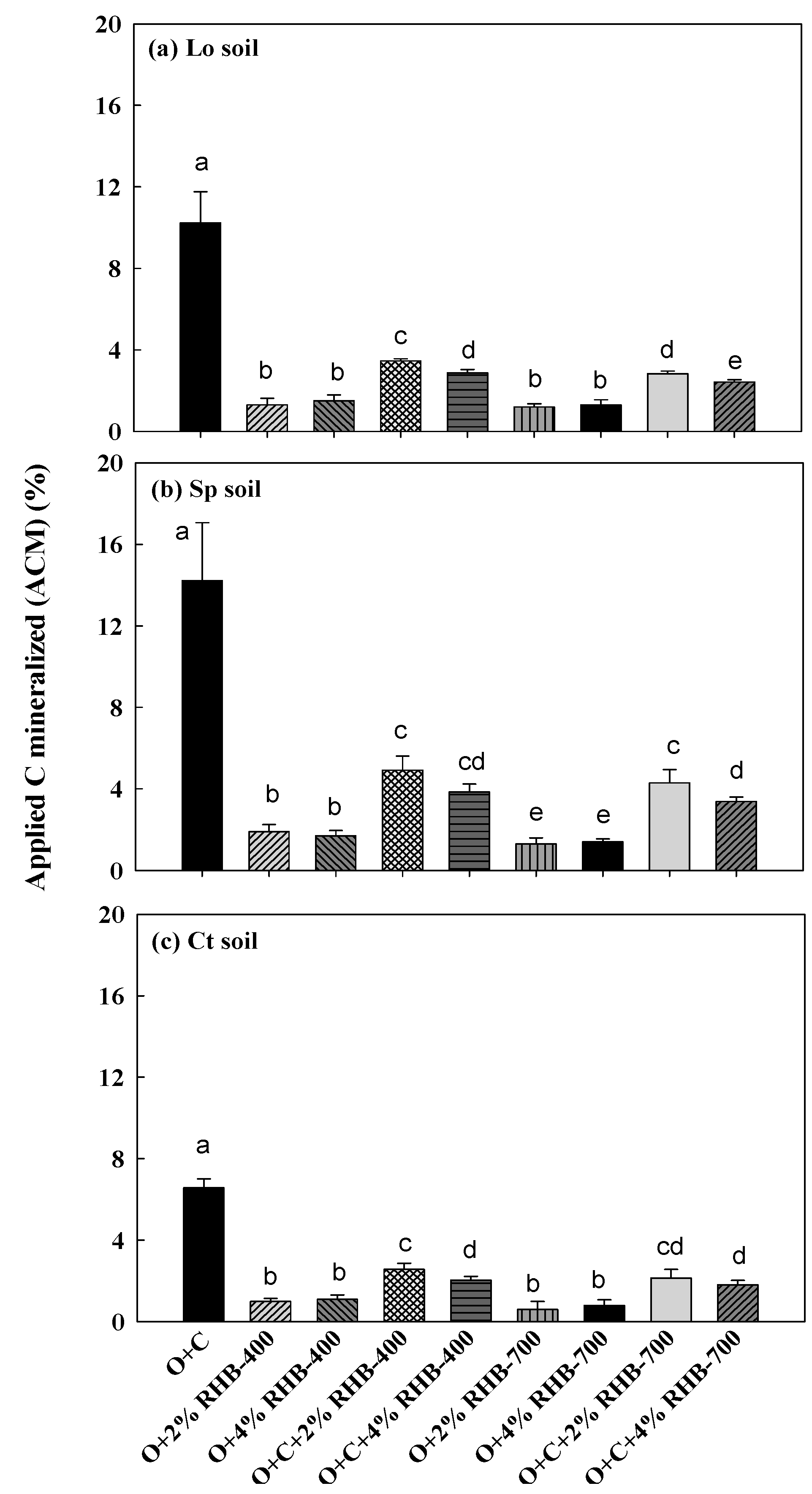
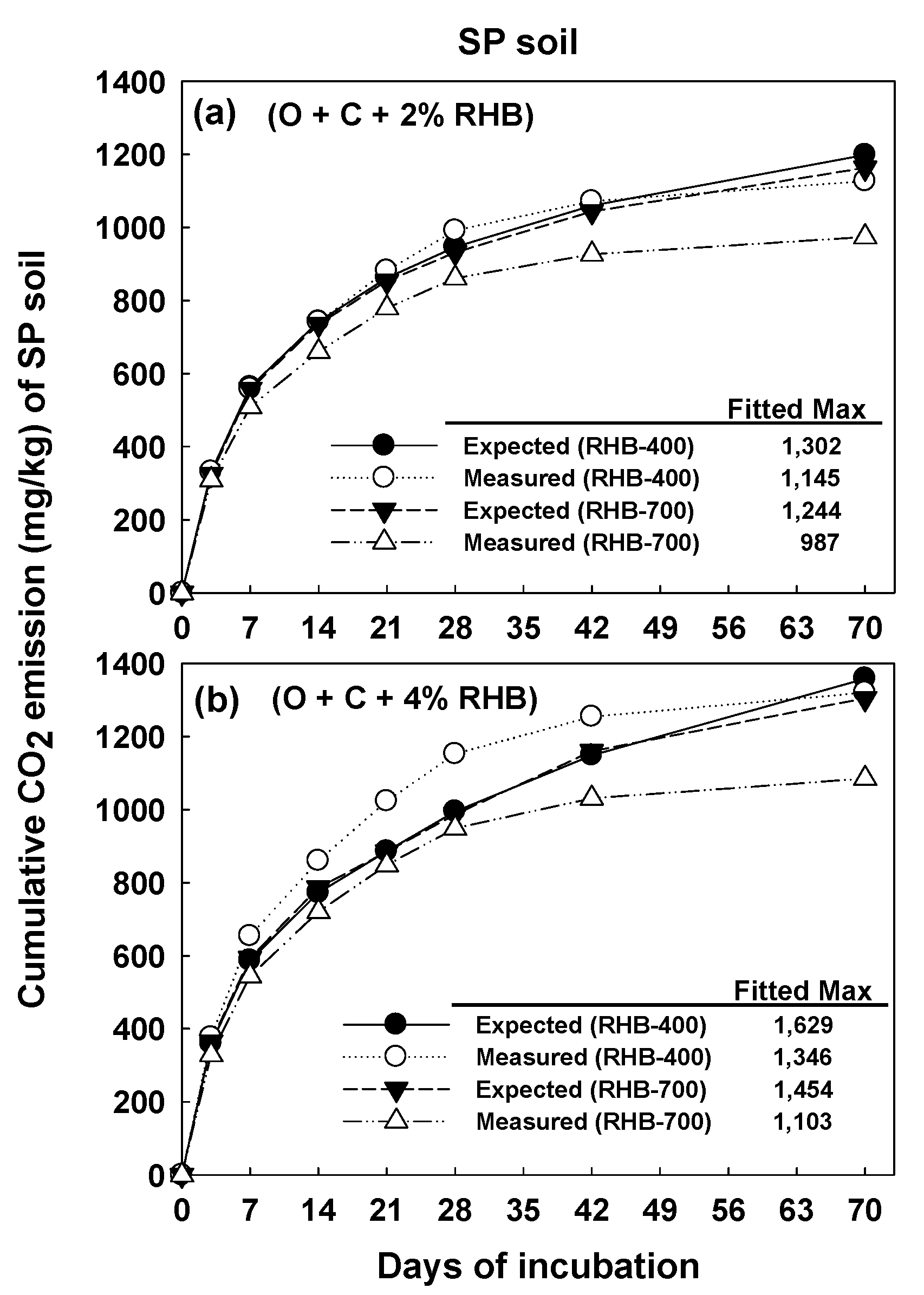
3.3. Kinetics of Carbon Mineralization
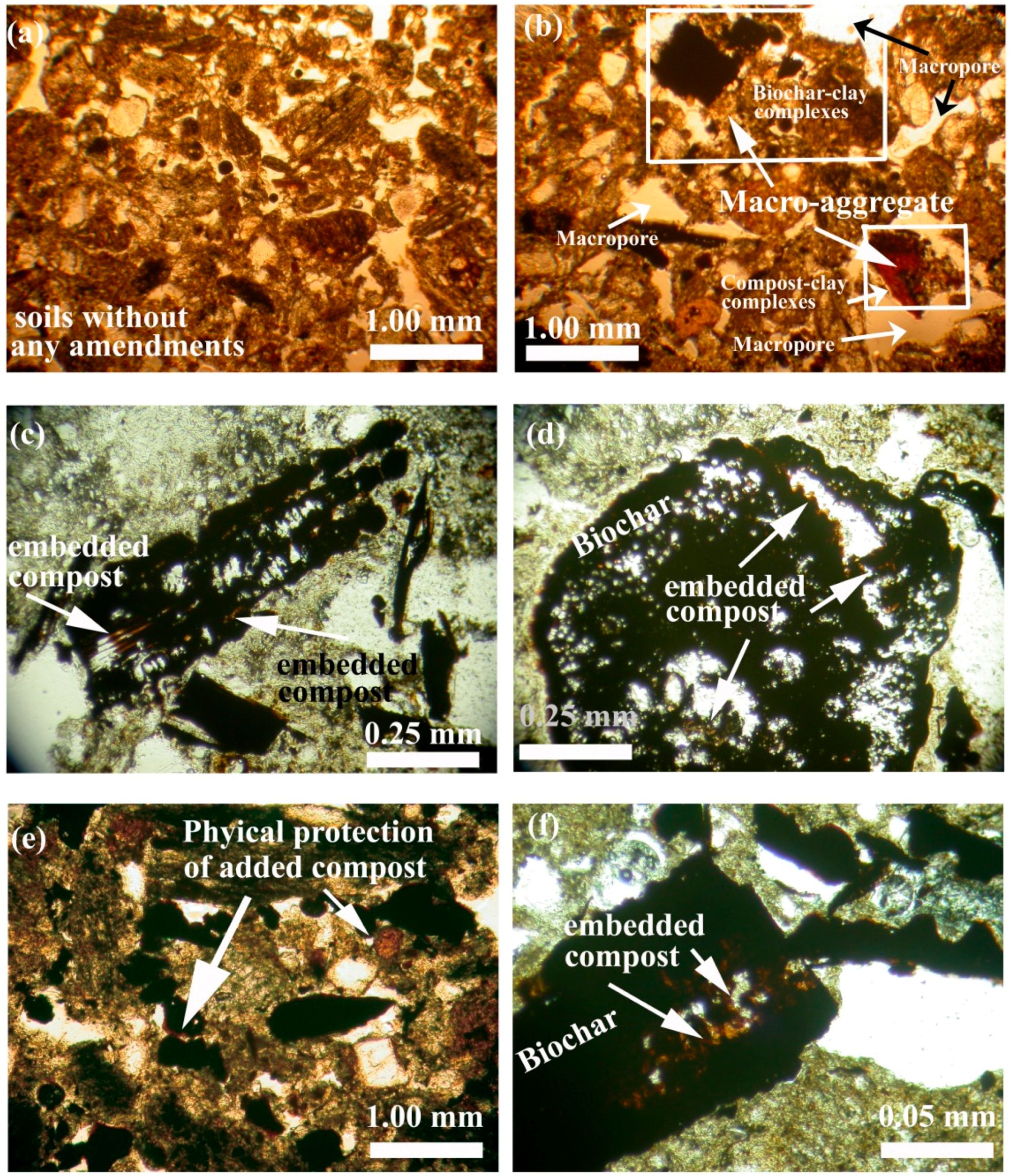
3.4. Soil Micromorphology
4. Discussions
4.1. CO2 Emissions from Soils Amended with the Compost and Biochars
4.2. Effect of Soil Type on Carbon Mineralization with Compost and Biochar Amendment
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mekuria, W.; Noble, A.; Sengtaheuanghoung, O.; Hoanh, C.T.; Bossio, D.; Sipaseuth, N.; McCartney, M.; Langan, S. Organic and Clay-Based Soil Amendments Increase Maize Yield, Total Nutrient Uptake, and Soil Properties in Lao PDR. Agroecol. Sustain. Food Syt. 2014, 38, 936–961. [Google Scholar] [CrossRef]
- Bolan, N.S.; Kunhikrishnan, A.; Choppala, G.K.; Thangarajan, R.; Chung, J.W. Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total Environ. 2012, 424, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Troy, S.M.; Lawlo, P.G.; O’Flynn, C.J.; Healy, M.G. Impact of biochar addition to soil on greenhouse gas emissions following pigmanure application. Soil Biol. Biochem. 2013, 60, 173–181. [Google Scholar] [CrossRef]
- Lehmann, J.; Czimczik, C.; Laird, D.; Sohi, S. Stability of biochar in the soil. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 183–205. [Google Scholar]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.W.; Xu, H.J.; Zhou, C.J.; Wang, S.Q.; Xin, G.X. Effects of crop-straw biochar on crop growth and soil fertility over a wheat-millet rotation in soils of China. Soil Use Manag. 2014, 30, 311–319. [Google Scholar] [CrossRef]
