Management Practices and Their Potential Influence on Johne’s Disease Transmission on Canadian Organic Dairy Farms—A Conceptual Analysis
Abstract
:1. Introduction
2. Canadian Organic Dairy Farming
2.1. Canadian Organic Standards
2.2. Organic Dairy Industry
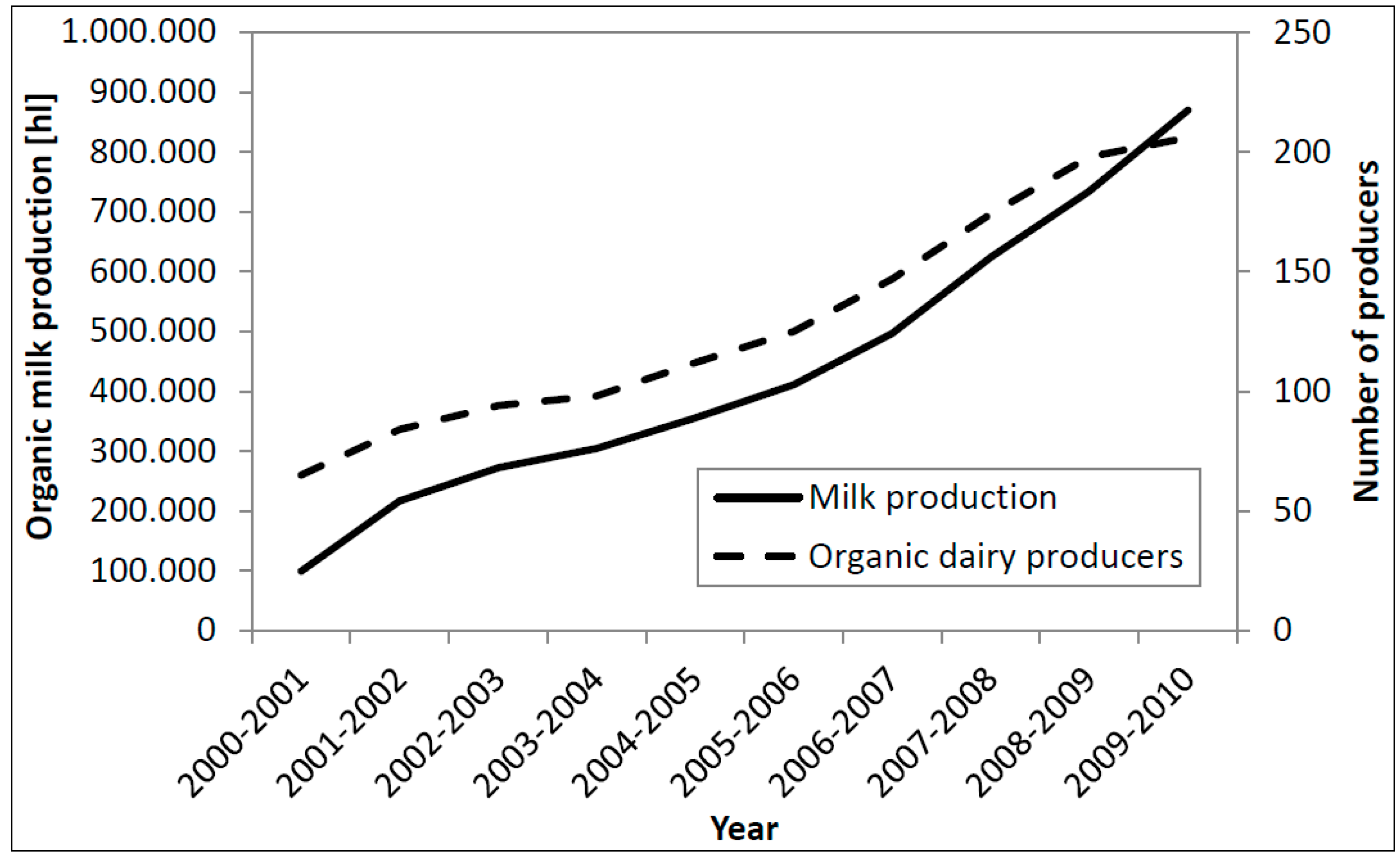
2.3. Scientific Investigations on Organic Dairy Production
3. Johne’s Disease
3.1. Etiology and Epidemiology
3.2. Diagnostic Tests
| Test | Reference Test | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|
| Milk ELISA | Fecal culture | 61.1 (48.9–72.4) | 94.7 a | [41] |
| 28.9 | 99.7 | [44] | ||
| Serum ELISA | Fecal culture | 73.6 (61.9–83.3) | 87.5 a | [41] |
| 16.7 (4.5–28.8) | 97.1 (96.0–98.1) | [45] c | ||
| 13.9 (2.6–25.2) | 95.9 (94.6–97.2) | [45] c | ||
| 27.8 (13.1–42.4) | 90.1 (88.2–92.0) | [45] c | ||
| 31.3 (20.6–43.8) | 97.8 (94.5–99.4) | [40] | ||
| 28.9 | 95.3 | [44] c | ||
| 28.4 | 99.7 | [44] c | ||
| 28.0 | 100.0 | [44] c | ||
| 44.5 | 84.9 | [44] c | ||
| 57 | 98.9 | [46] | ||
| Serum ELISA | Tissue culture | 8.8 (4.4–13.1) | 97.6 (96.6–98.6) | [45] c |
| 6.9 (3.0–10.8) | 96.0 (94.7–97.4) | [45] c | ||
| 16.9 (11.0–22.7) | 90.8 (88.8–92.7) | [45] c | ||
| Fecal culture | Repeated fecal culture | 38 | 100 | [47] |
| Direct fecal PCR | Fecal culture | 70.2 (57.7–80.7) | 85.3 (79.3–90.1) | [40] |
| Environmental culture d | Individual fecal culture | 71.4 (49.2–86.5) | 98.6 (94.8–99.6) | [48] |
| 76.0 | 80.0 a | [49] | ||
| Individual serum ELISA | 76.3 | 58.8 a | [49] | |
| Individual milk ELISA | 71.4 | 66.7 a | [49] | |
