Human Activities Inducing High CH4 Diffusive Fluxes in an Agricultural River Catchment in Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
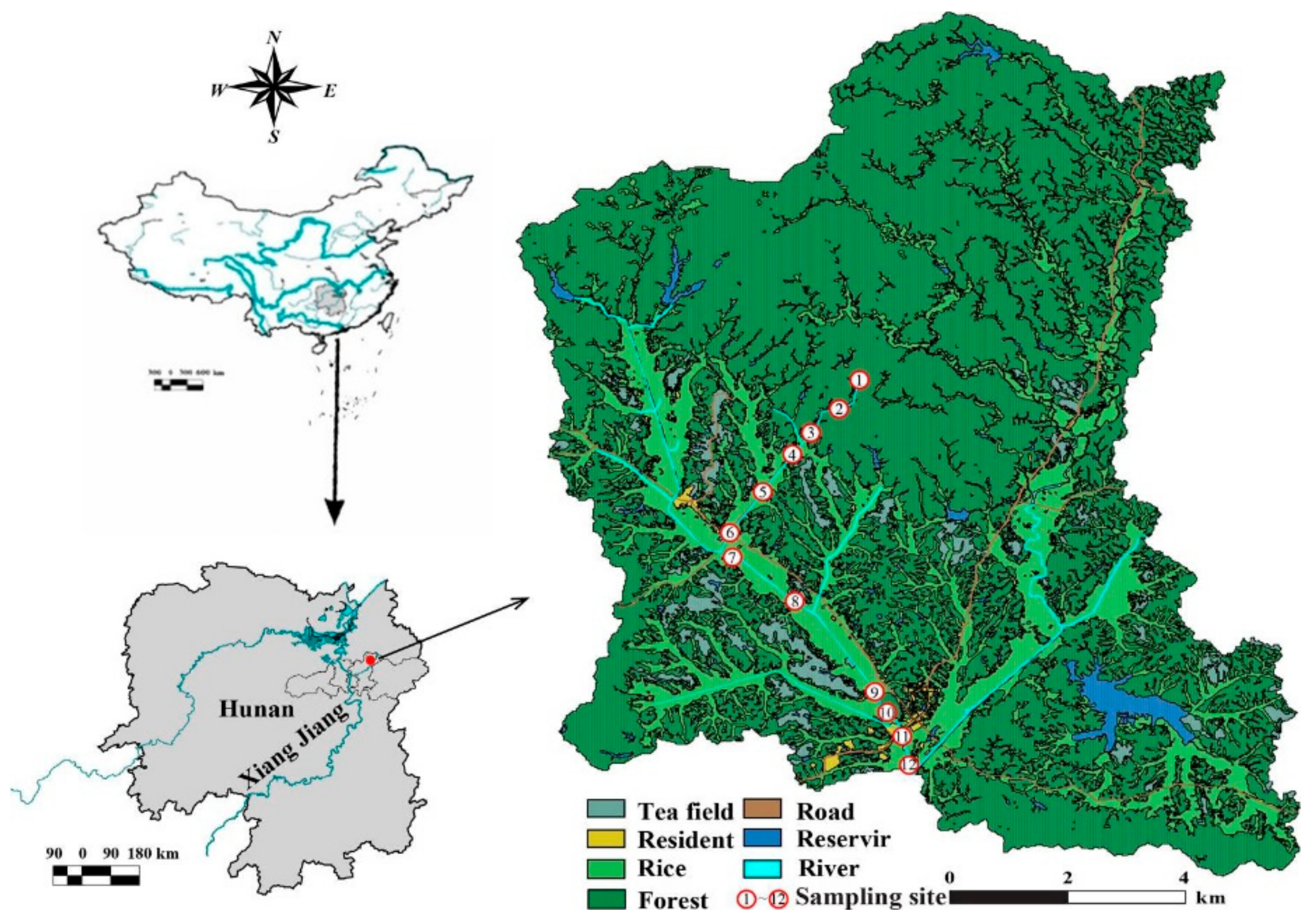
2.1. Experimental Sites
2.2. Sample Design and Collection
2.3. Dissolved CH4 Concentration
2.4. CH4 Diffusive Fluxes
2.5. Environmental Parameters
2.6. Statistical Analysis
3. Results
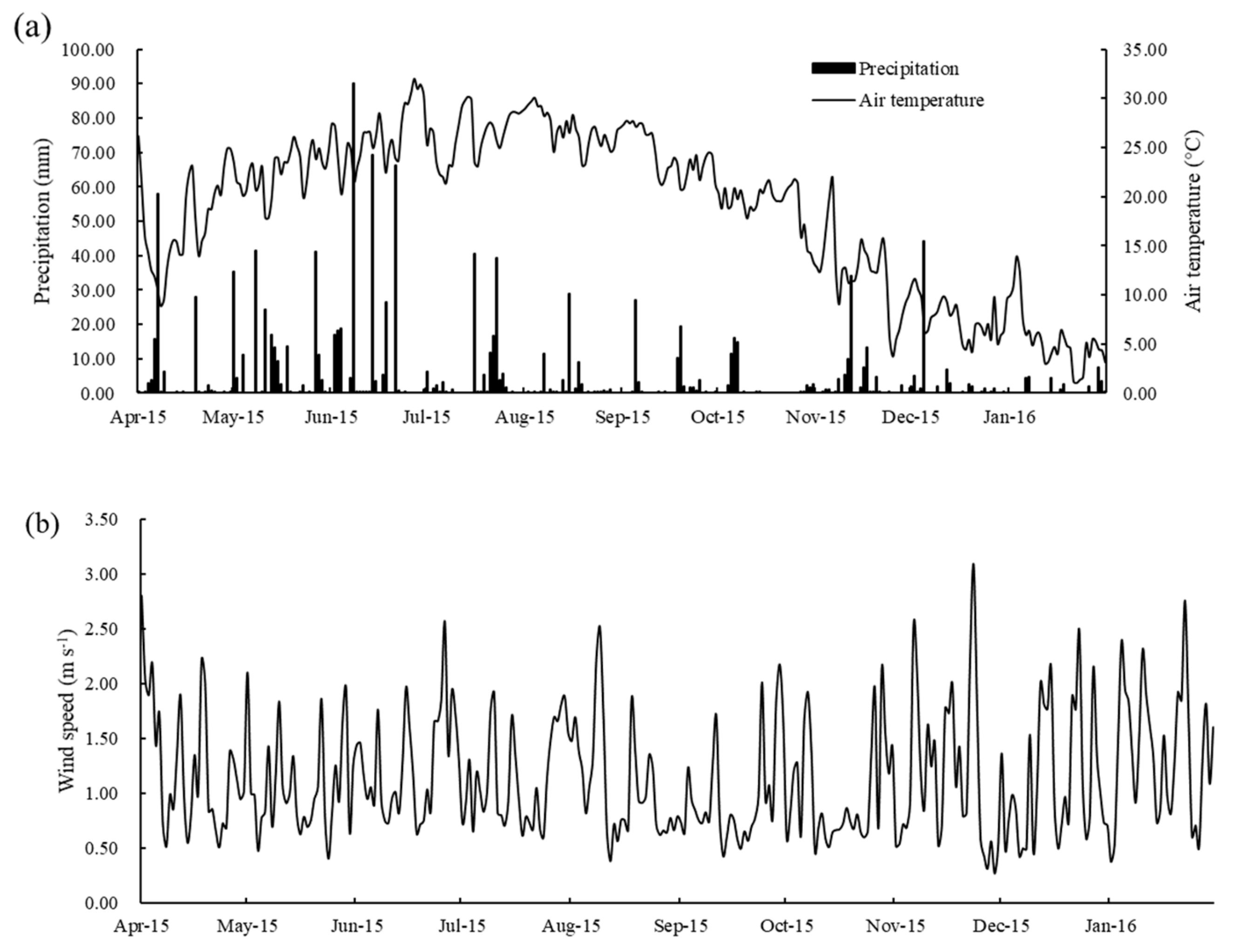
3.1. Precipitation, Air Temperature, and Wind Speed
3.2. Water Parameters
3.3. Water Quality Parameters and Socioeconomic Data
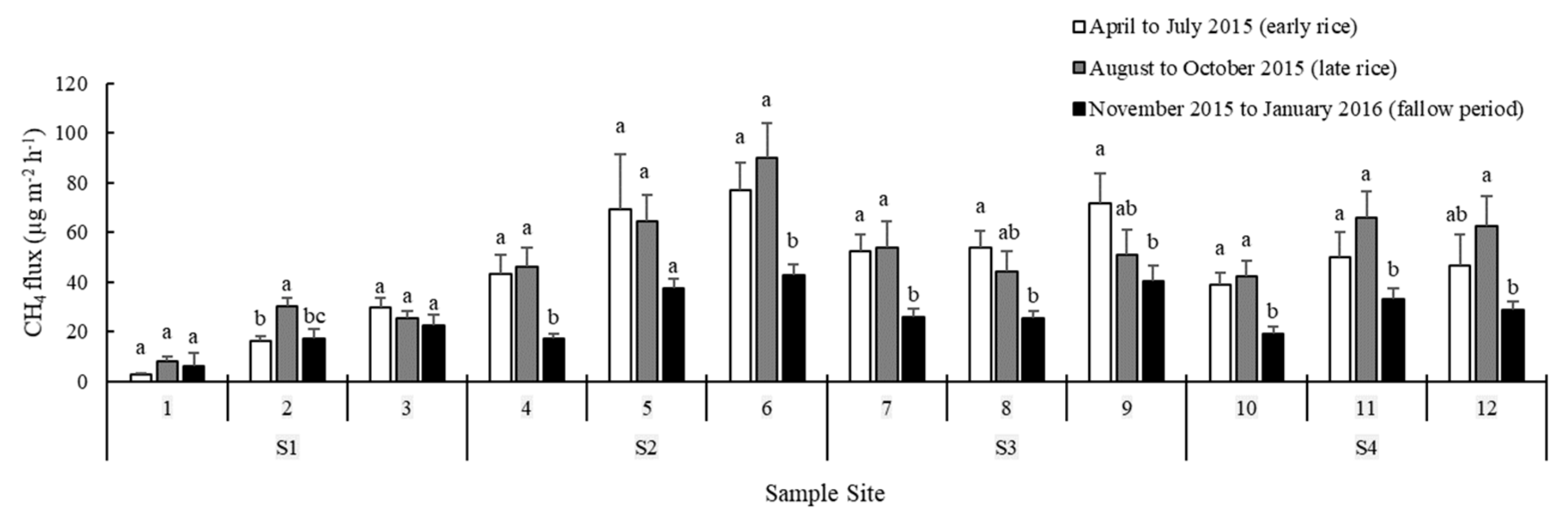
3.4. CH4 Concentration and Flux
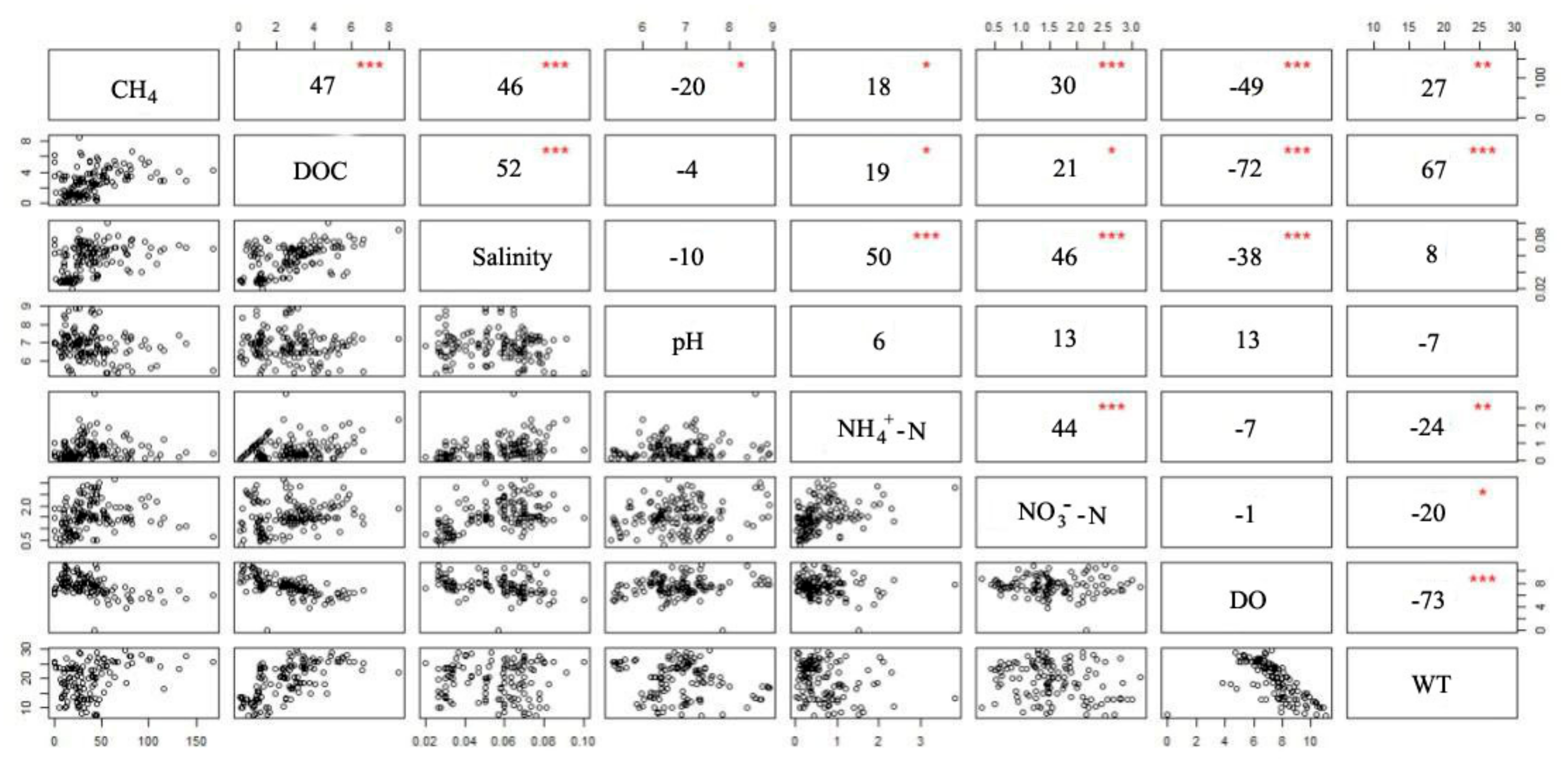
3.5. Dependence of CH4 Fluxes on Water Parameters
3.6. CH4 Production Pathway
4. Discussion
4.1. Comparison with Other Studies
4.2. Spatiotemporal Variation in CH4 Fluxes
4.3. CH4 Production Pathways
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. (Contribution of Working Group I to the Fifth Assessment. Report of the Intergovernmental Panel on Climate Change); Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Ganadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Anthony, S.E.; Prahl, F.G.; Peterson, T.D. Methane dynamics in the Willamette River, Oregon. Limnol. Oceanogr. 2012, 57, 1517–1530. [Google Scholar] [CrossRef]