- Gaunt, J.; Lehmann, J. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A. The charcoal vision: A win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of Biochar on Manure Carbon Stabilization and Greenhouse Gas Emissions. Soil Sci. Soc. Am. J. 2011, 75, 871–879. [Google Scholar] [CrossRef]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer, and biochar on decomposition of soilorganic matter and plant residues as determined by 14C and enzyme activities. Eur. J. Soil Biol. 2012, 48, 1–10. [Google Scholar] [CrossRef]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer and biochar on the decomposition of 14C-labelled maize residues and on their stabilization in soil aggregates. Eur. J. Soil Sci. 2013, 64, 488–499. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Steffens, D.; Reisenauer, H.P.; Schubert, S. Biochars influence differential distribution and chemical composition of soil organic matter. Plant Soil Environ. 2014, 60, 337–343. [Google Scholar]
- Fernández, J.M.; Nieto, M.A.; López-de-sá, E.G.; Gascó, G.; Méndez, A.; Plaza, C. Carbon dioxide emmisions from semi-arid soils amended with biochar alone or combined with mineral and organic fertilizers. Sci. Total Environ. 2014, 482–483, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbeal biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Van Veen, J.A.; Kuikman, P.J. Soil structure aspects of decomposition of organic matter by micro-organisms. Biogeochemistry 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B.; Brodowski, S.; Amelung, W. Interactive priming of black carbon and glucose mineralisation. Org. Geochem. 2004, 35, 823–830. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Keith, A.; Singh, B.; Singh, B.P. Interactive Priming of Biochar and Labile Organic Matter Mineralization in a Smectite-Rich Soil. Environ. Sci. Technol. 2011, 45, 9611–9618. [Google Scholar] [CrossRef] [PubMed]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of contrasting biochar types on five soils at increasing rates of application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; pp. 487–488. [Google Scholar]
- Rhoades, J.D. Soluble salts. In Methods of Soil Analysis Part 2—Chemical and Microbiological Properties; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 167–179. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1218–1220. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2—Chemical and Microbiological Properties; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Mulvaney, R.L. Nitrogen-Inorganic forms. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 3—Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis Part 2—Chemical and Microbiological Methods, 2nd ed.; Agronomy Monograph 9; America Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 437–451. [Google Scholar]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Conteh, A.; Lefroy, R.D.B.; Blair, G.J. Dynamics of organic matter in soils as determined by variations in 13C/12C isotopic ratios and fractionation by ease of oxidation. Aust. J. Soil Res. 1997, 35, 881–890. [Google Scholar] [CrossRef]
- Zibilske, L.M. Carbon mineralization. In Methods of Soil Analysis: Soil Science Society of America Book Series 5 Part 2—Microbial and Biochemical Properties; Weaver, R.W., Ed.; ASA and SSSA: Madison, WI, USA, 1994; pp. 835–863. [Google Scholar]
- Novak, J.M.; Busscher, D.W.; Watts, D.W.; Laird, D.A.; Ahmedna, M.A.; Niandou, M.A.S. Short-term CO2 mineralization after additions of biochar and switchgrass to a TypicKandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Riberio, H.M.; Fanqueiro, D.; Alves, F.; Vasconcelos, E.; Coutinho, J.; Bol, R.; Cabral, F. Carbon-mineralization kinetics in an organically managed Cambic Arenosol amended with organic fertilizers. J. Plant Nutr. Soil Sci. 2010, 173, 39–45. [Google Scholar] [CrossRef]
- Stoops, G. Guidelines for Analysis and Description of Soil and Regolith Thin Sections; Soil Science Society of Amenrica, Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 11th edn USDA-NRCS, Agricultural Handbook No. 436; US Government Printing Office: Washington, DC, USA, 2010.