| Bulk tank milk ELISA d | Sensitivity: Individual fecal culture from Danish herds; Specificity: bulk tank samples from Norwegian herds considered to be negative | 97.1 (83–100) b | 83.3 (74–90) b | [50] |
3.3. Johne’s Disease Prevalence and Control in Canada
| Region/Province | Duration | Budget | Main Sponsoring Body | Number of Farms Participating (%) | Number of Trained Dairy Veterinarians (%) |
|---|---|---|---|---|---|
| Atlantic Canada a | 2011–2014 | 1,000,000 | Public | 459 (69) | 49 (60) |
| Quebec | 2007–2014 | 1,600,000 | Public | 1362 (22) | 161 (47) |
| Ontario | 2010–2013 | 2,440,000 | Industry | 2339 (58) | 246 (>95) |
| Manitoba | 2010–2011 | 100,000 | Public | 200 (57) b | 20 b |
| Saskatchewan | 2012–2013 | 125,000 | Public | 20 (12) | 10 b |
| Alberta | 2010–2013 | 1,040,000 | Public | 350 (61) | 78 (95) |
| British Columbia | 2009–2012 | 250,000 | Public | 30 (6) | 11 (50) |
| Canada | 2007–2014 | 6,600,000 | Public | 4759 (>35) | 575 (>60) |
| Province | Test | Herd Level Prevalence (%) | Reference |
|---|---|---|---|
| New Brunswick | Serum ELISA | 43.3 a (24.5–62.2) | [54] |
| Nova Scotia | Serum ELISA | 53.3 a (34.4–72.2) | [54] |
| Prince Edward Island | Serum ELISA | 33.3 a (15.4–51.2) | [54] |
| Atlantic provinces d | Environmental culture | 20 | [55] c |
| Quebec | Serum ELISA | 42.5 | [56] c |
| Ontario | Serum ELISA | 58 a (44–72) | [57] |
| Milk ELISA | 34 a (21–47) | [57] | |
| Milk or Serum ELISA | 26 | [58] c | |
| Manitoba | Serum ELISA | 68.4 a (52.5–84.2) | [59] |
| Saskatchewan | Serum ELISA | 43.3 a (27.4–59.3) | [60] |
| Alberta | Serum ELISA | 40.0 ± 13.6 b | [61] |