- WMO. Greenhouse Gas Bulletin; WMO: Geneva, Switzerland, 2018. [Google Scholar]
- Khalil, M.A.K.; Rasmussen, R.A. Sources, sinks, and seasonal cycles of atmospheric methane. J. Geophys. Res. 1983, 88, 5131–5144. [Google Scholar] [CrossRef]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.M.; Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science 2011, 331, 50. [Google Scholar] [CrossRef] [Green Version]
- Stanley, E.H.; Casson, N.J.; Christel, S.T.; Crawford, J.T.; Loken, L.C.; Oliver, S.K. The ecology of methane in streams and rivers: Patterns, controls, and global significance. Ecol. Monogr. 2016, 86, 146–171. [Google Scholar] [CrossRef]
- Wu, S.; Li, S.Q.; Zou, Z.H.; Hu, T.; Hu, Z.Q.; Liu, S.W.; Zou, J.W. High methane emissions largely attributed to ebullitive fluxes from a subtropical river draining a rice paddy watershed in China. Environ. Sci. Technol. 2019, 53, 3499–3507. [Google Scholar] [CrossRef]
- Tan, Y.J. The Greenhouse Gases Emission and Production Mechanism from River Sediment in Shanghai; East China Normal University: Shanghai, China, 2017. [Google Scholar]
