Contribution and Driving Mechanism of N2O Emission Bursts in a Chinese Vegetable Greenhouse after Manure Application and Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Field Treatments
2.2. N2O Flux Measurement
2.3. Gas Kinetics during the Aerobic Incubations
2.4. Statistical Analysis
3. Results
3.1. Soil Moisture and N2O Flux during Field Observation
3.2. N2O and Mineral N Concentration during the Aerobic Incubation
3.3. Integrated Relationships Between the Gas Emissions and the Soil Properties
4. Discussion
4.1. Significance of the Bursts in N2O Emissions
4.2. Contribution of Denitrification to the N2O Emission Bursts
4.3. Implications for N2O Mitigation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dickinson, R.E.; Cicerone, R.J. Future global warming from atmospheric trace gases. Nature 1986, 319, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, L.; Huang, G.; Wu, L.; Chen, X.; Zhang, F. The Development and Contribution of Nitrogenous Fertilizer in China and Challenges Faced by the Country. Sci. Agric. Sin. 2013, 46, 3161–3171. (In Chinese) [Google Scholar]
- Heffer., P.; Prud’homme, M. Global nitrogen fertilizer demand and supply: Trend, current level and outlook. In Proceedings of the International Nitrogen Initiative Conference, Melbourne, Australia, 4–8 December 2016. [Google Scholar]
- Food and Agriculture Organization. FAOSTAT Database Collections; FAO: Rome, Italy, 2015; Available online: http://www.apps.fao.org (accessed on 28 February 2018).
- Liu, Q.; Qin, Y.; Zou, J.; Guo, Y.; Gao, Z. Annual nitrous oxide emissions from open-air and greenhouse vegetable cropping systems in China. Plant Soil 2013, 370, 223–233. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Christie, P.; Li, J. Environmental implications of low nitrogen use efficiency in excessively fertilized hot pepper (Capsicum frutescens L.) cropping systems. Agric. Ecosyst. Environ. 2005, 111, 70–80. [Google Scholar] [CrossRef]
- Xiong, Z.; Xie, Y.; Xing, G.; Zhu, Z.; Butenhoff, C. Measurements of nitrous oxide emissions from vegetable production in China. Atmos. Environ. 2006, 40, 2225–2234. [Google Scholar] [CrossRef]
- Ju, X.; Kou, C.; Zhang, F.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Guo, J.; Ren, T.; Chen, Q.; Li, B.; Wang, J. Increase of soil pH in a solar greenhouse vegetable production system. Soil Sci. Soc. Am. J. 2012, 76, 2074–2082. [Google Scholar] [CrossRef]
- Guo, J.H. Inputs of Irrigation Water, Fertilizers, Pesticides and Life Cycle Assessment of Environmental Impacts from Typica Greenhouse Vegetable Production Systems in China. Ph.D. Thesis, China Agricultural University, Beijing, China, 2016. (In Chinese with English Abstract). [Google Scholar]
- Sun, Y.; Hu, K.; Zhang, K.; Jiang, L.; Xu, Y. Simulation of nitrogen fate for greenhouse cucumber grown under different water and fertilizer management using the EU-Rotate_N model. Agric. Water Manag. 2012, 112, 21–32. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Tong, W.; Chen, F. The impact of local crops consumption on the water resources in Beijing. J. Clean. Prod. 2012, 21, 45–50. [Google Scholar] [CrossRef]
- He, F.; Jiang, R.; Chen, Q.; Zhang, F.; Su, F. Nitrous oxide emissions from an intensively managed greenhouse vegetable cropping system in Northern China. Environ. Pollut. 2009, 157, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Xing, G.; Chen, X.; Zhang, S.; Zhang, L.; Liu, X.; Cui, Z.; Yin, B.; Christie, P.; Zhu, Z.; et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [Green Version]
- Chinese Statistical Bureau. China Statistical Year Book; Chinese Statistical Bureau: Beijing, China, 2012.
