Life Cycle Assessment Comparison of Two Refractory Brick Product Systems for Ladle Lining in Secondary Steelmaking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodological Framework
2.2. Recipe Data
2.3. Brick Manufacturing Process
- (Optional) crushing and sieving (to achieve a defined particle-size distribution) of the raw materials, according to the recipe;
- Mixing of the components;
- Molding (a.k.a. shaping), by means of several pressing cycles, in order to obtain a compact and easy-to-handle “green-state” solid piece;
- Pre-heating and baking (in particular, “curing” for the reference MgO-C bricks) or drying and sintering (for the “carbonless” bricks);
- Cooling, packaging and shipping.
2.4. Modeling and Assumptions Regarding the Manufacturing Process of MgO-C Reference Bricks
2.4.1. Recipe Weight Percentage Adjustments
2.4.2. Emissions in the Heating Phase
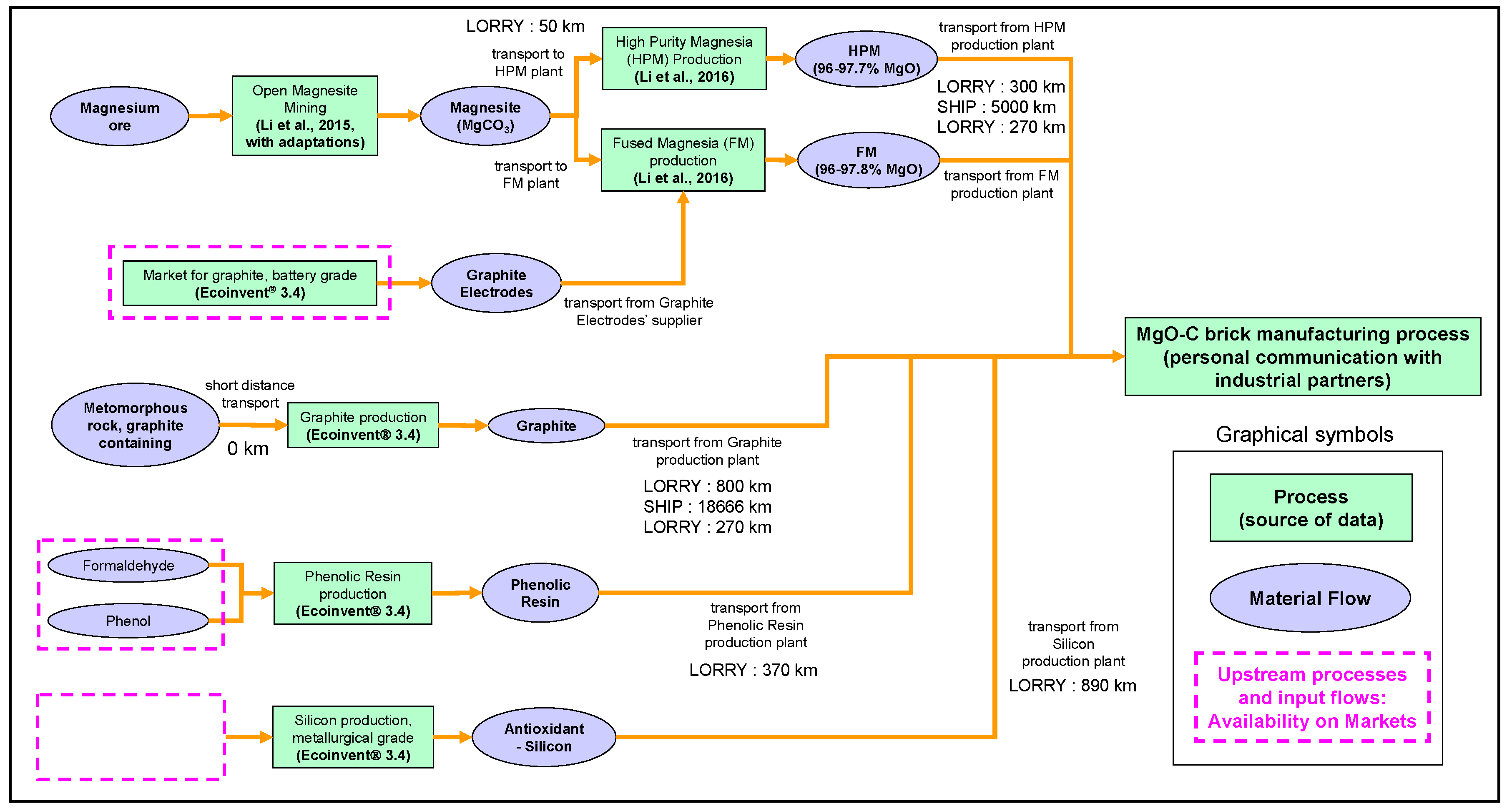
2.4.3. Modeling and Assumptions Regarding the Suppliers’ Chain
2.4.4. Magnesite Mining Process
2.4.5. Processes for the MgO-based Materials
- for electricity: “market for electricity, medium voltage|cut-off, U—TR”;
- for coal: “market for hard coal|cut-off, U—RoW”;
- for heavy oil: “market for heavy fuel oil|cut-off, U—RoW”.