- Molina, J.A.E.; Clapp, C.E.; Larson, W.E. Potentially mineralizable nitrogen in soil: the simple exponential model does not apply to the first 12 weeks of incubation. Soil Sci. Soc. Am. J. 1980, 44, 442–443. [Google Scholar] [CrossRef]
- Liang, B.Q.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Deenik, J.L.; Diarra, A.; Uehara, G.; Campell, S.; Sumiyoshi, Y.; Antal, M.J., Jr. Charcoal ash and volatile matter effects on soil properties and plant growth in an acid Ultisol. Soil Sci. 2011, 176, 336–345. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef]
- Hseu, Z.Y.; Jien, S.H.; Chien, W.S.; Liou, R.C. Impacts of biochar on physical properties and erosion potential of a mudstone slopeland soil. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G.; Gustafsson, O.; Bucheli, T.D.; Jonker, M.T.O.; Koelmans, A.A.; VanNoort, P.C.M. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 2005, 39, 6881–6895. [Google Scholar] [CrossRef] [PubMed]
- Sobek, A.; Stamm, N.; Bucheli, T.D. Sorption of phenyl urea herbicides to black carbon. Environ. Sci. Technol. 2009, 43, 8147–8152. [Google Scholar] [CrossRef] [PubMed]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef] [PubMed]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Cantrell, K.B.; Shumaker, P.D.; Szogi, A.A.; Johnson, M.G. Carbon mineralization in two ultisols amended with different sources and particle sizes of pyrolyzed biochar. Chemosphere 2014, 103, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sissoko, A.; Kpomblekou-A, K. Carbon decomposition in broiler litter-amended soils. Soil Biol. Biochem. 2010, 42, 543–550. [Google Scholar] [CrossRef]
- Sørensen, L.H. Size and persistence of the microbial biomass formed during the humification of glucose, hemicellulose, cellulose, and straw in soils containing different amounts of clay. Plant Soil 1983, 75, 121–130. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Palm, C.A.; Parton, W.J.; Elliott, E.T.; Frey, S.D. Soil pH and organic C dynamics in tropical forest soils: evidence from laboratory and simulation studies. Soil Biol. Biochem. 1995, 27, 1589–1599. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, Z.S. Carbon and nitrogen mineralization of sewage sludge compost in soils with different initial pH. Soil Sci. Plant Nutr. 2009, 55, 715–724. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jien, S.-H.; Wang, C.-C.; Lee, C.-H.; Lee, T.-Y. Stabilization of Organic Matter by Biochar Application in Compost-amended Soils with Contrasting pH Values and Textures. Sustainability 2015, 7, 13317-13333. https://doi.org/10.3390/su71013317
Jien S-H, Wang C-C, Lee C-H, Lee T-Y. Stabilization of Organic Matter by Biochar Application in Compost-amended Soils with Contrasting pH Values and Textures. Sustainability. 2015; 7(10):13317-13333. https://doi.org/10.3390/su71013317
Chicago/Turabian StyleJien, Shih-Hao, Chung-Chi Wang, Chia-Hsing Lee, and Tsung-Yu Lee. 2015. "Stabilization of Organic Matter by Biochar Application in Compost-amended Soils with Contrasting pH Values and Textures" Sustainability 7, no. 10: 13317-13333. https://doi.org/10.3390/su71013317