- Yao, Z.; Liu, C.; Dong, H.; Wang, R.; Zheng, X. Annual nitric and nitrous oxide fluxes from Chinese subtropical plastic greenhouse and conventional vegetable cultivations. Environ. Pollut. 2015, 196, 89–97. [Google Scholar] [CrossRef]
- Martins, M.R.; Jantalia, C.P.; Polidoro, J.C.; Batista, J.N.; Alves, B.J.; Boddey, R.M.; Urquiaga, S. Nitrous oxide and ammonia emissions from N fertilization of maize crop under no-till in a Cerrado soil. Soil Tillage Res. 2015, 151, 75–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, F.; Jin, Y.; Wang, X.; Liu, S.; Zou, J. Response of nitric and nitrous oxide fluxes to N fertilizer application in greenhouse vegetable cropping systems in southeast China. Sci. Rep. 2016, 6, 20700. [Google Scholar] [CrossRef] [Green Version]
- Hosono, T.; Hosoi, N.; Akiyama, H.; Tsuruta, H. Measurements of N2O and NO emissions during tomato cultivation using a flow-through chamber system in a glasshouse. Nutr. Cycl. Agroecosyst. 2006, 75, 115–134. [Google Scholar] [CrossRef]
- Song, H. Studies on Denitrification Process and Environmental Implications in Greenhouse Vegetable Cropping Systems. Ph.D. Thesis, China Agricultural University, Beijing, China, 2012. (In Chinese with English Abstract). [Google Scholar]
- Maharjan, B.; Venterea, R.T. Nitrite intensity explains N management effects on N2O emissions in maize. Soil Biol. Biochem. 2013, 66, 229–238. [Google Scholar] [CrossRef]
- Ma, L.; Shan, J.; Yan, X. Nitrite behavior accounts for the nitrous oxide peaks following fertilization in a fluvo-aquic soil. Biol. Fertil. Soils 2015, 51, 1–10. [Google Scholar]
- Venterea, R.T.; Clough, T.J.; Coulter, J.A.; Breuillin-Sessoms, F. Ammonium sorption and ammonia inhibition of nitrite-oxidizing bacteria explain contrasting soil N2O production. Sci. Rep. 2015, 5, 12153. [Google Scholar] [CrossRef]
- Ren, S.L. Characteristics of Nitrification and Nitrogen Mineralization in Greenhouse Vegetable Soil. Master’s Thesis, China Agricultural University, Beijing, China, 2012. (In Chinese). [Google Scholar]