| Serum ELISA | 70.2 a (53.7–86.6) | [62] | |
| Environmental culture | 70 | [63] c |
4. Johne’s Disease Prevalence on Organic Dairy Farms
5. Association between Johne’s Disease and Organic Dairy Farming
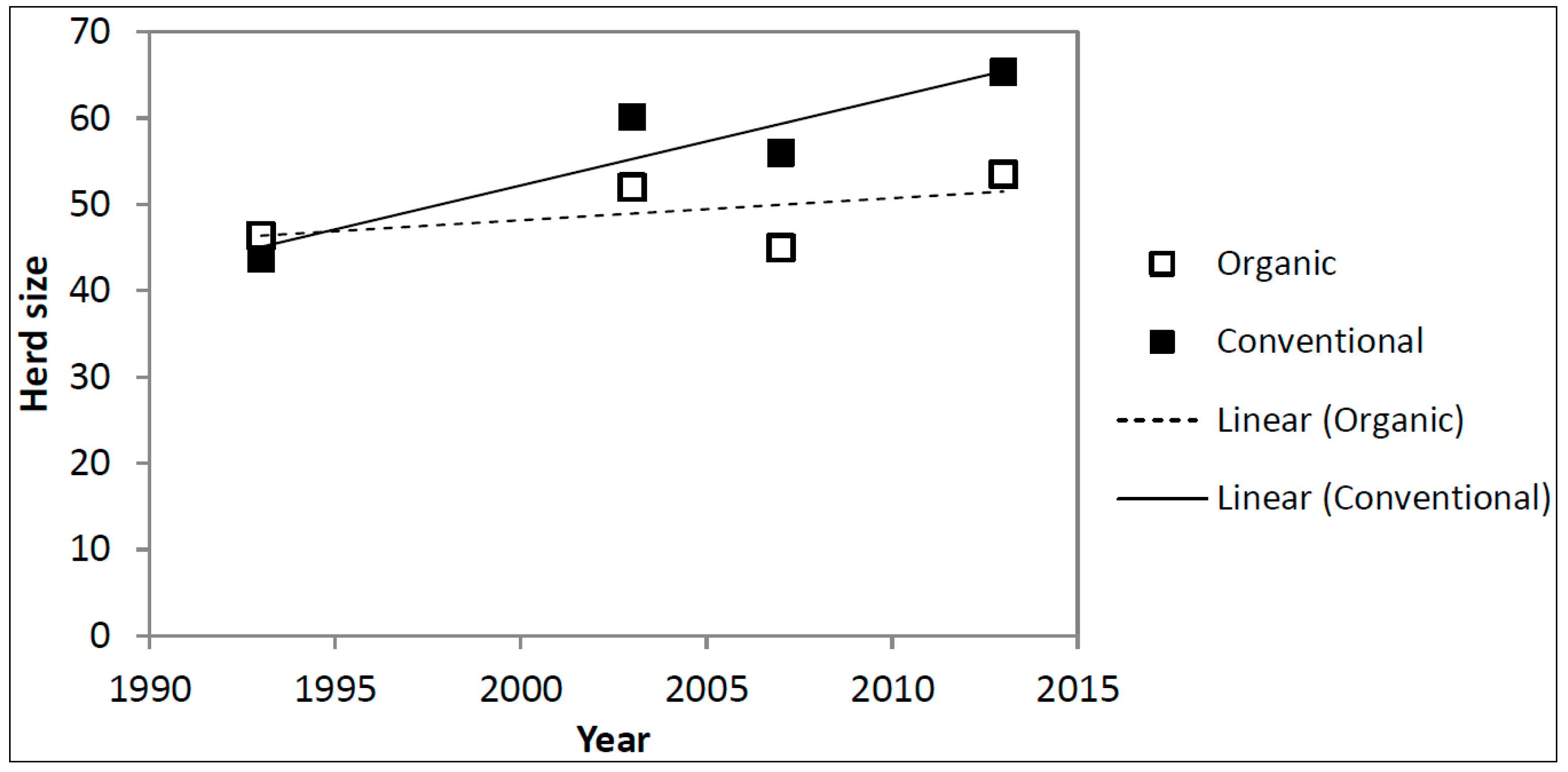
5.1. Farm Structure
5.2. Crop Management
5.3. Nutrition
5.4. Calving and Dairy Heifer Management
5.5. Veterinary Treatments
5.6. Biosecurity
5.7. Breeding Strategy
6. Knowledge Gaps
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agriculture and Agri-Food Canada (AAFC). Organic dairy industry in Canada. Available online: http://www.dairyinfo.gc.ca/pdf/organic_profile_eng.pdf (accessed on 5 January 2014).
- Government of Canada. CAN/CGSB-32.310-2006 Organic Production Systems-General Principles and Management Standards; Government of Canada: Ottawa, ON, Canada, 2006. [Google Scholar]
- Hill, S.B.; MacRae, R.J. Organic farming in Canada. Agric. Ecosyst. Environ. 1992, 39, 71–84. [Google Scholar]
- Government of Canada. CAN/CGSB-32.311-2006 Organic Production Systems-Permitted Substances Lists; Government of Canada: Ottawa, ON, Canada, 2006.
- Government of Canada. Organic Products Regulations, 2009 (SOR/2009-176); Government of Canada: Ottawa, ON, Canada, 2009.
- Agriculture and Agri-Food Canada (AAFC). Certified organic production statistics for Canada. Available online: http://www.agr.gc.ca/eng/industry-markets-and-trade/statistics-and-market-information/by-product-sector/organic-products/organic-production-canadian-industry/certified-organic-production-statistics-for-canada-2009/?id=1312385802597#a3 (accessed on 15 January 2014).
