Connectivity Predicts Presence but Not Population Density in the Habitat-Specific Mountain Lizard Iberolacerta martinezricai
Abstract
:1. Introduction
2. Materials and Methods
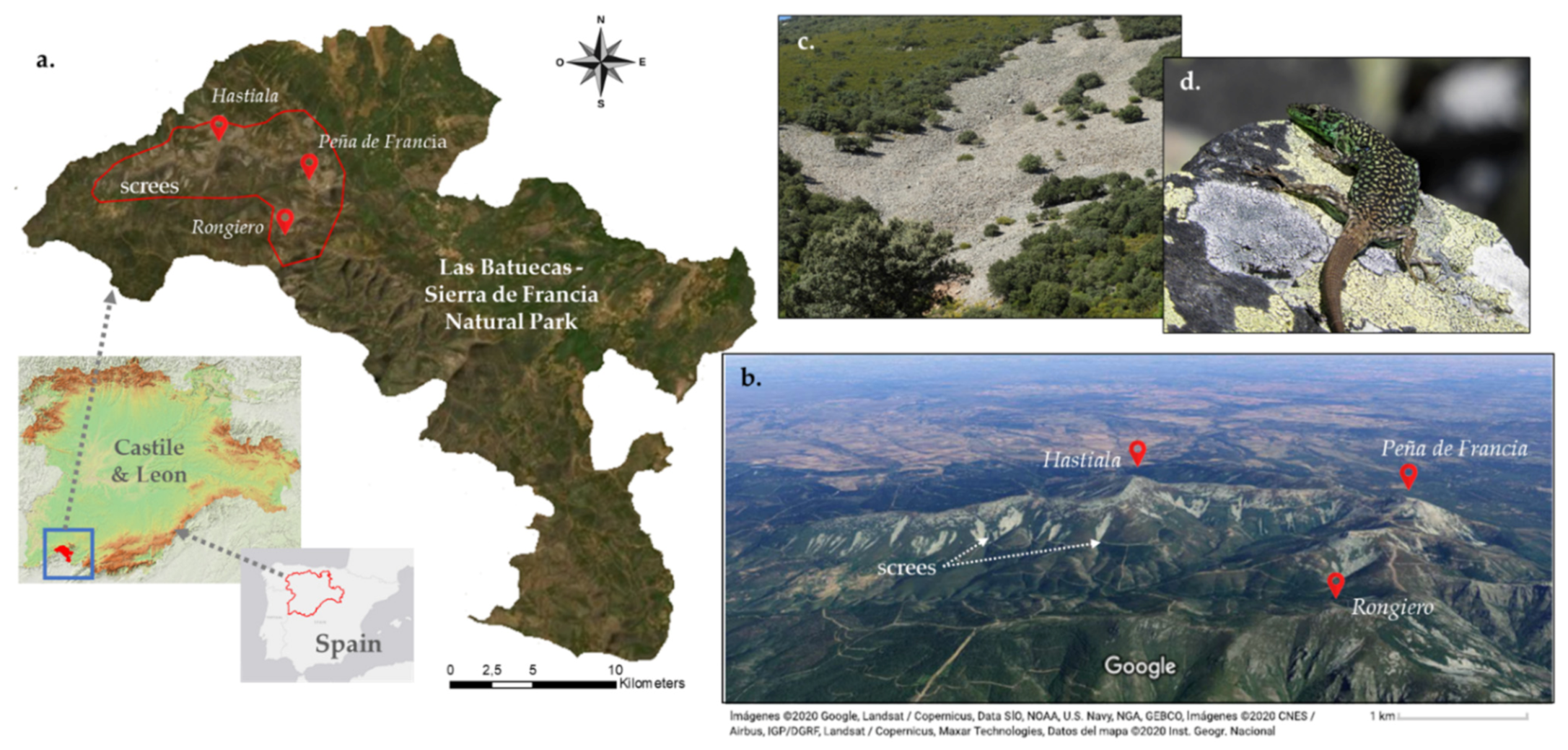
2.1. Study Area
2.2. Focal Species
2.3. Field Surveys and Density Estimation
2.4. Connectivity Analysis for I. martinezricai
2.5. Presence and Density Models for I. martinezricai
3. Results
Sampling Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Sinervo, B.; Mendez De La Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagran Santa Cruz, M.; Lara Resendiz, R.; Martinez Mendez, N.; Calderon Espinosa, M.L.; Meza Lazaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Foden, W.B.; Young, B.E.; Akçakaya, H.R.; Garcia, R.A.; Hoffmann, A.A.; Stein, B.A.; Thomas, C.D.; Wheatley, C.J.; Bickford, D.; Carr, J.A.; et al. Climate change vulnerability assessment of species. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, e551. [Google Scholar] [CrossRef] [Green Version]
- Aragón, P.; Rodríguez, M.A.; Olalla-Tárraga, M.A.; Lobo, J.M. Predicted impact of climate change on threatened terrestrial vertebrates in central Spain highlights differences between endotherms and ectotherms. Anim. Conserv. 2010, 13, 363–373. [Google Scholar] [CrossRef]
- Burraco, P.; Orizaola, G.; Monaghan, P.; Metcalfe, N.B. Climate change and ageing in ectotherms. Glob. Chang. Biol. 2020, 26, 5371–5381. [Google Scholar] [CrossRef]
- Maggini, R.; Lehmann, A.; Kéry, M.; Schmid, H.; Beniston, M.; Jenni, L.; Zbinden, N. Are Swiss birds tracking climate change? Detecting elevational shifts using response curve shapes. Ecol. Modell. 2011, 222, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Donelson, J.M.; Sunday, J.M.; Figueira, W.F.; Gaitán-Espitia, J.D.; Hobday, J.A.; Johnson, C.R.; Leis, J.M.; Ling, S.D.; Marshall, D.; Pandolfi, J.M.; et al. Understanding interactions between plasticity, adaptation, and range shifts in response to marine environmental change. Phil. Trans. R. Soc. B 2019, 374, 20180186. [Google Scholar] [CrossRef] [Green Version]
- Aguado, S.; Braña, F. Thermoregulation in a cold-adapted species (cyren’s rocklizard, Iberolacerta cyreni): Influence of thermal environment and associated costs. Can. J. Zool. 2014, 92, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, J.A.; Jess, M.; Williams, S.E. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology, and adaptation. Phil. Trans. R. Soc. B 2012, 367, 1665–1679. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.M.; Stimola, M.A.; Algar, A.C.; Conover, A.; Rodriguez, A.J.; Landestoy, M.A.; Bakken, G.S.; Losos, J.B. Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Phil. Trans. R. Soc. B 2014, 281, 20132433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunderson, A.R.; Stillman, J.H. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Phil. Trans. R. Soc. B 2015, 282, 20150401. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.B.; Ehrenberger, J.C.; Angilletta, M.J. Thermoregulatory behaviour limits local adaptation of thermal niches and confers sensitivity to climate change. Funct. Ecol. 2015, 29, 1038–1047. [Google Scholar] [CrossRef]
- Berg, M.P.; Kiers, E.T.; Driessen, G.; Van Der Heijden, M.; Kooi, B.W.; Kuenen, F.; Liefting, M.; Verhoef, H.A.; Ellers, J. Adapt or disperse: Understanding species persistence in a changing world. Glob. Chang. Biol. 2010, 16, 587–598. [Google Scholar] [CrossRef]
- McCain, C.M. Global analysis of reptile elevational diversity. Glob. Ecol. Biogeogr. 2010, 19, 541–553. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. Are mountain habitats becoming more suitable for generalist than cold-adapted lizards thermoregulation? PeerJ 2016, 4, e2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonero, J.; García-Díaz, P.; Ávila, C.; Arribas, O.; Lizana, M. Distribution, habitat characterization and conservation status of Iberolacerta martinezricai (ARRIBAS, 1996), in the Sierra de Francia, Salamanca. Spain (Squamata: Sauria: Lacertilidae). Herpetozoa 2016, 28, 149–165. [Google Scholar]
- Araújo, M.B.; Guilhaumon, F.; Neto, D.R.; Pozo, I.; Calmaestra, R. Impactos, Vulnerabilidad y Adaptación al Cambio Climático de la Biodiversidad Española. Fauna de Vertebrados; Dirección General de Medio Natural y Política Forestal: Madrid, Spain, 2011. [Google Scholar]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from climate change to terrestrial vertebrate hotspots in Europe. PLoS ONE 2013, 8, e2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogués-Bravo, D.; Araújo, M.B.; Lasanta, T.; Moreno, J.L. Climate change in Mediterranean mountains during the 21st century. Ambio 2008, 37, 280–285. [Google Scholar] [CrossRef]
- Moreno-Rueda, G.; Pleguezuelos, J.M.; Pizarro, M.; Montori, A. Northward shifts of the distributions of Spanish reptiles in association with climate change. Conserv. Biol. 2012, 26, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos, J.M. Vulnerabilidad de los reptiles ibéricos al cambio climático. In Los Bosques y la Biodiversidad Frente al Cambio Climático: Impactos, Vulnerabilidad y Adaptación en España; Herrero, A., Zavala, M.A., Eds.; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2015; pp. 143–151. [Google Scholar]