- FAO/Unesco. Soil Map of the World, Revised Legend; FAO: Rome, Italy, 1988. [Google Scholar]
- Fan, Z.; Lin, S.; Zhang, X.; Jiang, Z.; Yang, K.; Jian, D.; Chen, Y.; Li, J.; Chen, Q.; Wang, J. Conventional flooding irrigation causes an overuse of nitrogen fertilizer and low nitrogen use efficiency in intensively used solar greenhouse vegetable production. Agric. Water Manag. 2014, 144, 11–19. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Livingston, G.P. Use of chamber systems to measure trace gas fluxes. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; Wiley: Ney York, NY, USA, 1993; pp. 63–78. [Google Scholar]
- Ding, W.; Cai, Y.; Cai, Z.; Yagi, K.; Zheng, X. Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the North China Plain. Sci. Total Environ. 2007, 373, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hillel, D. Environmental Soil Physics; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Molstad, L.; Dörsch, P.; Bakken, L.R. Robotized incubation system for monitoring gases (O2, NO, N2O, N2) in denitrifying cultures. J. Microbiol. Methods 2007, 71, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wang, J.; Almøy, T.; Bakken, L.R. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+ N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Chang. Biol. 2014, 20, 1685–1698. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Zhang, X.; Guo, J. Abiotic nitrate loss and nitrogenous trace gas emission from Chinese acidic forest soils. Environ. Sci. Pollut. Res. 2017, 24, 22679–22687. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chen, H.; Cai, H.; Yang, F.; Li, D.; Wang, F. CO2 and N2O emissions from Lou soils of greenhouse tomato fields under aerated irrigation. Atmos. Environ. 2016, 132, 69–76. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Wei, J.; Shi, J.; Tan, D.; Wang, M.; Jiang, L. Emission Characteristics of Soil Nitrous Oxide from Typical Greenhouse Vegetable Fields in North China. Agric. Sci. Technol. 2017, 18, 438–442. (In Chinese) [Google Scholar]