- Rozzi, P.; Miglior, F.; Hand, K.J. A total merit selection index for Ontario organic dairy farmers. J. Dairy Sci. 2007, 90, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J.; Martin, R.C.; Juurlink, S.D.; Lynch, D.H.; Voroney, R.P. Nutrient budgets of Ontario organic dairy farms. Can. J. Soil Sci. 2008, 88, 107–114. [Google Scholar] [CrossRef]
- Stonehouse, D.P.; Clark, E.A.; Ogini, Y.A. Organic and conventional dairy farm comparisons in Ontario, Canada. Biol. Agric. Hortic. 2001, 19, 115–125. [Google Scholar] [CrossRef]
- Ogini, Y.O.; Clark, A.; Stonehouse, D.P. Comparison of organic and conventional dairy farms in Ontario. Am. J. Altern. Agric. 1999, 14, 122–128. [Google Scholar] [CrossRef]
- Sholubi, Y.O.; Stonehouse, D.P.; Clark, E.A. Profile of organic dairy farming in Ontario. Am. J. Altern. Agric. 1997, 12, 133. [Google Scholar] [CrossRef]
- Lynch, D. Environmental impacts of organic agriculture: A Canadian perspective. Can. J. Plant Sci. 2009, 89, 621–628. [Google Scholar] [CrossRef]
- Machackova, M.; Svastova, P.; Lamka, J.; Parmova, I.; Liska, V.; Smolik, J.; Fischer, O.A.; Pavlik, I. Paratuberculosis in farmed and free-living wild ruminants in the Czech Republic (1999–2001). Vet. Microbiol. 2004, 101, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nebbia, P.; Robino, P.; Ferroglio, E.; Rossi, L.; Meneguz, G.; Rosati, S. Paratuberculosis in Red Deer (Cervus elaphus hippelaphus) in the Western Alps. Vet. Res. Commun. 2000, 24, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nugent, G.; Whitford, E.J.; Hunnam, J.C.; Wilson, P.R.; Cross, M.L.; de Lisle, G.W. Mycobacterium avium subsp. paratuberculosis infection in wildlife on three deer farms with a history of Johne’s disease. N. Zeal. Vet. J. 2011, 59, 293–298. [Google Scholar]
- Judge, J.; Kyriazakis, I.; Greig, A.; Allcroft, D.J.; Hutchings, M.R. Clustering of Mycobacterium avium subsp. paratuberculosis in rabbits and the environment: How hot is a hot spot? Appl. Environ. Microbiol. 2005, 71, 6033–6038. [Google Scholar]
- Rolle, M.; Mayr, A. Medical Microbiology and Epidemiology (Medizinische Mikrobiologie, Infektions- und Seuchenlehre); Enke: Stuttgart, Germany, 2007; Volume 8. [Google Scholar]
- Tiwari, A.; VanLeeuwen, J.A.; McKenna, S.L.B.; Keefe, G.P.; Barkema, H.W. Johne’s disease in Canada Part I: Clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds. Can. Vet. J. 2006, 47, 874–882. [Google Scholar] [PubMed]
- Streeter, R.N.; Hoffsis, G.F.; Bech-Nielsen, S.; Shulaw, W.P.; Rings, D.M. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 1995, 56, 1322–1324. [Google Scholar]
- Khol, J.L.J.; Kralik, P.P.; Slana, I.I.; Beran, V.V.; Aurich, C.C.; Baumgartner, W.W.; Pavlik, I.I. Consecutive excretion of Mycobacterium avium subspecies paratuberculosis in semen of a breeding bull compared to the distribution in feces, tissue and blood by IS900 and F57 quantitative real-time PCR and culture examinations. J. Vet. Med. Sci. Jpn. Soc. Vet. Sci. 2010, 72, 1283–1288. [Google Scholar] [CrossRef]
- Buergelt, C.D.; Donovan, G.A.; Williams, J.E. Identification of Mycobacterium avium subspecies paratuberculosis by Polymerase Chain Reaction in Blood and Semen of a Bull with Clinical Paratuberculosis. Int. J. Appl. Res. Vet. Med. 2004, 2, 130–134. [Google Scholar]
- Ayele, W.Y.; Bartos, M.; Svastova, P.; Pavlik, I. Distribution of Mycobacterium avium subsp. paratuberculosis in organs of naturally infected bull-calves and breeding bulls. Vet. Microbiol. 2004, 103, 209–217. [Google Scholar]
- Larsen, A.B.; Stalheim, O.H.V.; Hughes, D.E.; Appell, L.H.; Richards, W.D.; Himes, E.M. Mycobacterium paratuberculosis in the Semen and Genital Organs of a Semen-Donor Bull. J. Am. Vet. Med. Assoc. 1981, 179, 169–171. [Google Scholar] [PubMed]
- Larsen, A.B.; Kopecky, K.E. Mycobacterium paratuberculosis in reproductive organs and semen of bulls. Am.J. Vet. Res. 1970, 31, 255–258. [Google Scholar] [PubMed]
- Sorge, U.S.; Kurnick, S.; Sreevatsan, S. Detection of Mycobacterium avium subspecies paratuberculosis in the saliva of dairy cows: A pilot study. Vet. Microbiol. 2013, 164, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.A.; Whittington, R.J. Evidence for age susceptibility of cattle to Johne’s disease. Vet. J. 2010, 184, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.M.; Medley, G.F.; Collins, M.T.; Schukken, Y.H. A meta-analysis of the effect of dose and age at exposure on shedding of Mycobacterium avium subspecies paratuberculosis (MAP) in experimentally infected calves and cows. Epidemiol. Infect. 2011, 28, 1–16. [Google Scholar]
- Merkal, R.S.; Miller, J.M.; Hintz, A.M.; Bryner, J.H. Intrauterine inoculation of Mycobacterium paratuberculosis into guinea pigs and cattle. Am. J. Vet. Res. 1982, 43, 676–678. [Google Scholar] [PubMed]
- Owen, W.J.; Thoen, C.O. Experimental exposure of cattle to Mycobacterium paratuberculosis orally and intrauterine with attempted culture of the organs and detection of humoral antibodies. Proc. USA Health Assoc. 1983, 87, 570–581. [Google Scholar]
