Analysis of the Adaptative Strategy of Cirsium vulgare (Savi) Ten. in the Colonization of New Territories
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Target Species: Cirsium vulgare (Savi) Ten. (Asteraceae)
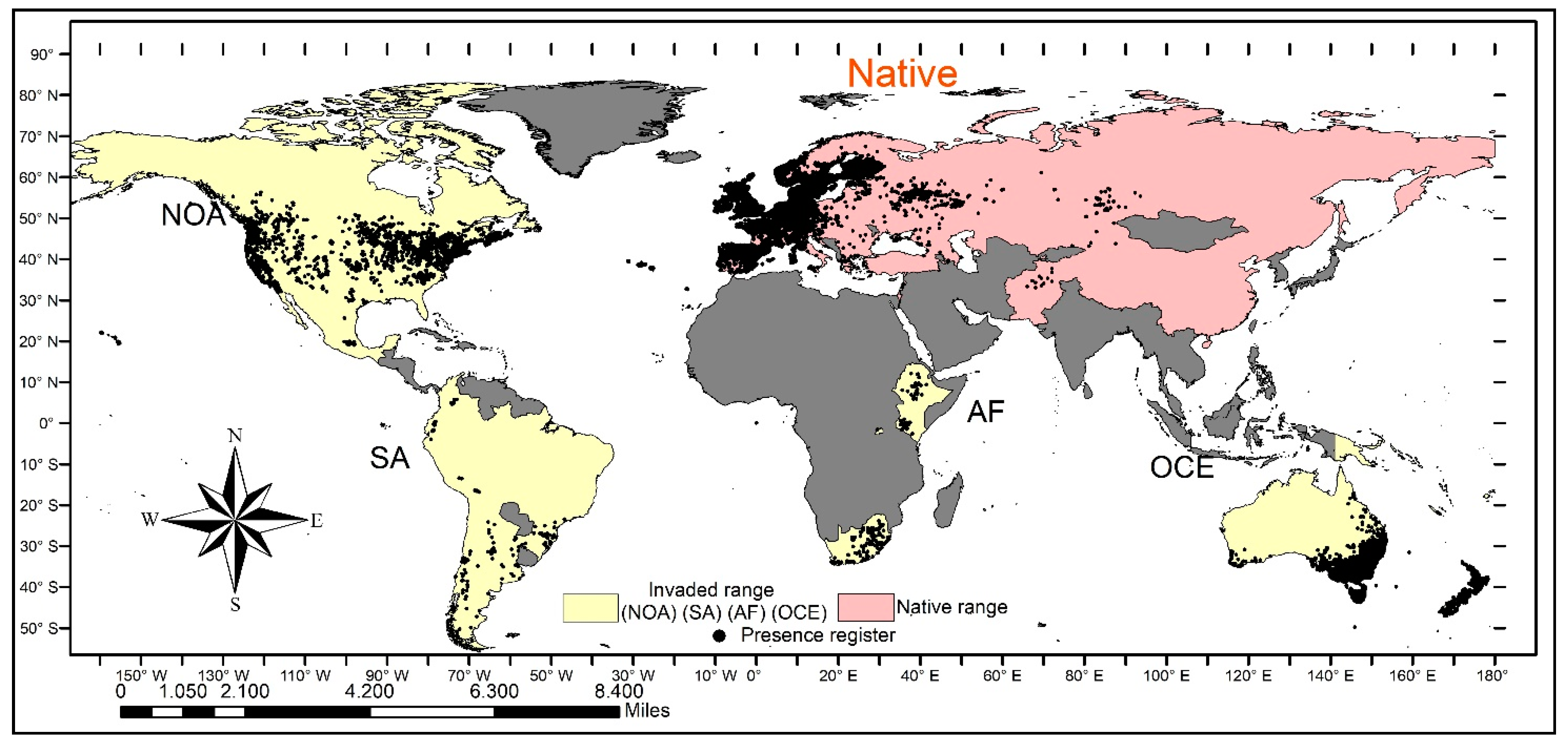
2.2. Occurrence Datasets
2.3. Environmental Variables
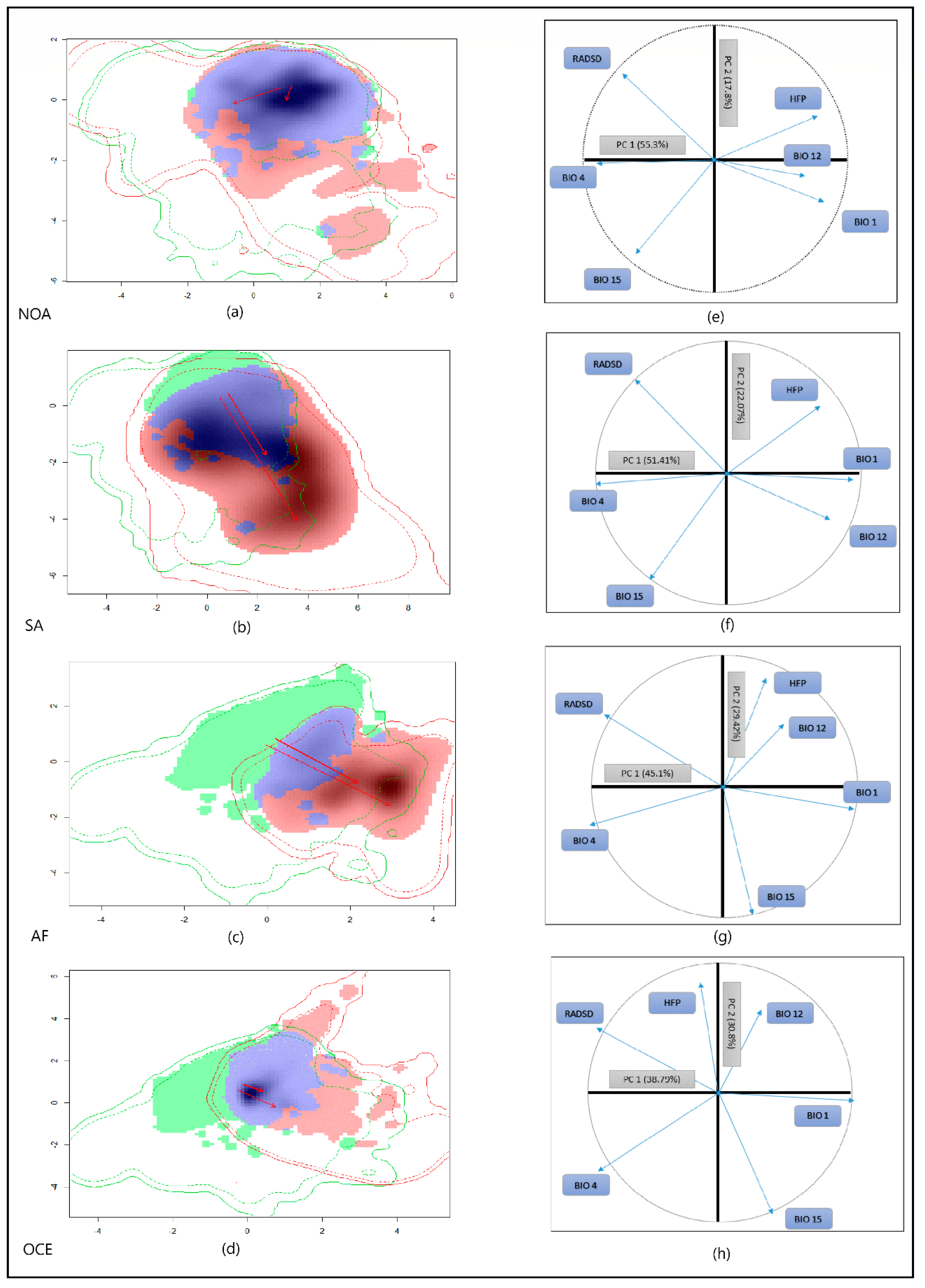
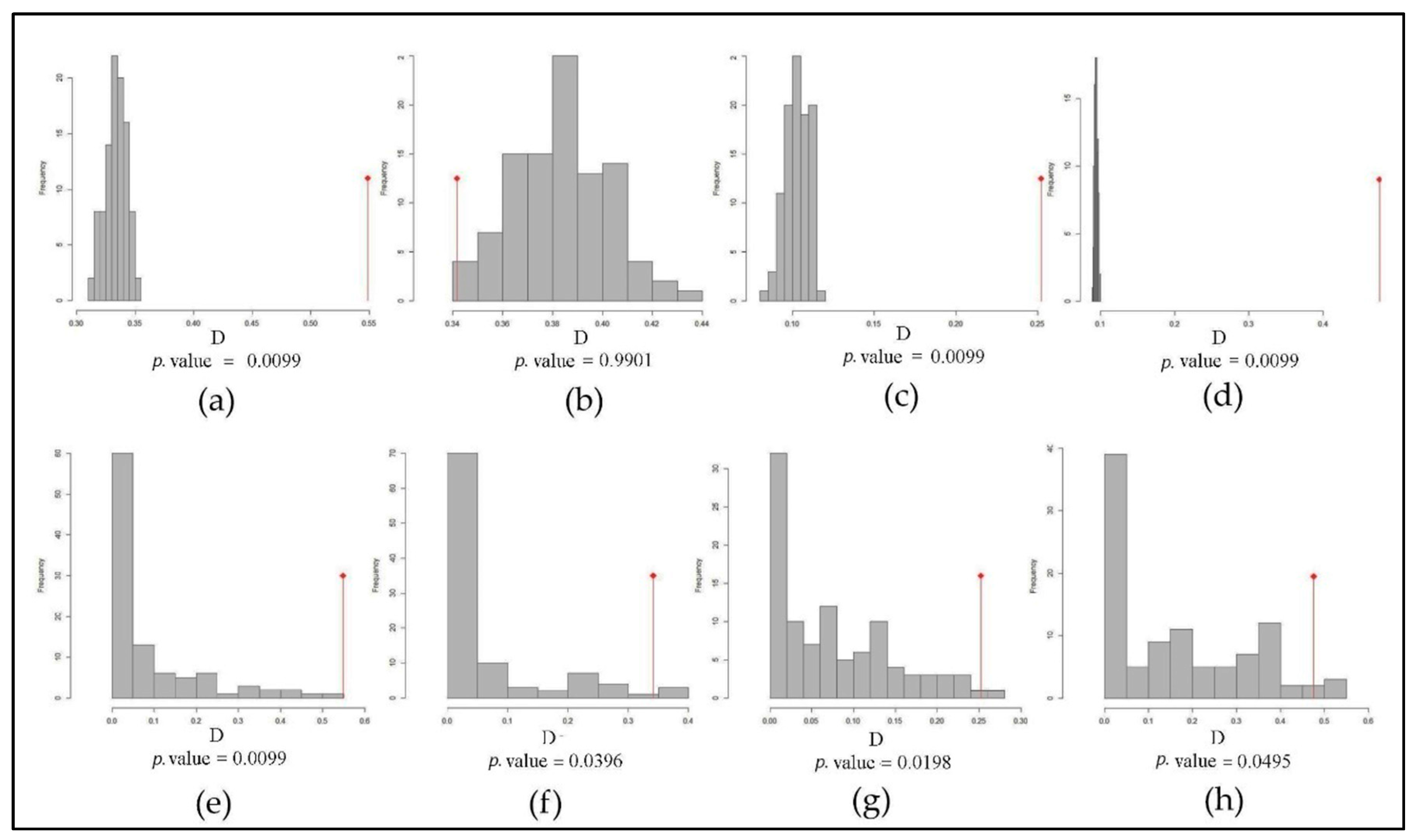
2.4. Niche Shift Measurement
2.5. Reciprocal Ecological Niche Models: Calibration and Evaluation
3. Results
3.1. Environmental Niche Analysis
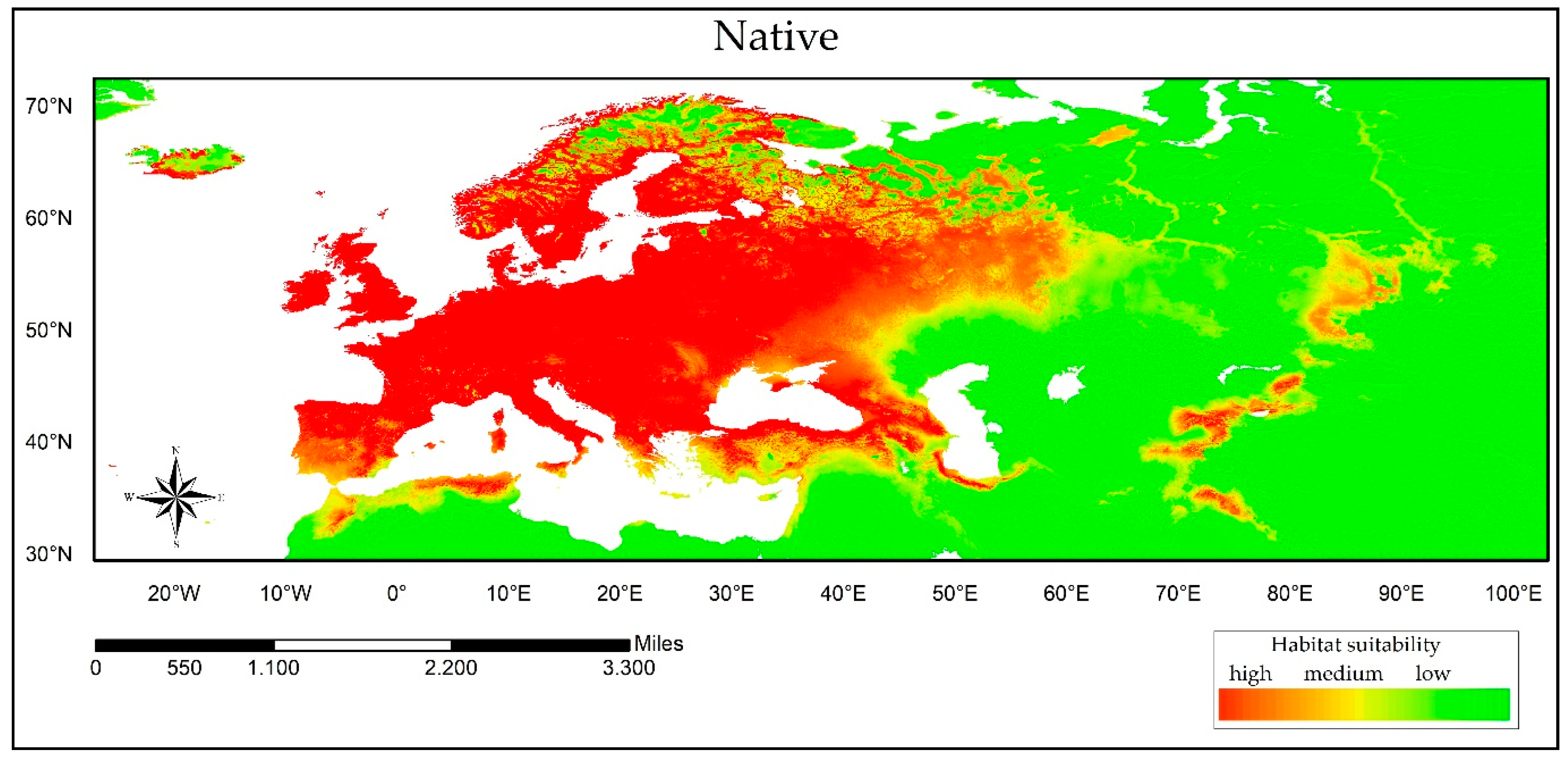