- Arribas, O. New data on the Peña de Francia Mountain Lizard ‘Lacerta’ cyreni martinezricai. Arribas, 1996. Herpetozoa 1999, 12, 119–128. [Google Scholar]
- Elsen, P.R.; Monahan, W.B.; Merenlender, A.M. Topography and human pressure in mountain ranges alter expected species responses to climate change. Nat. Commun. 2020, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.F.; Wood, J.T.; Lindenmayer, D.B. The effects of winter recreation on alpine and subalpine fauna: A systematic review and meta-analysis. PLoS ONE 2013, 8, e64282. [Google Scholar] [CrossRef]
- Courtney, L.; Sarah, E.; Reed, S.E.; Merenlender, A.M.; Crooks, K.R. A meta-analysis of recreation effects on vertebrate species richness and abundance. Conserv. Sci. Pract. 2019, 1, e93. [Google Scholar]
- Amo, L.; Lopez, P.; Martin, J. Habitat deterioration affects body condition of lizards: A behavioral approach with Iberolacerta cyreni lizards inhabiting ski resorts. Biol. Conserv. 2007, 135, 77–85. [Google Scholar] [CrossRef]
- Sato, C.F.; Wood, J.T.; Schroder, M.; Green, K.; Osborne, W.S.; Michael, D.R.; Lindenmayer, D.B. An experiment to test key hypotheses of the drivers of reptile distribution in subalpine ski resorts. J. Appl. Ecol. 2014, 51, 13–22. [Google Scholar] [CrossRef]
- Arribas, O.J. Thermoregulation, activity and microhabitat selection in the rare and endangered Batuecan Rock Lizard, Iberolacerta martinezricai (Arribas, 1996) (Squamata: Sauria: Lacertidae). Herpetozoa 2013, 26, 77–90. [Google Scholar]
- Meek, P. Patterns of reptile road-kills in the Vendée region of western France. Herpetol. J. 2009, 19, 135–142. [Google Scholar]
- Gonçalves, L.O.; Alvares., D.J.; Teixeira, F.Z.; Schuck, G.G.; Coelho, I.P.; Esperandio, I.B.; Anza, J.; Beduschi, J.; Bastazini, V.A.G.; Kindel, A. Reptile road-kills in Southern Brazil: Composition, hot moments and hotspots. Sci. Total Environ. 2018, 615, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Colino-Rabanal, V.J.; Lizana, M. Herpetofauna and roads: A review. Basic Appl. Herpetol. 2012, 26, 5–31. [Google Scholar] [CrossRef]
- Arribas, O. Lagartija batueca Iberolacerta martinezricai. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2015; Available online: http://www.vertebradosibericos.org/ (accessed on 15 April 2020).