- Wells, S.J.; Wagner, B.A. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. JAVMA 2000, 219, 1450–1457. [Google Scholar] [CrossRef]
- Tiwari, A.; VanLeeuwen, J.A.; Dohoo, I.R.; Keefe, G.P.; Haddad, J.P.; Scott, H.M.; Whiting, T. Risk factors associated with Mycobacterium avium subspecies paratuberculosis seropositivity in Canadian dairy cows and herds. Prev. Vet. Med. 2009, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wraight, M.D.; McNeil, J.; Beggs, D.S.; Greenall, R.K.; Humphris, T.B.; Irwin, R.J.; Jagoe, S.P.; Jemmeson, A.; Morgan, W.F.; Brightling, P.; et al. Compliance of Victorian dairy farmers with current calf rearing recommendations for control of Johne’s disease. Vet. Microbiol. 2000, 77, 429–442. [Google Scholar] [CrossRef]
- Marcé, C.; Ezanno, P.; Seegers, H.; Pfeiffer, D.U.; Fourichon, C. Within-herd contact structure and transmission of Mycobacterium avium subspecies paratuberculosis in a persistently infected dairy cattle herd. Prev. Vet. Med. 2011, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Diéguez, F.J.; Arnaiz, I.; Sanjuán, M.L.; Vilar, M.J.; Yus, E. Management practices associated with Mycobacterium avium subspecies paratuberculosis infection and the effects of the infection on dairy herds. Vet. Rec. 2008, 162, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Van Roermund, H.J.W.; Bakker, D.; Willemsen, P.T.J.; de Jong, M.C.M. Horizontal transmission of Mycobacterium avium subsp. paratuberculosis in cattle in an experimental setting: Calves can transmit the infection to other calves. Vet. Microbiol. 2007, 122, 270–279. [Google Scholar]
- Nielsen, S.S.; Bjerre, H.; Toft, N. Colostrum and Milk as Risk Factors for Infection with Mycobacterium avium subspecies paratuberculosis in Dairy Cattle. J. Dairy Sci. 2008, 91, 4610–4615. [Google Scholar] [CrossRef] [PubMed]
- Pithua, P.; Godden, S.M.; Wells, S.J.; Oakes, M.J. Efficacy of feeding plasma-derived commercial colostrum replacer for the prevention of transmission of Mycobacterium avium subsp paratuberculosis in Holstein calves. J. Am. Vet. Med. Assoc. 2009, 234, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Muskens, J.; Elbers, A.R.W.; Van Weering, H.J.; Noordhuizen, J.P.T.M. Herd Management Practices Associated with Paratuberculosis Seroprevalence in Dutch Dairy Herds. J. Vet. Med. Ser. B 2003, 50, 372–377. [Google Scholar] [CrossRef]
- Word Organisation for Animal Health (OIE). Paratuberculosis (Johne’s disease). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th ed.; Word Organization for Animal Health: Paris, France, 2012; Volume 1, pp. 276–291. [Google Scholar]
- Clark, D.L., Jr.; Koziczkowski, J.J.; Radcliff, R.P.; Carlson, R.A.; Ellingson, J.L.E. Detection of Mycobacterium avium Subspecies paratuberculosis: Comparing Fecal Culture Versus Serum Enzyme-Linked Immunosorbent Assay and Direct Fecal Polymerase Chain Reaction. J. Dairy Sci. 2008, 91, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, S.; Duffield, T.; Kelton, D.; Leslie, K.; Lissemore, K.; Archambault, M. Evaluation of enzyme-linked immunosorbent assays performed on milk and serum samples for detection of paratuberculosis in lactating dairy cows. J. Am. Vet. Med. Assoc. 2005, 226, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Ersbøll, A.K. Age at Occurrence of Mycobacterium avium Subspecies paratuberculosis in Naturally Infected Dairy Cows. J. Dairy Sci. 2006, 89, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.F.; Kogut, J.; de Bree, J.; van Schaik, G.; Nielen, M. Age at which dairy cattle become Mycobacterium avium subsp. paratuberculosis faecal culture positive. Prev. Vet. Med. 2010, 97, 29–36. [Google Scholar] [CrossRef]
- Collins, M.T.; Wells, S.J.; Petrini, K.R.; Collins, J.E.; Schultz, R.D.; Whitlock, R.H. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin. Diagn. Lab. Immunol. 2005, 12, 685–692. [Google Scholar] [PubMed]
- McKenna, S.L.B.; Keefe, G.P.; Barkema, H.W.; Sockett, D.C. Evaluation of three ELISAs for Mycobacterium avium subsp. paratuberculosis using tissue and fecal culture as comparison standards. Vet. Microbiol. 2005, 110, 105–111. [Google Scholar]
- Milner, A.R.; Mack, W.N.; Coates, K.J.; Hill, J.; Gill, I.; Sheldrick, P. The sensitivity and specificity of a modified ELISA for the diagnosis of Johne’s disease from a field trial in cattle. Vet. Microbiol. 1990, 25, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.H.; Wells, S.J.; Sweeney, R.W.; Van Tiem, J. ELISA and fecal culture for paratuberculosis (Johne’s disease): Sensitivity and specificity of each method. Vet. Microbiol. 2000, 77, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Lavers, C.; Keefe, G.P.; Mckenna, S.; Chaffer, M. Evaluation of environmental culture to determine herd status for Johne’s disease on Atlantic Canadian dairy farms. In Proceedings of the 11th International Colloquium on Paratuberculosis (11ICP), Sydney, Australia, 5–10 February 2012; pp. 52–53.