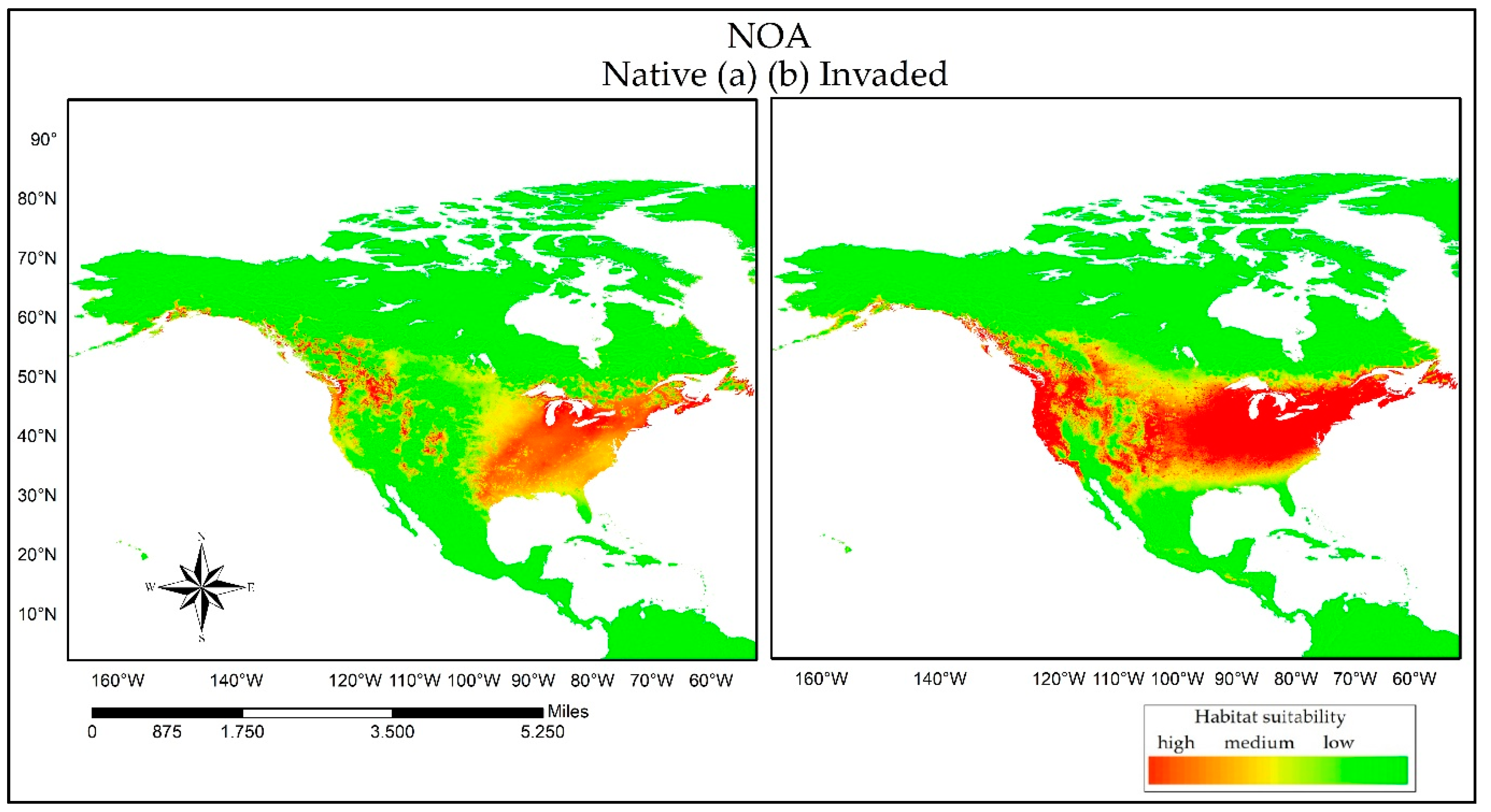
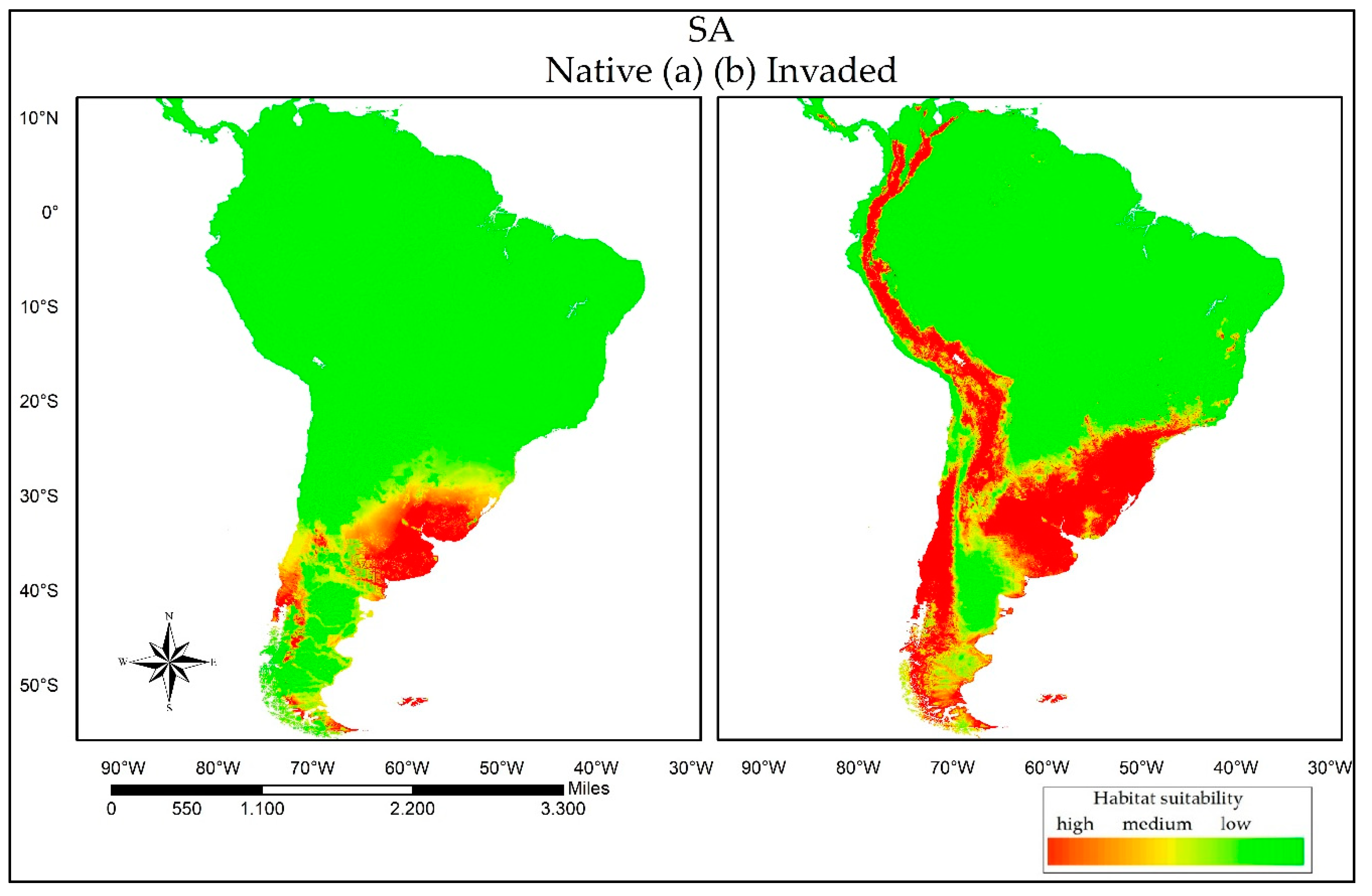
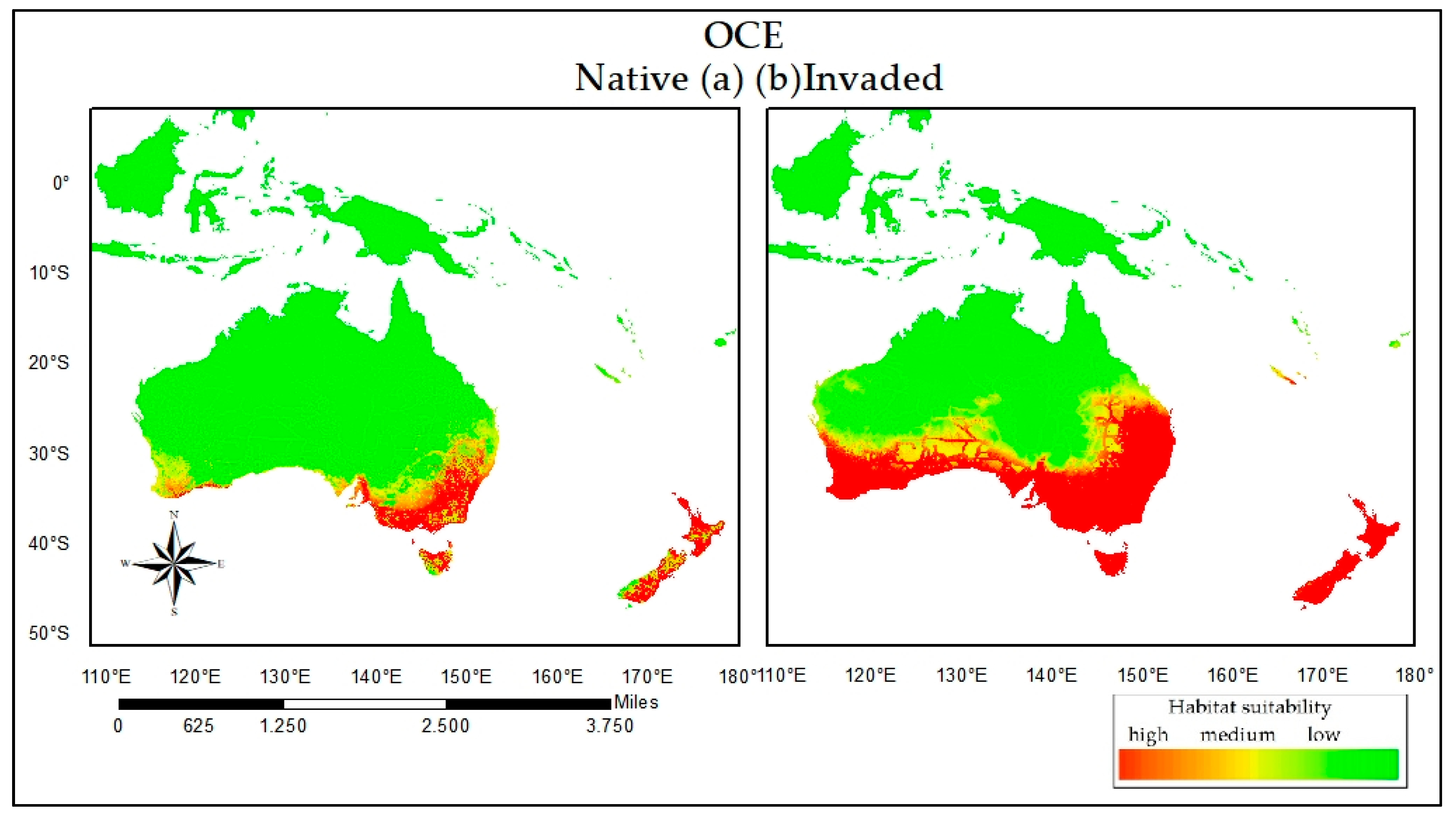
3.2. Reciprocal Ecological Niche Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [Green Version]
- Rybicki, J.; Hanski, I. Species-Area Relationships and Extinctions Caused by Habitat Loss and Fragmentation. Ecol. Lett. 2013, 16, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, J.N. Causas de la pérdida global de biodiversidad. Rev. Asoc. Colomb. Cienc. Biol. 2020, 183–198. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No Saturation in the Accumulation of