- Green, K.; Sanecki, G. Immediate and short-term responses of bird and mammal assemblages to a subalpine wildfire in the Snowy Mountains, Australia. Austral Ecol. 2006, 31, 673–681. [Google Scholar] [CrossRef]
- Young, M.E.; Ryberg, W.A.; Fitzgerald, L.A.; Hibbitts, T.J. Fragmentation alters home range and movements of the Dunes Sagebrush Lizard (Sceloporus arenicolus). Can. J. Zool. 2018, 96, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Walkup, D.K.; Leavitt, D.J.; Fitzgerald, L.A. Effects of habitat fragmentation on population structure of dune-dwelling lizards. Ecosphere 2017, 8, e01729. [Google Scholar] [CrossRef] [Green Version]
- Leavitt, D.J.; Fitzgerald, L.A. Disassembly of a dune-dwelling lizard community due to landscape fragmentation. Ecosphere 2013, 4, 97. [Google Scholar] [CrossRef]
- Vega, L.E.; Bellagamba, P.J.; Fitzgerald, L.A. Long-term effects of anthropogenic habitat disturbance on a lizard assemblage inhabiting coastal dunes in Argentina. Can. J. Zool. 2000, 78, 1653–1660. [Google Scholar] [CrossRef]
- Henle, K.; Davies, K.F.; Kleyer, M.; Margules, C.; Settele, J. Predictors of species sensitivity to fragmentation. Biodivers. Conserv. 2004, 13, 207–251. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Fernandez-González, F.; Sánchez, D. El Sistema Central Español. De la sierra de Ayllón a la Serra da Estrela. In La vegetación de España; Peinado, M., Rivas-Martínez, S., Eds.; Universidad de Alcalá: Alcalá de Henares, Madrid, Spain, 1987; pp. 419–452. [Google Scholar]
- Crochet, P.A.; Chaline, O.; Surget-Groba, Y.; Debain, C.; Cheylan, M. Speciation in mountains: Phylogeography and phylogeny of the rock lizards genus Iberolacerta(Reptilia: Lacertidae). Mol. Phylogenet. Evol. 2004, 30, 860–886. [Google Scholar] [CrossRef] [PubMed]
- Carranza, S.; Arnold, E.N.; Amat, F. DNA phylogeny of Lacerta (Iberolacerta) and other lacertine lizards (Reptilia: Lacertidae): Did competition cause long-term mountain restriction? Syst. Biodivers. 2004, 2, 57–77. [Google Scholar] [CrossRef] [Green Version]
- Carbonero, J.; Lizana, M.; García, P.; Arribas, O. Distribución, Estado de Conservación y Medidas de Gestión para la Lagartija Serrana de la Peña de Francia (Iberolacerta martinezricai) en el Parque Natural de Batuecas-sierra de Francia (Unpublished Report); Fundación Patrimonio Natural-Junta de Castilla y León: Salamanca, Spain, 2008. [Google Scholar]
- Pérez-Mellado, V.; Márquez, R.; Martínez-Solano, I. Iberolacerta martinezricai. IUCN Red List of Threatened Species. 2009: E.T61516A12499291. Available online: www.iucnredlist.org (accessed on 12 March 2020).