- Rajkumar, A.N.; Barnes, J.; Ramesh, R.; Purvaja, R.; Upstill-Goddard, R.C. Methane and nitrous oxide fluxes in the polluted Adyar River and estuary, SE India. Mar. Pollut. Bull. 2008, 56, 2043–2051. [Google Scholar] [CrossRef]
- Yang, L.B.; Li, X.Y.; Yan, W.J.; Ma, P.; Wang, J.N. CH4 concentrations and emissions from three rivers in the Chaohu Lake watershed in Southeast China. J. Integr. Agric. 2012, 11, 665–673. [Google Scholar] [CrossRef]
- Liu, K.H.; Hu, Z.B.; Wei, J.Q.; Jiang, Z.; Lu, H.; Wang, C. Analysis of methane flux produced by city black odor river in summer–An example of Chaoyang Creek in Nanjing city, China. Earth Environ. 2015, 43, 415–419. [Google Scholar]
- Qu, B.; Aho, K.S.; Li, C.L.; Kang, S.C.; Sillanpää, M.; Yan, F.P.; Aymond, P.A. Greenhouse gases emissions in rivers of the Tibetan Plateau. Sci. Rep. 2017, 7, 16573. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.L.; Pan, K.W.; Zhang, L.; Wang, Y.J.; Li, W.; He, X.J.; Luo, H.Y. Warming and nitrogen deposition are interactive in shaping surface soil microbial communities near the alpine timberline zone on the eastern Qinghai-Tibet Plateau, southwestern China. Appl. Soil Ecol. 2016, 101, 72–83. [Google Scholar] [CrossRef]