- Lombard, J.E.; Wagner, B.A.; Smith, R.L.; McCluskey, B.J. Evaluation of Environmental Sampling and Culture to Determine Mycobacterium avium subspecies paratuberculosis Distribution and Herd Infection Status on US Dairy Operations. J. Dairy Sci. 2006, 89, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Thamsborg, S.M.; Houe, H.; Bitsch, V. Bulk-tank milk ELISA antibodies for estimating the prevalence of paratuberculosis in Danish dairy herds. Prev. Vet. Med. 2000, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Groenendaal, H.; Nielen, M.; Hesselink, J.W. Development of the Dutch Johne’s disease control program supported by a simulation model. Prev. Vet. Med. 2003, 60, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Barkema, H.W.; Fecteau, G.; Keefe, G.P.; Kelton, D.F. Johne’s Disease Control in Canada-Coordinated Nationally-Delivered Provincially. In Proceedings of the 3rd ParaTB Forum, Sydney, Australia, 4 February 2012.
- Barker, R. Optimising Canadian Dairy Farm Biosecurity-Leveraging Lessons Learned from the Canadian Johne’s Disease Initiative (CJDI: 2010–2013); Canadian Animal Health Coalition (CJDI): Guelph, ON, Canada, 2013; unpublished work. [Google Scholar]
- VanLeeuwen, J.A.; Keefe, G.P.; Tremblay, R.; Power, C.; Wichtel, J.J. Seroprevalence of infection with Mycobacterium avium subspecies paratuberculosis, bovine leukemia virus, and bovine viral diarrhea virus in maritime Canada dairy cattle. Can. Vet. J. 2001, 42, 193–198. [Google Scholar] [PubMed]
- Atlantic Johne’s Disease Initiative. Johne’s Control Program Reaches Milestone. Available online: http://www.atlanticjohnes.ca/files/documents/InsertSpring2012V1.pdf (accessed on 24 April 2014).
- Ministère de l’Agriculture des Pêcheries et de l’Alimentation (MAPAQ). Paratuberculose. Available online: http://www.mapaq.gouv.qc.ca/fr/Productions/santeanimale/maladiesanimales/paratuberculose/Pages/paratuberculose.aspx (accessed on 24 April 2014).
- Hendrick, S.; Duffield, T.; Leslie, K.; Lissemore, K.; Archambault, M.; Kelton, D. The prevalence of milk and serum antibodies to Mycobacterium avium subspecies paratuberculosis in dairy herds in Ontario. Can. Vet. J. 2005, 46, 1126–1129. [Google Scholar] [PubMed]
- Ontario Johne’s Education and Management Assistance Program. Program Results. Available online: http://www.johnes.ca/program%20results.htm (accessed on 24 April 2014).
- VanLeeuwen, J.A.; Tiwari, A.; Plaizier, J.C.; Whiting, T.L. Seroprevalences of antibodies against bovine leukemia virus, bovine viral diarrhea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in beef and dairy cattle in Manitoba. Can. Vet. J. 2006, 47, 783–786. [Google Scholar] [PubMed]
- VanLeeuwen, J.A.; Forsythe, L.; Tiwari, A.; Chartier, R. Seroprevalence of antibodies against bovine leukemia virus, bovine viral diarrhea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in dairy cattle in Saskatchewan. Can. Vet. J. 2005, 46, 56–58. [Google Scholar] [PubMed]
- Sorensen, O.; Rawluk, S.; Wu, J.; Manninen, K.; Ollis, G. Mycobacterium paratuberculosis in dairy herds in Alberta. Can. Vet. J. 2003, 44, 221–226. [Google Scholar] [PubMed]
- Scott, H.M.; Sorensen, O.; Wu, J.T.; Chow, E.Y.; Manninen, K.; VanLeeuwen, J.A. Seroprevalence of Mycobacterium avium subspecies paratuberculosis, Neospora caninum, Bovine leukemia virus, and Bovine viral diarrhea virus infection among dairy cattle and herds in Alberta and agroecological risk factors associated with seropositivity. Can. Vet. J. 2006, 47, 981–991. [Google Scholar] [PubMed]
- Alberta Johne’s Disease Initiative. Johne’s Disease-What’s the Scoop? Available online: http://www.albertajohnes.ca/JDabout/MT_1401a.pdf (accessed on 24 April 2014).