Alien Species Worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.; Morales-Castilla, I.; Caplat, P.; Early, R. Usefulness of Species Traits in Predicting Range Shifts. Trends Ecol. Evol. 2016, 31, 190–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathakutha, R.; Steyn, C.; le Roux, P.C.; Blom, I.J.; Chown, S.L.; Daru, B.H.; Ripley, B.S.; Louw, A.; Greve, M. Invasive Species Differ in Key Functional Traits from Native and Non-Invasive Alien Plant Species. J. Veg. Sci. 2019, 30, 994–1006. [Google Scholar] [CrossRef]
- Alharbi, W.; Petrovskii, S. Patterns of Invasive Species Spread in a Landscape with a Complex Geometry. Ecol. Complex. 2018, 33, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Lambraño, R.E.; González-Moreno, P.; Sánchez-Agudo, J.Á. Towards the Top: Niche Expansion of Taraxacum Officinale and Ulex Europaeus in Mountain Regions of South America. Austral Ecol. 2017, 42, 577–589. [Google Scholar] [CrossRef]
- Born-Schmidt, G.; De Alba, F.; Servole, J.; Koleff, P. Espaldon, maria victoria Principales Retos Que Enfrenta Mexico Ante Las Especies Exóticas Invasoras; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio), el Programa de las Naciones Unidas para el Desarrollo (PNUD) y el Centro de Estudios Sociales y de Opinión Pública (CESOP): Mexico City, Mexico, 2017. [Google Scholar]
- Zilio, M.I. El Impacto Económico de las Invasiones Biológicas en Argentina: Cuánto Cuesta no Proteger la Biodiversidad; Asociación Argentina de Economía Política (AAEP): Buenos Aires, Argentina, 2019. [Google Scholar]
- Hoffmann, B.; Broadhurst, L. The Economic Cost of Managing Invasive Species in Australia. NeoBiota 2016, 31, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jackson, T. Addressing the Economic Costs of Invasive Alien Species: Some Methodological and Empirical Issues. Int. J. Sustain. Soc. 2015, 7, 221–240. [Google Scholar] [CrossRef]
- Broennimann, O.; Guisan, A. Predicting Current and Future Biological Invasions: Both Native and Invaded Ranges Matter. Biol. Lett. 2008, 4, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.M. Aplicación de Modelos de Nicho Ecológico y Sistemas de Información Geográfica para la Conservación de la Biodiversidad. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2018. Available online: http://purl.org/dc/dcmitype/Text (accessed on 3 April 2020).