- Gong, D.L.; Hong, X.; Zeng, G.J.; Wang, Y.; Zuo, S.M.; Liu, X.L.; Wu, J.S. Prediction of water quality in rivers in agricultural regions typical of subtropical in China using Multivariate Linear Regression Model. J. Ecol. Rural Environ. 2017, 33, 509–518. [Google Scholar]
- Wang, Y.; Li, Y.; Liu, F.; Li, Y.Y.; Song, L.F.; Li, H.; Meng, C.; Wu, J.S. Linking rice agriculture to nutrient chemical composition, concentration and mass flux in catchment streams in subtropical central China. Agric. Ecosyst. Environ. 2014, 184, 9–20. [Google Scholar] [CrossRef]
- Wu, H.B.; Lyu, C.W.; Li, Y.E.; Qin, X.B.; Liao, Y.L.; Li, Y. The spatial-temporal distribution of nitrogen and N2O emission from soil and sediment in agricultural watershed of Tuojia River. Acta Sci. Circumstantiae 2017, 37, 1539–1546. [Google Scholar]
- Holmes, M.E.; Chanton, J.P.; Bae, H.S.; Ogram, A. Effect of nutrient enrichment on δ13CH4 and the methane production pathway in the Florida Everglades. Biogeosciences 2013, 119, 1267–1280. [Google Scholar]
- Lü, F.; Hao, L.P.; Guan, D.X.; Qi, Y.J.; Shao, L.M.; He, P.J. Synergetic stress of acids and ammonium on the shift in the methanogenic pathways during thermophilic anaerobic digestion of organics. Water Res. 2013, 47, 2297–2306. [Google Scholar] [CrossRef]