- Kijlstra, A.; Eijck, I. Animal health in organic livestock production systems: A review. Njas-Wageningen J. Life Sci. 2006, 54, 77–94. [Google Scholar] [CrossRef]
- Ramanantoanina, F.; Francoz, D.; Côté, G.; Labrecque, O.; Gagnon, A.C.; Beauchamp, G.; Fecteau, G. Seroprevalence of Mycobacterium avium subsp. Paratuberculosis, Neospora caninum, bovine viral diarrhea and infectious bovine rhinotracheitis viruses in Québec organic herds. In Proceedings of the XXVII World Buiatrics Congress, Lissabon, Portugal, 3–8 June 2012.
- Sorge, U.S. An Overview Over the Organic Dairy Industry in Minnesota. In Proceedings of the 117th Annual Meeting of the Minnesota Veterinary Medical Association, Minneapolis, MN, USA, 6–8 February 2014.
- Zwald, A.G.; Ruegg, P.L.; Kaneene, J.B.; Warnick, L.D.; Wells, S.J.; Fossler, C.; Halbert, L.W. Management practices and reported antimicrobial usage on conventional and organic dairy farms. J. Dairy Sci. 2004, 87, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kaneene, J.B.; Erskine, R.J.; Bartlett, P.C. A comparison of production and management between Wisconsin organic and conventional dairy herds. Livest. Prod. Sci. 2005, 93, 105–115. [Google Scholar] [CrossRef]
- Pieper, L.; Godkin, A.; Sorge, U.; Lissemore, K.; DeVries, T.J.; Kelton, D. Prevalence of Mycobacterium avium subsp. paratuberculosis (MAP) ELISA-Positive Cows and Assessment of MAP Transmission Risk on Organic Dairy Farms in Ontario, Canada. In Proceedings of the 46th Annual Conference of the American Association of Bovine Practitioners (AABP), Milwaukee, WI, USA, 19–21 September 2013; pp. 156–157.
- Stiglbauer, K.E.; Cicconi-Hogan, K.M.; Richert, R.; Schukken, Y.H.; Ruegg, P.L.; Gamroth, M. Assessment of herd management on organic and conventional dairy farms in the United States. J. Dairy Sci. 2013, 96, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Hardeng, F.; Edge, V.L. Mastitis, ketosis, and milk fever in 31 organic and 93 conventional Norwegian dairy herds. J. Dairy Sci. 2001, 84, 2673–2679. [Google Scholar] [CrossRef] [PubMed]
- Sorge, U.S.; Lissemore, K.; Godkin, A.; Hendrick, S.; Wells, S.; Kelton, D. Associations between paratuberculosis milk ELISA result, milk production, and breed in Canadian dairy cows. J. Dairy Sci. 2011, 94, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Jubb, T.F.; Sergeant, E.S.G.; Callinan, A.P.L.; Galvin, J.W. Estimate of the sensitivity of an ELISA used to detect Johne’s disease in Victorian dairy cattle herds. Aust. Vet. J. 2004, 82, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Dhand, N.K.; Eppleston, J.; Whittington, R.J.; Toribio, J.-A.L.M.L. Association of farm soil characteristics with ovine Johne’s disease in Australia. Prev. Vet. Med. 2009, 89, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Grewal, S.K.; Rajeev, S.; Sreevatsan, S.; Michel, F.C., Jr. Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl. Environ. Microbiol. 2006, 72, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk, V.L.; Krause, D.O.; McAllister, T.A.; Buckley, K.E.; Reuter, T.; Hendrick, S.; Ominski, K.H. Assessing the inactivation of Mycobacterium avium subsp. paratuberculosis during composting of livestock carcasses. Appl. Environ. Microbiol. 2013, 79, 3215–3224. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Sorge, U.S.; Molitor, T.; Linn, J.; Gallaher, D.; Wells, S.W. Cow-level association between serum 25-hydroxyvitamin D concentration and Mycobacterium avium subspecies paratuberculosis antibody seropositivity: A pilot study. J. Dairy Sci. 2013, 96, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, B.; Erdogan, H.M.; Morgan, K.L. Relationships between the presence of Johne’s disease and farm and management factors in dairy cattle in England. Prev. Vet. Med. 1997, 32, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and Dormancy of Mycobacterium avium subsp. paratuberculosis in the Environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef]
- Thamsborg, S.M.; Roepstorff, A.; Larsen, M. Integrated and biological control of parasites in organic and conventional production systems. Vet. Parasitol. 1999, 84, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Marsh, I.B.; Reddacliff, L.A. Survival of Mycobacterium avium subsp. paratuberculosis in dam water and sediment. Appl. Environ. Microbiol. 2005, 71, 5304–5308. [Google Scholar] [CrossRef]
- Johnson-Ifearulundu, Y.J.; Kaneene, J.B. Management-related risk factors for M. paratuberculosis infection in Michigan, USA, dairy herds. Prev. Vet. Med. 1998, 37, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.M.; Nielsen, S.S.; Toft, N. Association Between the Presence of Antibodies to Mycobacterium avium subspecies paratuberculosis and Somatic Cell Count. J. Dairy Sci. 2008, 91, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Tavornpanich, S.; Johnson, W.O.; Anderson, R.J.; Gardner, I.A. Herd characteristics and management practices associated with seroprevalence of Mycobacterium avium subsp. paratuberculosis infection in dairy herds. Am. J. Vet. Res. 2008, 69, 904–911. [Google Scholar] [CrossRef]