- Chen, J.; Song, H.; Cao, W.; Wang, Y.; Wang, J. Nitrous Oxide Production in Response to Oxygen in a Solar Greenhouse Vegetable Soil. Environ. Sci. 2018, 30, 3826–3834. (In Chinese) [Google Scholar]
- Wang, Y.; Cao, W.; Zhang, X.; Guo, J. Rate of denitrification and stoichiometry of its products in fluvo-aquic Cambisols for sterilized and unsterilized incubations. Eur. J. Soil Sci. 2019. [Google Scholar] [CrossRef]
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soil at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P.M. Soil Microbiology, Biochemistry, and Ecology; Paul, E.A., Ed.; Springer: New York, NY, USA, 2007; pp. 341–364. [Google Scholar]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Meijide, A.; Díez, J.A.; Sánchez-Martín, L.; López-Fernández, S.; Vallejo, A. Nitrogen oxide emissions from an irrigated maize crop amended with treated pig slurries and composts in a Mediterranean climate. Agric. Ecosyst. Environ. 2007, 121, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, T.; Decock, C.; Six, J. Assessing drivers of N2O production in California tomato cropping systems. Sci. Total Environ. 2013, 465, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Kirk, T.; Beare, M.H.; Meenken, E.D.; Condron, L.M. Soil organic matter and texture affect responses to dry/wet cycles: Effects on carbon dioxide and nitrous oxide emissions. Soil Biol. Biochem. 2013, 57, 43–55. [Google Scholar] [CrossRef]
- Owens, J.; Clough, T.J.; Laubach, J.; Hunt, J.E.; Venterea, R.T. Nitrous oxide fluxes and soil oxygen dynamics of soil treated with cow urine. Soil Sci. Soc. Am. J. 2017, 81, 289–298. [Google Scholar] [CrossRef]
- Uchida, Y.; Wang, Y.; Akiyama, H.; Nakajima, Y.; Hayatsu, M. Expression of denitrification genes in response to a waterlogging event in a Fluvisol and its relationship with large nitrous oxide pulses. FEMS Microbiol. Ecol. 2014, 88, 407–423. [Google Scholar] [CrossRef] [Green Version]