- Arribas, O.J. Growth, sex-dimorphism and predation pressure in the Batuecan Lizard, Iberolacerta martinezricai (Arribas, 1996). Butllet. Soc. Catal. Herpetol. 2014, 21, 147–173. [Google Scholar]
- Arribas, O.J. Reproductive characteristics of the Batuecan Lizard, Iberolacerta martinezricai (ARRIBAS, 1996) (Squamata: Sauria: Lacertidae). Herpetozoa 2018, 30, 187–202. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling. Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Advanced Distance Sampling. Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.; Laake, J.L.; Rexstad, E.A.; Strindberg, S.; Marques, F.; Buckland, S.; Borchers, D.; Anderson, D.; Burnham, K.; Burt, M. User’s Guide Distance 6.0 Release 2; Research Unit for Wildlife Population Assessment, University of St. Andrews: St. Andrews, UK, 2009. [Google Scholar]
- Taylor, P.D.; Fahrig, L.; With, K.A. Landscape connectivity: A return to the basics. In Connectivity Conservation; Crooks, K.R., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Bennett, G. Integrating Biodiversity Conservation and Sustainable Use: Lessons Learned from Ecological Networks; IUCN: Gland, Switzerland; Cambridge, UK, 2004. [Google Scholar]
- Urban, D.L.; Minor, M.S.; Treml, E.A.; Schick, R.S. Graph models of habitat mosaics. Ecol. Lett. 2009, 12, 260–273. [Google Scholar] [CrossRef]
- Kool, J.T.; Moilanen, A.; Treml, E.A. Population connectivity: Recent advances and new perspectives. Landsc. Ecol. 2013, 28, 165–185. [Google Scholar] [CrossRef]
- Harris, K.M.; Dickinson, K.J.M.; Whigham, P.A. Functional connectivity and matrix quality: Network analysis for a critically endangered New Zealand lizard. Landsc. Ecol. 2014, 29, 41–53. [Google Scholar] [CrossRef]
- Urban, D.; Keitt, T. Landscape connectivity: A graph theoretic perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- Pascual-Hortal, L.; Saura, S. Comparison and development of new graph-based landscape connectivity indices: Towards the priorization of habitat patches and corridors for conservation. Landsc. Ecol. 2006, 21, 959–967. [Google Scholar] [CrossRef]
- Decout, S.; Manel, S.; Miaud, C.; Luque, S. Integrative approach for landscape-based graph connectivity analysis: A case study with the common frog (Rana temporaria) in human-dominated landscapes. Landsc. Ecol. 2012, 27, 267–279. [Google Scholar] [CrossRef]
- Clauzel, C.; Bannwarth, C.; Foltete, J.C. Integrating regional-scale connectivity in habitat restoration: An application for amphibian conservation in eastern France. J. Nat. Conserv. 2015, 23, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Saura, S.; Pascual-Hortal, L. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparising with existing indices and application to a case study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Saura, S.; Torne, J. Conefor Sensinode 2.2: A software package for quantifying the importance of habitat patches for landscape connectivity. Environ. Model. Softw. 2009, 24, 135–139. [Google Scholar] [CrossRef]
- Saura, S.; Rubio, L. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography 2010, 33, 523–537. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; Stevens, G.G.; Kaufman, D.M. The geographic range: Size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 1996, 27, 597–623. [Google Scholar] [CrossRef] [Green Version]