- Avery, G.B.; Shannon, R.D.; White, J.R.; Martens, C.S.; Alperin, M.J. Controls on methane production in a tidal freshwater estuary and a peatland: Methane production via acetate fermentation and CO2 reduction. Biogeochemistry 2002, 62, 19–37. [Google Scholar] [CrossRef]
- Hines, M.E.; Duddleston, K.N.; Rooney-Varga, J.; Fields, D.; Chanton, J.P. Uncoupling of acetate degradation from methane formation in Alaskan wetlands: Connections to vegetation distribution. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Hao, L.P.; Lü, F.; He, P.J.; Li, L.; Shao, L.M. Predominant contribution of syntrophic acetate oxidation to thermophilic methane formation at high acetate concentrations. Environ. Sci. Technol. 2011, 45, 508–513. [Google Scholar] [CrossRef]
- Wu, K.; Lu, B.; Yuan, Z. The recent developments and the contribution of farmland irrigation to national grain safeness in China. J. Irrig. Drain. 2006, 25, 7–10. [Google Scholar]
- Zhang, W.; Yu, Y.Q.; Huang, Y.; Li, T.T.; Wang, P. Modeling methane emissions from irrigated rice cultivation in China from 1960 to 2050. Glob. Chang. Biol. 2011, 17, 3511–3523. [Google Scholar] [CrossRef]
- FAO. Statistical databases, Food and Agriculture Organization (FAO) of the United Nations. 2014. Available online: http://faostat.fao.org/ (accessed on 2 March 2020).