- Riya, S.; Takeuchi, Y.; Zhou, S.; Terada, A.; Hosomi, M. Nitrous oxide production and mRNA expression analysis of nitrifying and denitrifying bacterial genes under floodwater disappearance and fertilizer application. Environ. Sci. Pollut. Res. 2017, 24, 15852–15859. [Google Scholar] [CrossRef]
- Min, J.; Shi, W.; Xing, G.; Powlson, D.; Zhu, Z. Nitrous oxide emissions from vegetables grown in a polytunnel treated with high rates of applied nitrogen fertilizers in Southern China. Soil Use Manag. 2012, 28, 70–77. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, Y.; Deng, J.; Wang, L. Multiple-year nitrous oxide emissions from a greenhouse vegetable field in China: Effects of nitrogen management. Sci. Total Environ. 2018, 616, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Davidson, E.A. Soil water content and the ratio of nitrous oxide to nitric oxide emitted from soil. In Biogeochemistry of Global Change; Oremland, R.S., Ed.; Springer: New York, NY, USA, 1993; pp. 368–386. [Google Scholar]
- Sanchez-Martín, L.; Meijide, A.; Garcia-Torres, L.; Vallejo, A. Combination of drip irrigation and organic fertilizer for mitigating emissions of nitrogen oxides in semiarid climate. Agric. Ecosyst. Environ. 2010, 137, 99–107. [Google Scholar] [CrossRef]
- Kennedy, T.L.; Suddick, E.C.; Six, J. Reduced nitrous oxide emissions and increased yields in California tomato cropping systems under drip irrigation and fertigation. Agric. Ecosyst. Environ. 2013, 170, 16–27. [Google Scholar] [CrossRef]
- Vallejo, A.; Meijide, A.; Boeckx, P.; Arce, A.; García-torres, L.; Aguado, P.L.; Sanchez-Martín, L. Nitrous oxide and methane emissions from a surface drip-irrigated system combined with fertilizer management. Eur. J. Soil Sci. 2014, 65, 386–395. [Google Scholar] [CrossRef]
- Guardia, G.; Cangani, M.T.; Sanz-Cobena, A.; Junior, J.L.; Vallejo, A. Management of pig manure to mitigate NO and yield-scaled N2O emissions in an irrigated Mediterranean crop. Agric. Ecosyst. Environ. 2017, 238, 55–66. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, Y.; Mu, Y.; Zhou, Y.; Zhang, C.; Liu, J. The effect of drip irrigation and drip fertigation on N2O and NO emissions, water saving and grain yields in a maize field in the North China Plain. Sci. Total Environ. 