- Soberón, J.; Osorio-Olvera, L.; Peterson, T. Diferenias conceptuales entre modelación de nichos y modelación de áreas de distribución. Rev. Mex. Biodivers. 2017, 88, 437–441. [Google Scholar] [CrossRef]
- Mota Vargas, C.; Encarnación Luévano, A.; Ortega Andrade, H.M.; Prieto Torres, D.A.; Peña Peniche, A.; Rojas Soto, O.R. Una Breve Introducción a los Modelos de Nicho Ecológico; Universidad Autónoma del Estado de Hidalgo: Pachuca, Mexico, 2020; ISBN 978-607-482-598-5. [Google Scholar]
- Colwell, R.K.; Rangel, T.F. Hutchinson’s Duality: The Once and Future Niche. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. 2), 19651–19658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, G.E. Concluding Remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, E.; Herrera, I.; Duarte, M.; Bustamante, R.; Lampo, M.; Squez, G.; Sharma, G.; Garcia-Rangel, S. Global Invasion of Lantana Camara: Has the Climatic Niche Been Conserved across Continents? PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battini, N.; Farias, N.; Giachetti, C.; Schwindt, E.; Bortolus, A. Staying Ahead of Invaders: Using Species Distribution Modeling to Predict Alien Species’ Potential Niche Shifts. Mar. Ecol. Prog. Ser. 2019, 612, 127–140. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.; Anderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.; Huettmann, F.; Leathwick, J.; Lehmann, A. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche Dynamics in Space and Time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T. Predicting the Geography of Species’ Invasions via Ecological Niche Modeling. Q. Rev. Biol. 2003, 78, 419–433. [Google Scholar] [CrossRef] [Green Version]
- Carlos-Júnior, L.A.; Barbosa, N.P.U.; Moulton, T.P.; Creed, J.C. Ecological Niche Model Used to Examine the Distribution of an Invasive, Non-Indigenous Coral. Mar. Environ. Res. 2015, 103, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Parravicini, V.; Azzurro, E.; Kulbicki, M.; Belmaker, J. Niche Shift Can Impair the Ability to Predict Invasion Risk in the Marine Realm: An Illustration Using Mediterranean Fish Invaders. Ecol. Lett. 2015, 18, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying Niche Shift Studies: Insights from Biological Invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hierro, J.L.; Maron, J.L.; Callaway, R.M. A Biogeographical Approach to Plant Invasions: The Importance of Studying Exotics in Their Introduced and Native Range. J. Ecol. 2005, 93, 5–15. [Google Scholar] [CrossRef]
- Richardson, D.M.; Allsopp, N.; D’Antonio, C.M.; Milton, S.J.; Rejmánek, M. Plant Invasions—The Role of Mutualisms. Biol. Rev. 2000, 75, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic Niche Shifts Are Rare Among Terrestrial Plant Invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.J.; Frankton, C. The Thistles of Canada; Research Branch, Canada Department of Agriculture: Ottawa, ON, Canada, 1974. [Google Scholar]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia; Inkata Press: Melbourne, VIC, Australia, 1992; ISBN 978-0-909605-81-0. [Google Scholar]
- Mitich, L.W. Bull Thistle, Cirsium vulgare. Weed Technol. 1998, 12, 761–763. [Google Scholar] [CrossRef] [Green Version]
- Cripps, M.; Navukula, J.; Casonato, S.; van Koten, C. Impact of the Gall Fly, Urophora Stylata, on the Pasture Weed, Cirsium Vulgare, in New Zealand. BioControl 2020, 65, 501–513. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring Ecological Niche Overlap from Occurrence and Spatial Environmental Data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef] [Green Version]
- Holm, L.; Doll, J.; Holm, E.; Pancho, J.V.; Herberger, J.P. World Weeds: Natural Histories and Distribution; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Herrera, I.; Goncalves, E.; Pauchard, A.; Bustamante, R.O. Manual de Plantas Invasoras de Sudamérica; IEB Chile, Instituto de Ecología y Biodiversidad: Región de O’Higgins, Chile, 2016. [Google Scholar]
- Petryna, L.; Moora, M.; Nuñes, C.O.; Cantero, J.J.; Zobel, M. Are Invaders Disturbance-Limited? Conservation of Mountain Grasslands in Central Argentina. Appl. Veg. Sci. 2002, 5, 195–202. [Google Scholar] [CrossRef]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO Publishing: Collingwood, VIC, Australia, 2001; ISBN 978-0-643-06514-7. [Google Scholar]
- Klinkhamer, P.G.; De Jong, T.J. Cirsium Vulgare (Savi) Ten. J. Ecol. 1993, 177–191. [Google Scholar] [CrossRef]
- R: El Proyecto R Para Computación Estadística. Available online: https://www.r-project.org/index.html (accessed on 4 February 2021).
- About ArcGIS|Mapping & Analytics Software and Services. Available online: https://www.esri.com/en-us/arcgis/about-arcgis/overview (accessed on 31 January 2021).
- Cola, V.D.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R Package to Support Spatial Analyses and Modeling of Species Niches and Distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of Climatic Niche Shift during Biological Invasion. Ecol. Lett 2007, 10, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- González-Moreno, P.; Delgado, J.; Vilà, M. Una Visión a Escala de Paisaje de Las Invasiones Biológicas. Ecosistemas 2015, 24, 84–92. [Google Scholar] [CrossRef]
- Webber, B.; Le Maitre, D.; Kriticos, D. Comment on “Climatic Niche Shifts Are Rare Among Terrestrial Plant Invaders”. Science 2012, 338, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack of All Trades, Master of Some? On the Role of Phenotypic Plasticity in Plant Invasions-Richards-2006-Ecology Letters-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1461-0248.2006.00950.x (accessed on 15 December 2020).