- Zhu, Z.Y.; Zhang, X.B.; Li, M.X. China’s pork market in 2018 and its prospects for 2019. Agric. Outlook 2019, 4, 6–9. [Google Scholar]
- Song, L.F. Characteristic and Influencing Factorings of Nitrogen and Phosphorus Export in Jinjing Town in Changsha County; Huazhong Agricultural University: Wuhan, China, 2014. [Google Scholar]
- Seitzinger, S.P.; Kroeze, C. Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Glob. Biogeochem. Cycles 1998, 12, 93–113. [Google Scholar] [CrossRef]
- Zou, J.W.; Huang, Y.; Jiang, J.Y.; Zheng, X.H.; Sass, R.L. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Glob. Biogeochem. Cycles 2005, 19, 1–9. [Google Scholar] [CrossRef]
- Li, J.L.; Li, Y.E.; Wan, Y.F.; Wang, B.; Waqas, M.A.; Cai, W.W.; Guo, C.; Zhou, S.H.; Su, R.S.; Qin, X.B.; et al. Combination of modified nitrogen fertilizers and water saving irrigation can reduce greenhouse gas emissions and increase rice yield. Geoderma 2018, 315, 1–10. [Google Scholar] [CrossRef]
- Yuan, M.D. Methanogenesis and Nitrogen Removal in the Biological Stabilization Ponds for Large Scale Swine Wastewater Treatment; Zhejiang University: Hangzhou, China, 2016. [Google Scholar]
- Xu, W.Q. Study and Estimation on the Biochemical Methane Potential of Livestock Manure in Anaerobic Digestion; Chinese Academy of Agricultural Sciences Dissertation: Beijing, China, 2017. [Google Scholar]
- Tian, D.; Jiang, L.; Ma, S.H.; Fang, W.J.; Schmid, B.; Xu, L.C.; Zhu, J.X.; Li, P.; Losapio, G.; Jing, X.; et al. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in China. Sci. Total Environ. 2017, 607–608, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.V.; Darchambeau, F.; Lambert, T.; Bouillon, S.; Morana, C.; Brouyère, S.; Hakoun, V.; Jurado, A.; Tseng, H.C.; Descy, J.P.; et al. Effects of agricultural land use on fluvial carbon dioxide methane and nitrogen oxide concentrations in a large European river, the Meuse (Belgium). Sci. Total Environ. 2018, 610–611, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B. Greenhouse Gas Emission and Transport of Carbon and Nitrogen in Typical River Ecosystem; Anhui Normal University: Wuhu, China, 2017. [Google Scholar]
- Qin, X.B.; Li, Y.; Goldberg, S.; Wan, Y.F.; Fan, M.R.; Liao, Y.L.; Wang, B.; Gao, Q.Z.; Li, Y.E. Assessment of indirect N2O emission factors from agricultural river networks based on long-term study at high temporal resolution. Environ. Sci. Technol. 2019, 53, 10781–10791. [Google Scholar] [CrossRef]
- Qin, X.B.; Li, Y.E.; Wan, Y.F.; Fan, M.R.; Liao, Y.L.; Li, Y.; Wang, B.; Gao, Q.Z. Diffusive flux of CH4 and N2O from agricultural river networks: Regression tree and importance analysis. Sci. Total Environ. 2020, 717, 137244. [Google Scholar] [CrossRef]
- Zhao, Q.; Lyu, C.W.; Qin, X.B.; Wu, H.B.; Wan, Y.F.; Liao, Y.L.; Lu, Y.H.; Wang, B.; Li, Y. Key pathway of methane production and characteristics of stable carbon isotope of the Tuojia River waterbody. Chin. J. Appl. Ecol. 2018, 29, 1450–1460. [Google Scholar]
- Liss, P.S.; Merlivat, L. Air-sea gas exchange rates: Introduction and synthesis. Role Air Sea Exch. Geochem. Cycl. 1986, 185, 113–127. [Google Scholar]
- Zhang, Y.; Li, Y.; Qin, X.B.; Kong, F.L.; Chi, M.; Li, Y.E. Dissolved methane concentration and emission flux in agricultural watershed of subtropics. Sci. Agric. Sin. 2016, 49, 3968–3980. [Google Scholar]
- Raymond, P.A.; Cole, J.J. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuary Coast 2001, 24, 312–317. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res. Ocean. 1992, 97, 7373–7382. [Google Scholar] [CrossRef]
- Huttunen, J.T.; Väisänen, T.S.; Hellsten, S.K.; Martikainen, P.J. Methane fluxes at the sediment-water interface in some boreal lakes and reservoirs. Boreal Environ. Res. 2006, 11, 27–34. [Google Scholar]