2017, 575, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Han, B.; Li, W.; Zhang, X.; Zhang, Y.; Lin, X.; Zou, H. Effects of different irrigation methods on nitrous oxide emissions and ammonia oxidizers microorganisms in greenhouse tomato fields. Agric. Water Manag. 2018, 203, 115–123. [Google Scholar] [CrossRef]
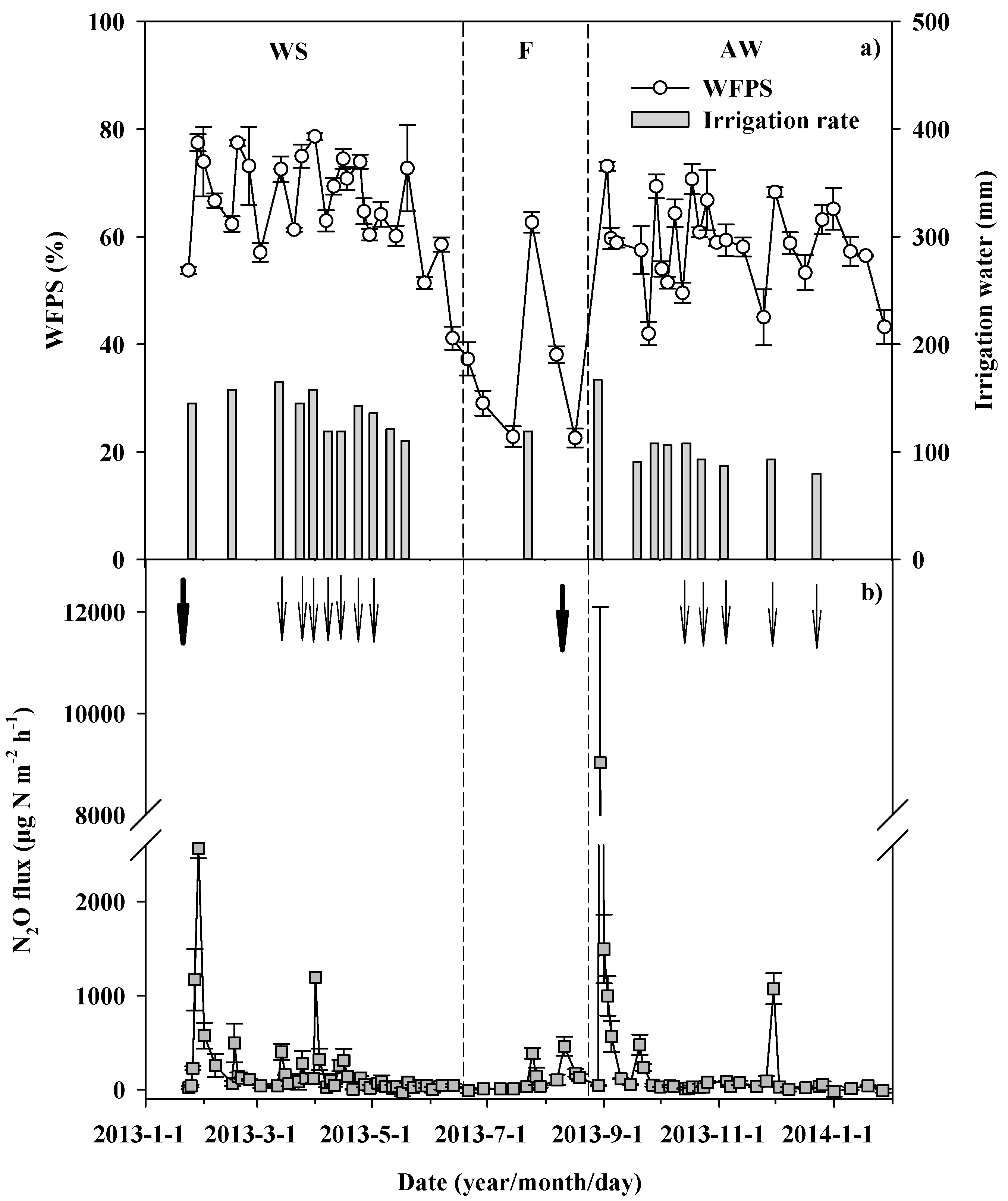

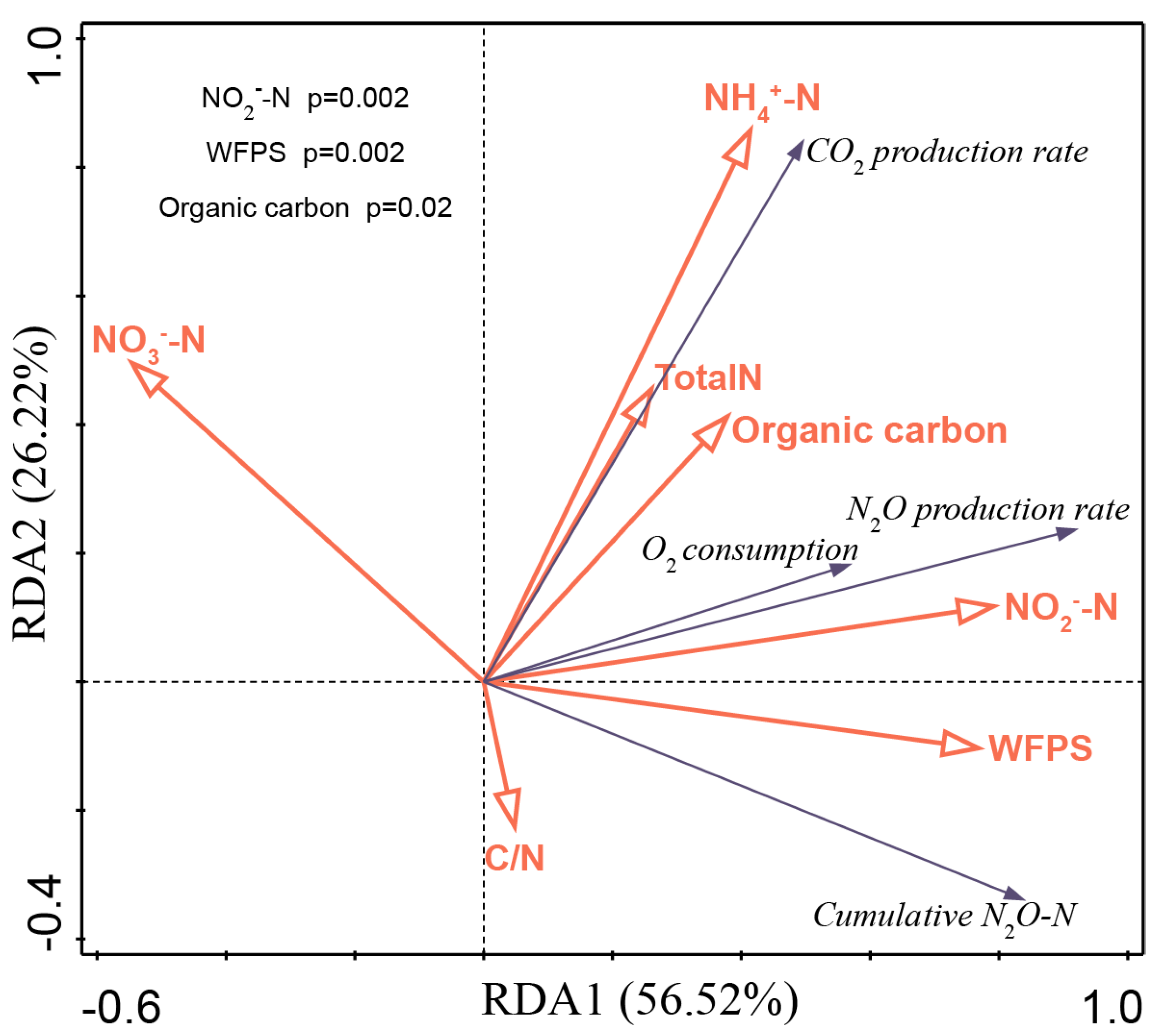

| Treatment | pH † | Soil Organic Carbon (g kg−1) | Total Nitrogen (g kg−1) | Olsen-P (mg kg−1) | Exchangeable Potassium (mg kg−1) | NH4+-N (mg kg−1) | NO3−N (mg kg−1) |
|---|---|---|---|---|---|---|---|
| CFF | 8.03 ± 0.03 | 11.0 ± 0.24 | 1.10 ± 0.06 | 172 ± 3.7 | 496 ± 3.9 | 3.54 ± 1.3 | 43.1 ± 2.1 |
| Soil Moisture | N2O Emission Rate (nmol N g−1 h−1) | |
|---|---|---|
| M0 | M260 | |
| 65% WFPS | 0.009 ± 0.00 bB | 0.31 ± 0.11 aB |
| 75% WFPS | 0.014 ± 0.00 bB | 2.14 ± 0.57 aAB |
| 85% WFPS | 1.76 ± 0.47 bA | 8.46 ± 2.89 aA |
| ANOVA | ||
| Chicken manure (CM) | 9.35 ** | |
| Soil moisture (WFPS) | 9.35 ** | |
| CM × WFPS | 3.66 * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Liu, S.; Qu, Z.; Song, H.; Qin, W.; Guo, J.; Chen, Q.; Lin, S.; Wang, J. Contribution and Driving Mechanism of N2O Emission Bursts in a Chinese Vegetable Greenhouse after Manure Application and Irrigation. Sustainability 2019, 11, 1624. https://doi.org/10.3390/su11061624
Cao W, Liu S, Qu Z, Song H, Qin W, Guo J, Chen Q, Lin S, Wang J. Contribution and Driving Mechanism of N2O Emission Bursts in a Chinese Vegetable Greenhouse after Manure Application and Irrigation. Sustainability. 2019; 11(6):1624. https://doi.org/10.3390/su11061624
Chicago/Turabian StyleCao, Wenchao, Su Liu, Zhi Qu, He Song, Wei Qin, Jingheng Guo, Qing Chen, Shan Lin, and Jingguo Wang. 2019. "Contribution and Driving Mechanism of N2O Emission Bursts in a Chinese Vegetable Greenhouse after Manure Application and Irrigation" Sustainability 11, no. 6: 1624. https://doi.org/10.3390/su11061624