- Hampe, A.; Jump, A.S. Climate Relicts Past, Present, Future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Buckley, L.B.; Jetz, W. Insularity and the determinants of lizard population density. Ecol. Lett. 2007, 10, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Novosolov, M.; Rodda, G.H.; Feldman, A.; Kadison, A.E.; Dor, R.; Meiri, S. Power in numbers. Drivers of high population density in insular lizards. Glob. Ecol. Biogeogr. 2016, 25, 87–95. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Quinn, P.F.; Beven, K.J.; Lamb, R. The ln(a/tan beta) index: How to calculate it and how to use it within the TOPMODEL framework. Hydrol. Process. 1995, 9, 161–182. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Core Team R: Vienna, Austria, 2004. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Introduction, Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1. [Google Scholar] [CrossRef] [Green Version]
- Dibner, R.R.; Doak, D.F.; Murphy, M. Discrepancies in occupancy and abundance approaches to identifying and protecting habitat for an at-risk species. Ecol. Evol. 2017, 7, 5692–5702. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Gaston, K.J. Occupancy–Abundance relationships and sampling scales. Ecography 2000, 23, 503–511. [Google Scholar] [CrossRef]
- Santos, T.; Díaz, J.A.; Pérez-Tris, J.; Carbonell, R.; Tellería, J.L. Habitat quality predicts the distribution of a lizard in fragmented woodlands better than habitat fragmentation. Anim. Conserv. 2008, 11, 46–56. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation Ecology; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Addicott, J.F.; Aho, J.M.; Antolin, M.F.; Padilla, D.K.; Richardson, J.S.; Soluk, D.A. Ecological neighborhoods: Scaling environmental patterns. Oikos 1987, 49, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Ryberg, W.A.; Hill, M.T.; Painter, C.W.; Fitzgerald, L.A. Landscape pattern determines neighborhood size and structure within a lizard population. PLoS ONE 2013, 8, e56856. [Google Scholar] [CrossRef] [Green Version]
- Munguia-Vega, A.; Rodriquez-Estrella, R.; Shaw, W.W.; Culver, M. Localized extinction of an arboreal desert lizard caused by habitat fragmentation. Biol. Conserv. 2013, 157, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Gomes, M.; Duarte Rocha, C.F. Diversity and distribution of lizards in fragmented Atlantic forest landscape in Southeastern Brazil. J. Herpetol. 2014, 48, 423–429. [Google Scholar] [CrossRef]
- Crooks, K.R.; Sanjayan, M.A. Connectivity conservation: Maintaining connections for nature. In Connectivity Conservation; Crooks, K.R., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–20. [Google Scholar]
- Boitani, L.; Falcucci, A.; Maiorano, L.; Rondinini, C. Ecological networks as conceptual frameworks or operational tools in conservation. Conserv. Biol. 2007, 21, 1414–1422. [Google Scholar] [CrossRef]
- Minor, E.S.; Urban, D.L. A graph-theory framework for evaluating landscape connectivity and conservation planning. Conserv. Biol. 2008, 22, 297–307. [Google Scholar] [CrossRef]
- Bodin, O.; Saura, S. Ranking individual habitat patches as connectivity providers: Integrating network analysis and patch removal experiments. Ecol. Modell. 2010, 221, 2393–2405. [Google Scholar] [CrossRef]
- Beninde, J.; Feldmeier, S.; Werner, M.; Peroverde, D.; Schulte, U.; Hochkirch, A.; Veith, M. Cityscape genetics: Structural vs. functional connectivity of an urban lizard population. Mol. Biol. 2016, 25, 4984–5000. [Google Scholar]
- Storfer, A.; Murphy, M.A.; Evans, J.S.; Goldberg, C.S.; Robinson, S.; Spear, S.F.; Dezzani, R.; Delmelle, E.; Vierling, L.; Waits, L.P. Putting the landscape in landscape genetics. Heredity 2007, 98, 128–142. [Google Scholar] [CrossRef]

| Variable | Abbrev. | Description |
|---|---|---|
| Altitude | ALT | Obtained from a 5 metre resolution DEM, developed by the Spanish National Geographic Institute through interpolation from LIDAR data. The minimum (ALTmin), average (ALTave), maximum elevation (ALTmax), and altitudinal range (ALTran) were considered. |