- Koné, Y.J.M.; Abril, G.; Delille, B.; Borges, A.V. Seasonal variability of methane in the rivers and lagoons of Ivory Coast (West Africa). Biogeochemistry 2010, 100, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Teodoru, C.R.; Nyoni, F.C.; Borges, A.V.; Darchambeau, F.; Nyambe, I.; Bouillon, S. Spatial variability and temporal dynamics of greenhouse gas (CO2, CH4, N2O) concentrations and fluxes along the Zambezi River mainstem and major tributaries. Biogeosci. Discuss. 2014, 11, 16391–16445. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.L.; Zhang, J.; Liu, S.M.; Ren, J.L.; Xu, J.; Zhang, F. Methane in the Changjiang (Yangtze River) Estuary and its adjacent marine area: Riverine input, sediment release and atmospheric fluxes. Biogeochemistry 2008, 91, 71–84. [Google Scholar] [CrossRef]
- Schade, J.D.; Bailio, J.; McDowell, H. Greenhouse gas flux from headwater streams in New Hampshire, USA: Pattern and drivers. Limnol. Oceanogr. 2016, 61, S165–S174. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.X. Study on Methane Flux Emitted from Manure Pond on Medium Scale Pig Farm; Chinese Academy of Agricultural Science: Beijing, China, 2007. [Google Scholar]
- Sieczko, A.K.; Demeter, K.; Singer, G.A.; Tritthart, M.; Preiner, S.; Mayr, M.; Meisterl, K.; Peduzzi, P. Aquatic methane dynamics in a human-impacted river-floodplain of the Danube. Limnol. Oceanogr. 2016, 61, S175–S181. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhou, S.; Wu, N.; Wang, Y.F.; Luo, P.; Shi, F.S. Advance in studies on production, oxidation and emission flux of methane from wetlands. Chin. J. Appl. Environ. Biol. 2006, 12, 726–733. [Google Scholar]
- Yang, S.S.; Chen, I.C.; Liu, C.P.; Liu, L.Y.; Chang, C.H. Carbon dioxide and methane emissions from Tanswei River in Northern. Atmos. Pollut. Res. 2015, 6, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Schrier-Uijl, A.P.; Veraart, A.J.; Leffelaar, P.A.; Berendse, F.; Veenendaal, E.M. Release of CO2 and CH4 from lakes and drainage ditches in temperate wetlands. Biogeochemistry 2011, 102, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Mer, J.L.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Galbally, I.E.; Kirstine, W.V.; Meyer, C.P.; Wang, Y.P. Soil-atmosphere trace gas exchange in semiarid and arid zones. J. Environ. Qual. 2008, 37, 599–607. [Google Scholar] [CrossRef]
- Kuivila, K.M.; Murray, J.W.; Devol, A.H. Methane production, sulfate reduction and competition for substrates in the sediments of Lake Washington. Geochim. Cosmochim. Acta 1989, 53, 409–416. [Google Scholar] [CrossRef]
- Heilman, M.A.; Carlton, R.G. Ebullitive release of lacunar gases from floral spikes of Potamogeton angustifolius and Potamogeton amplifolius: Effects on plant aeration and sediment CH4 flux. Aquat. Bot. 2001, 71, 19–33. [Google Scholar] [CrossRef]
- Crowe, S.A.; Katsev, S.; Leslie, K.; Sturm, A.; Magen, C.; Nomosatryo, S.; Pack, M.A.; Kessler, J.D.; Reeburgh, W.S.; Roberts, J.A.; et al. The methane cycle in ferruginous Lake Matano. Geobiology 2011, 9, 61–78. [Google Scholar] [CrossRef]
- Oswald, K.; Milucka, J.; Brand, A.; Littmann, S.; Wehrli, B.; Kuypers, M.M.M.; Schubert, C.J. Light-dependent aerobic methane oxidation reduces methane emission from seasonally stratified lakes. PLoS ONE 2015, 10, e0132574. [Google Scholar] [CrossRef]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.X.; Cai, Z.C.; Tsuruta, H. Plant species effects on methane emissions from freshwater marshes. Atmos. Environ. 2005, 39, 3199–3207. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Xing, G.X. Nitrogen Cycling; Tsinghua University Press: Beijing, China, 2002. [Google Scholar]