- Duve, L.R.; Weary, D.M.; Halekoh, U.; Jensen, M.B. The effects of social contact and milk allowance on responses to handling, play, and social behavior in young dairy calves. J. Dairy Sci. 2012, 95, 6571–6581. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, S.H.; Kelton, D.F.; Leslie, K.E.; Lissemore, K.D.; Archambault, M.; Bagg, R.; Dick, P.; Duffield, T.F. Efficacy of monensin sodium for the reduction of fecal shedding of Mycobacterium avium subsp. paratuberculosis in infected dairy cattle. Prev. Vet. Med. 2006, 75, 206–220. [Google Scholar] [CrossRef]
- Hendrick, S.H.; Duffield, T.F.; Leslie, K.E.; Lissemore, K.D.; Archambault, M.; Bagg, R.; Dick, P.; Kelton, D.F. Monensin might protect Ontario, Canada dairy cows from paratuberculosis milk-ELISA positivity. Prev. Vet. Med. 2006, 76, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.P.; Klocke, P.; Butler, G.; Smolders, G.; Nielsen, J.H.; Canever, A.; Leifert, C. Effect of production system, alternative treatments and calf rearing system on udder health in organic dairy cows. Njas-Wageningen J. Life Sci. 2011, 58, 157–162. [Google Scholar] [CrossRef]
- Ruegg, P.L. Management of mastitis on organic and conventional dairy farms. J. Anim. Sci. 2009, 87, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Richert, R.M.; Cicconi, K.M.; Gamroth, M.J.; Schukken, Y.H.; Stiglbauer, K.E.; Ruegg, P.L. Management factors associated with veterinary usage by organic and conventional dairy farms. J. Am. Vet. Med. Assoc. 2013, 242, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Kelton, D.; Perkins, N.; Godkin, A.; MacNaughton, G.; Cantin, R.; Hand, K. Comparison of Participants and Non-Participants in a Voluntary Johne’s Disease Control Program in Ontario, Canada. In Proceedings of the 11th International Colloquium on Paratuberculosis (11ICP), Sydney, Australia, 5–10 February 2012.
- Thompson-Crispi, K.A.; Hine, B.; Quinton, M.; Miglior, F.; Mallard, B.A. Short communication: Association of disease incidence and adaptive immune response in Holstein dairy cows. J. Dairy Sci. 2012, 95, 3888–3893. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, P.J.; Donovan, G.A.; Rae, O.; de la Paz, J. Association between Paratuberculosis Infection and General Immune Status in Dairy Cattle. In Proceedings of the 10th International Colloquium for Paratuberculosis, Minneapolis, MN, USA, 9–14 August 2009.
- Van Hulzen, K.J.E.; Koets, A.P.; Nielen, M.; Heuven, H.C.M.; van Arendonk, J.A.M.; Klinkenberg, D. The effect of genetic selection for Johne’s disease resistance in dairy cattle: Results of a genetic-epidemiological model. J. Dairy Sci. 2014, 97, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; VanLeeuwen, J.A.; Weersink, A.; Keefe, G.P. Management factors related to seroprevalences to bovine viral-diarrhoea virus, bovine-leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in dairy herds in the Canadian Maritimes. Prev. Vet. Med. 2002, 55, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Glawischnig, W.; Awad-Masalmeh, M.; Khaschabi, D.; Schoenbauer, M. [Detection fo Mycobacterium avium subsp. paratuberculosis from the testicles of a clinically infected breeding bull]. Berl. Muench. Tieraerztl. Wschr. 2004, 117, 136–139. (In German) [Google Scholar]
- Lombard, J.E.; Byrem, T.M.; Wagner, B.A.; McCluskey, B.J. Comparison of milk and serum enzyme-linked immunosorbent assays for diagnosis of Mycobacterium avium subspecies paratuberculosis infection in dairy cattle. J. Vet. Diagn. Invest. 2006, 18, 448–458. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieper, L.; Sorge, U.; Godkin, A.; DeVries, T.; Lissemore, K.; Kelton, D. Management Practices and Their Potential Influence on Johne’s Disease Transmission on Canadian Organic Dairy Farms—A Conceptual Analysis. Sustainability 2014, 6, 8237-8261. https://doi.org/10.3390/su6118237
Pieper L, Sorge U, Godkin A, DeVries T, Lissemore K, Kelton D. Management Practices and Their Potential Influence on Johne’s Disease Transmission on Canadian Organic Dairy Farms—A Conceptual Analysis. Sustainability. 2014; 6(11):8237-8261. https://doi.org/10.3390/su6118237
Chicago/Turabian StylePieper, Laura, Ulrike Sorge, Ann Godkin, Trevor DeVries, Kerry Lissemore, and David Kelton. 2014. "Management Practices and Their Potential Influence on Johne’s Disease Transmission on Canadian Organic Dairy Farms—A Conceptual Analysis" Sustainability 6, no. 11: 8237-8261. https://doi.org/10.3390/su6118237