| Slope | SLP | Obtained from the same DEM, expressed in degrees. |
| Orientation | ORI | From the 5 metre DEM using the ArcGIS 10.6 “aspect” tool. Five orientations were defined: North (N), South (S), East (E), West (W), and flat. |
| Probability of connectivity | PC | An indicator of landscape connectivity based on habitat availability, dispersal probabilities between habitat patches and graph structures (see Section Section 2.4). Obtained from SS cartography and CONEFOR software. |
| Topographic wetness index | TWI | Describes the tendency of an area to accumulate water. Obtained from the 5 m DEM using ArcGIS geoprocesses. |
| Lichen cover | LIC | Average coverage of lichens and bryophytes on the rocks. |
| Rock size | ROS | Each SS was classified in relation to the average size of the rock fragments. Three categories were considered: 1 (<50 cm), 2 (50–100 cm), 3 (>100 cm). |
| SS Positive (Average) | SS Negative (Average) | U Mann Whitney | Z | p-Value | |
|---|---|---|---|---|---|
| ALTmin | 1330.1 | 1180.5 | 241.5 | −3.612 | <0.001 |
| ALTmax | 1510.7 | 1344.8 | 189 | −4.319 | <0.001 |
| ALTave | 1421.6 | 1263.9 | 198 | −4.197 | <0.001 |
| ALTran | 180.7 | 164.3 | 463 | −0.632 | 0.527 |
| SLP | 24.77 | 26.50 | 340 | −2.287 | 0.022 |
| TWI | 9.41 | 9.03 | 408 | −1.372 | 0.170 |
| PC10000 | 2.05 | 1.13 | 318 | −2.583 | 0.010 |
| PC1000 | 3.46 | 1.31 | 264 | −3.310 | 0.001 |
| PC500 | 3.61 | 1.30 | 268 | −3.256 | 0.001 |
| PC250 | 3.43 | 1.26 | 287 | −3.001 | 0.003 |
| PC100 | 3.39 | 1.29 | 228 | −3.311 | 0.001 |
| PC50 | 3.53 | 1.26 | 253 | −3.331 | 0.001 |
| PC25 | 3.49 | 1.29 | 228 | −3.211 | 0.001 |
| PCintra * | 0.13 | 0.06 | 318 | −2.583 | 0.010 |
| PCflux * | 2.50 | 0.92 | 262 | −3.336 | 0.001 |
| PCconnector * | 0.98 | 0.32 | 327 | −2.464 | 0.014 |
| LIC | 275.5 | −2.542 | 0.011 | ||
| ROS | 205.5 | −3.334 | 0.001 | ||
| χ2 | d.f. | p-value | |||
| ORI | 3.871 | 3 | 0.276 |
| Candidate Presence Models | ∆AICc | %∑dev | Candidate Density Models | ∆AICc | %∑dev |
|---|---|---|---|---|---|
| ALTave+PC50+ROS+SLP | 0 | 48.29 | LIC+ALTave | 0 | 30.63 |
| ALTave+PC50+ROS+SLP+LIC | 1.63 | ALTave (38.1) | LIC | 0.50 | LIC (85.7) |
| ALTave+PC50+ROS | 3.64 | LIC+ALTave+PC25 | 2.00 | ||
| ALTave+PC50 | 10.35 | LIC+ALTave+PC25+SLP | 3.50 | ||
| ALTave | 20.46 | LIC+ALTave+PC25+SLP+ROS | 5.50 | ||
| PC50 | 22.91 | ALTave | 7.00 | ||
| PC25 | 24.05 | PC25 | 7.00 | ||
| PC100 | 24.29 | SLP | 7.10 | ||
| PC1000 | 25.85 | ORI | 7.10 | ||
| PC500 | 26.12 | PC500 | 7.40 | ||
| PC250 | 26.54 | PC250 | 7.40 | ||
| PC10000 | 26.87 | ROS | 7.40 | ||
| ROS | 27.11 | PC10000 | 7.50 | ||
| SLP | 33.14 | PC100 | 7.50 | ||
| LIC | 34.33 | PC1000 | 7.60 | ||
| ORI | 38.90 | PC50 | 7.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizana-Ciudad, D.; Colino-Rabanal, V.J.; Arribas, Ó.J.; Lizana, M. Connectivity Predicts Presence but Not Population Density in the Habitat-Specific Mountain Lizard Iberolacerta martinezricai. Sustainability 2021, 13, 2647. https://doi.org/10.3390/su13052647
Lizana-Ciudad D, Colino-Rabanal VJ, Arribas ÓJ, Lizana M. Connectivity Predicts Presence but Not Population Density in the Habitat-Specific Mountain Lizard Iberolacerta martinezricai. Sustainability. 2021; 13(5):2647. https://doi.org/10.3390/su13052647
Chicago/Turabian StyleLizana-Ciudad, Diego, Víctor J. Colino-Rabanal, Óscar J. Arribas, and Miguel Lizana. 2021. "Connectivity Predicts Presence but Not Population Density in the Habitat-Specific Mountain Lizard Iberolacerta martinezricai" Sustainability 13, no. 5: 2647. https://doi.org/10.3390/su13052647