- Bachoon, D.; Jones, R.D. Potential rates of methanogenesis in sawgrass marshes with peat and marl soils in the Everglades. Soil Biol. Biochem. 1992, 24, 21–27. [Google Scholar] [CrossRef]
- Wright, A.L.; Reddy, K.R. Phosphorus loading effects on extracellular enzyme activity in Everglades wetland soils. Soil Sci. Soc. Am. J. 2001, 65, 588–595. [Google Scholar] [CrossRef]
- Kelly, C.A.; Chynoweth, D.P. The Contributions of temperature and of the input of organic matter in controlling rates of sediment methanogenesis. Limnol. Oceanogr. 1981, 26, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.J.; Lukow, T.; Conrad, R. Effect of temperature on structure and function of the methanogenic archaeal community in an anoxic rice field soil. Appl. Environ. Microbiol. 1999, 65, 2341–2349. [Google Scholar] [CrossRef] [Green Version]
- Venkiteswaran, J.J.; Rosamond, M.S.; Schiff, S.L. Nonlinear response of riverine N2O fluxes to oxygen and temperature. Environ. Sci. Technol. 2014, 48, 1566–1573. [Google Scholar] [CrossRef]
- Zeng, F.W.; Masiello, C.A.; Hockaday, W.C. Controls on the origin and cycling of riverine dissolved inorganic carbon in the Brazos River, Texas. Biogeochemistry 2011, 104, 275–291. [Google Scholar] [CrossRef]
- Dyson, K.E.; Billett, M.F.; Dinsmore, K.J.; Harvey, F.; Thomson, A.M.; Piirainen, S.; Kortelainen, P. Release of aquatic carbon from two peatland catchments in E. Finland during the spring snowmelt period. Biogeochemistry 2011, 103, 125–142. [Google Scholar] [CrossRef]
- Conrad, R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]


| KW Models | Formula | Wind U10 (m s−1) | References |
|---|---|---|---|
| LM86 | 0 < U10 ≤ 3.6 | ||
| 3.6 < U10 ≤ 13 | [39] | ||
| U10 > 13 | |||
| W92a | Long term U10 | [42] | |
| W92b | Short term U10 | ||
| RC01 | Arbitrarily U10 | [41] |
| Reach | pH | DO (mg L−1) | Salinity | WT (°C) | DOC (mg L−1) | NH4+–N (mg L−1) | NO3−–N (mg L−1) |
|---|---|---|---|---|---|---|---|
| S1 | 6.88 ± 0.08 | 8.21 ± 0.12 | 0.03 ± 0.001 | 18.90 ± 0.51 | 1.11 ± 0.09 | 0.28 ± 0.05 | 0.84 ± 0.04 |
| S2 | 6.82 ± 0.07 | 7.03 ± 0.21 | 0.06 ± 0.001 | 19.73 ± 0.62 | 2.81 ± 0.13 | 0.90 ± 0.09 | 1.72 ± 0.08 |
| S3 | 6.77 ± 0.07 | 7.18 ± 0.19 | 0.06 ± 0.001 | 20.06 ± 0.67 | 3.30 ± 0.16 | 0.66 ± 0.05 | 1.78 ± 0.05 |
| S4 | 6.85 ± 0.07 | 7.35 ± 0.17 | 0.07 ± 0.001 | 19.60 ± 0.65 | 3.74 ± 0.19 | 0.67 ± 0.06 | 1.85 ± 0.05 |
| Mean | 6.83 ± 0.01 | 7.45 ± 0.09 | 0.06 ± 0.001 | 19.57 ± 0.31 | 2.74 ± 0.09 | 0.63 ± 0.03 | 1.55 ± 0.03 |
| Reach | Population | Pig | Poultry | Nitrogen fertilizer (kg ha−1) |
|---|---|---|---|---|
| S1 | 36 | N/A | 16 | N/A |
| S2 | 3863 | 426 | 365 | 150 |
| S3 | 2739 | 82 | 131 | 135 |
| S4 | 21,776 | N/A | N/A | 142.5 |
| S1 | S2 | S3 | S4 | |
|---|---|---|---|---|
| fermentation of acetate | 87% | 78% | 76% | 81% |
| reduction of carbon dioxide | 13% | 22% | 24% | 19% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhao, Q.; Gao, Q.; Li, Y.; Wan, Y.; Li, Y.; Tian, D.; Liao, Y.; Fan, M.; Ganjurjav, H.; et al. Human Activities Inducing High CH4 Diffusive Fluxes in an Agricultural River Catchment in Subtropical China. Sustainability 2020, 12, 2114. https://doi.org/10.3390/su12052114
Wu H, Zhao Q, Gao Q, Li Y, Wan Y, Li Y, Tian D, Liao Y, Fan M, Ganjurjav H, et al. Human Activities Inducing High CH4 Diffusive Fluxes in an Agricultural River Catchment in Subtropical China. Sustainability. 2020; 12(5):2114. https://doi.org/10.3390/su12052114
Chicago/Turabian StyleWu, Hongbao, Qiang Zhao, Qingzhu Gao, Yu’e Li, Yunfan Wan, Yong Li, Di Tian, Yulin Liao, Meirong Fan, Hasbagan Ganjurjav, and et al. 2020. "Human Activities Inducing High CH4 Diffusive Fluxes in an Agricultural River Catchment in Subtropical China" Sustainability 12, no. 5: 2114. https://doi.org/10.3390/su12052114