- Martín, J.S.; Ramírez, C.; Martín, C.S. La flora de las dunas chilenas y sus adaptaciones morfológicas. Bosque 1992, 13, 29–39. [Google Scholar] [CrossRef]
- Sheidai, M.; Zanganeh, S.; Haji-Ramezanali, R.; Nouroozi, M.; Noormohammadi, Z.; Ghsemzadeh-Baraki, S. Genetic Diversity and Population Structure in Four Cirsium (Asteraceae) Species. Biologia 2013, 68. [Google Scholar] [CrossRef]
- Suwa, T.; Louda, S.M.; Leland Russell, F. No Interaction between Competition and Herbivory in Limiting Introduced Cirsium Vulgare Rosette Growth and Reproduction. Oecologia 2010, 162, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyo, C.; Harrington, K.C.; Kemp, P.D. Effectiveness of Spraying Herbicides in the Centre Compared to All over Rosettes of Cirsium Vulgare and Jacobaea Vulgaris. In Proceedings of the 19th Australasian Weeds Conference, Hobart, Australia, 1–4 September 2014; pp. 235–238. [Google Scholar]
- Chauhan, B.S.; Jha, P. Glyphosate Resistance in Sonchus Oleraceus and Alternative Herbicide Options for Its Control in Southeast Australia. Sustainability 2020, 12, 8311. [Google Scholar] [CrossRef]
- Paulo De Marco, J.; Diniz-Filho, J.A.F.; Bini, L.M. Spatial Analysis Improves Species Distribution Modelling during Range Expansion. Biol. Lett. 2008, 4, 577–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horo, J.T.; Tessema, T. Abundance and Distribution of Invasive Alien Plant Species in Illu Ababora Zone of Oromia National Regional State, Ethiopia. J. Agric. Sci. Food Technol. 2015, 1, 94–100. [Google Scholar] [CrossRef]
- Cabra-Rivas, I.; Saldaña, A.; Castro-Díez, P.; Gallien, L. A Multi-Scale Approach to Identify Invasion Drivers and Invaders’ Future Dynamics. Biol. Invasions 2016, 18. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H.; Zhao, Z.; Liu, J.; Zhang, Q.; Zhang, X.; Gu, W. The Global Potential Distribution of Invasive Plants: Anredera Cordifolia under Climate Change and Human Activity Based on Random Forest Models. Sustainability 2020, 12, 1491. [Google Scholar] [CrossRef] [Green Version]

| Code | Name | Type |
|---|---|---|
| Bio 1 | Annual mean Temperature | Climatic |
| Bio 4 | Temperature Seasonality | |
| Bio 12 | Annual Precipitation | |
| Bio 15 | Precipitation Seasonality | |
| Rad_sd | Standard deviance of Radiation | Non-Climatic |
| HFP | Human Footprint |
| Range | Schoener’s D Metric | Marginal Environments | Whole Environmental Extent | ||||
|---|---|---|---|---|---|---|---|
| D. Overlap | Stability | Expansion | Unfilling | Stability | Expansion | Unfilling | |
| Native vs. NOA | 0.5486 | 0.8668 | 0.1331 | 0.0002 | 0.8642 | 0.1357 | 0.0002 |
| Native vs. SA | 0.3417 | 0.5411 | 0.4588 | 0.0557 | 0.4197 | 0.5802 | 0.0567 |
| Native vs. AF | 0.2522 | 0.2434 | 0.7565 | 0.3613 | 0.2291 | 0.7708 | 0.8097 |
| Native vs. OCE | 0.4761 | 0.9440 | 0.0559 | 0.1491 | 0.9410 | 0.0589 | 0.2713 |
| AUC Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Training | Test | ||||||||
| Native | NOA | SA | AF | OCE | Native | NOA | SA | AF | OCE |
| 0.67 | 0.89 | 0.98 | 0.98 | 0.81 | 0.67 | 0.88 | 0.97 | 0.98 | 0.81 |
| Variable | Native Range | Invaded Range | |||
|---|---|---|---|---|---|
| NOA | SA | AF | OCE | ||
| Bio 1 | 0.79 ± 8.37 | 15.3 ± 1.4 | 25.9 ± 4.4 | 31.4 ± 1.1 | 1.6 ± 4.4 |
| Bio 4 | 4.4 ± 6.8 | 4.8 ± 5.0 | 43.8 ± 4.6 | 37 ± 2.3 | 47.8 ± 9.8 |
| Bio 12 | 14.7 ± 6.4 | 12.9 ± 1.14 | 2.5 ± 6.8 | 5.3 ± 1.0 | 3.6 ± 1.4 |
| Bio 15 | 2.2 ± 3.9 | 2.7 ± 2.6 | 3.6 ± 3.1 | 2.9 ± 1.9 | 13.8 ± 1.2 |
| Rad_sd | 29.7 ± 4.8 | 22.1 ± 2.1 | 5.4 ± 4.1 | 4.7 ± 2.9 | 25 ± 1.8 |
| HFP | 48.0 ± 2.4 | 41.9 ± 4.4 | 19.1 ± 1.2 | 18.4 ± 1.1 | 8.1 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, J.F.C.; Hernández-Lambraño, R.E.; Rodríguez de la Cruz, D.; Sánchez Agudo, J.Á. Analysis of the Adaptative Strategy of Cirsium vulgare (Savi) Ten. in the Colonization of New Territories. Sustainability 2021, 13, 2384. https://doi.org/10.3390/su13042384
Román JFC, Hernández-Lambraño RE, Rodríguez de la Cruz D, Sánchez Agudo JÁ. Analysis of the Adaptative Strategy of Cirsium vulgare (Savi) Ten. in the Colonization of New Territories. Sustainability. 2021; 13(4):2384. https://doi.org/10.3390/su13042384
Chicago/Turabian StyleRomán, Jhony Fernando Cruz, Ricardo Enrique Hernández-Lambraño, David Rodríguez de la Cruz, and José Ángel Sánchez Agudo. 2021. "Analysis of the Adaptative Strategy of Cirsium vulgare (Savi) Ten. in the Colonization of New Territories" Sustainability 13, no. 4: 2384. https://doi.org/10.3390/su13042384