- for the road transport: “market for transport, freight, lorry, unspecified| cut-off, U—GLO” (with a total distance of 570 km) and
- for the sea transport: “market for transport, freight, sea, transoceanic ship|cut-off, U—GLO”.
2.4.6. Processes Associated with the Other Raw Materials of MgO-C Reference Bricks
2.5. Modeling and Assumptions Regarding the Manufacturing Process of Carbonless Bricks
2.5.1. Assumed Emissions and LCI of the Manufacturing Process
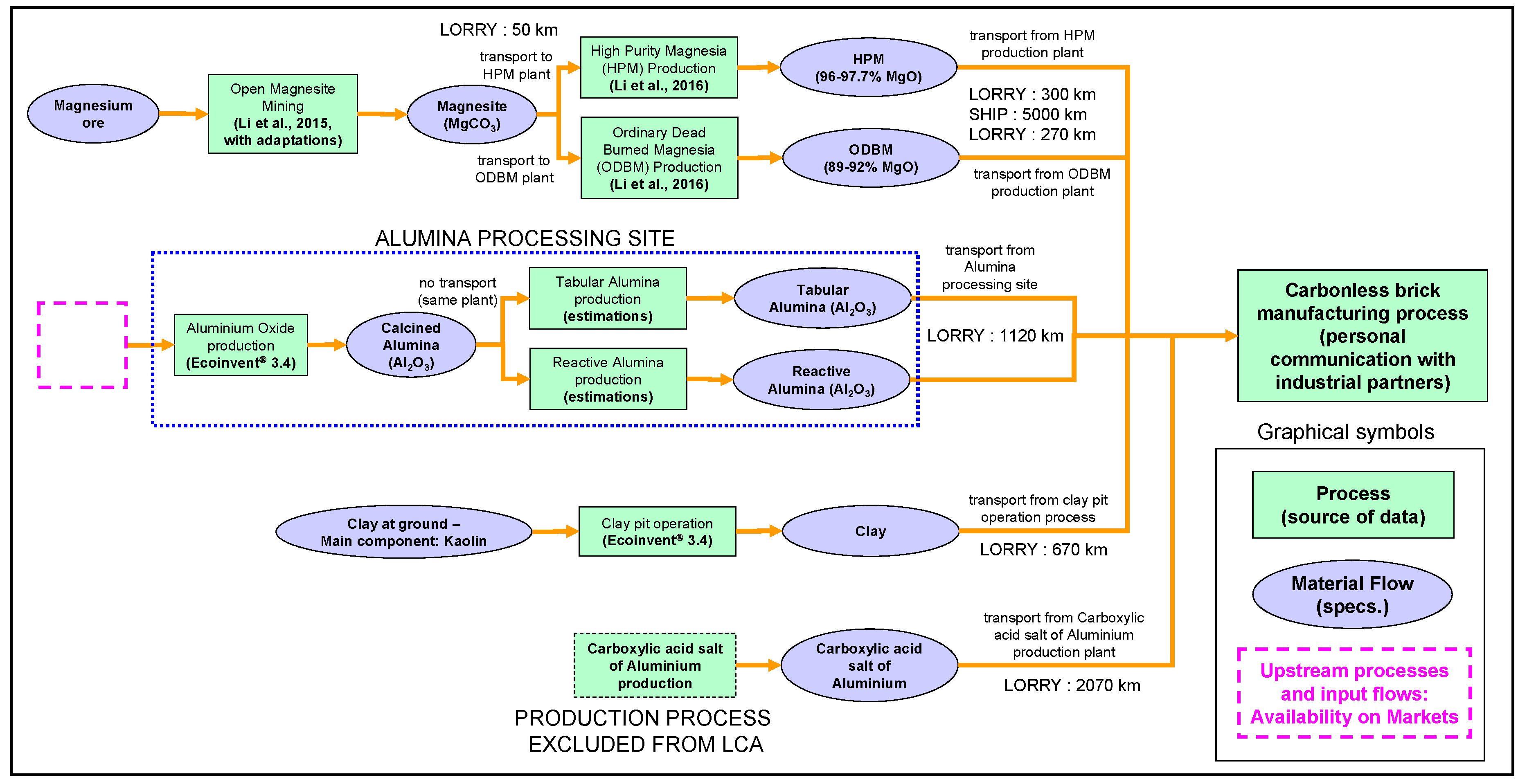
2.5.2. Modeling and Assumptions Regarding the Suppliers’ Chain
2.5.3. Processes for Alumina-based Materials
- W is the specific grinding energy [kWh/tshort];
- Wi is the Bond ball mill work index [kWh/tshort];
- F80 is the feed 80% passing size in [μm];
- P80 is the product 80% passing size in [μm].
2.5.4. Processes Associated with the Other Raw Materials of Carbonless Bricks
3. Results
3.1. Results of the Comparison in Relative and Absolute Terms
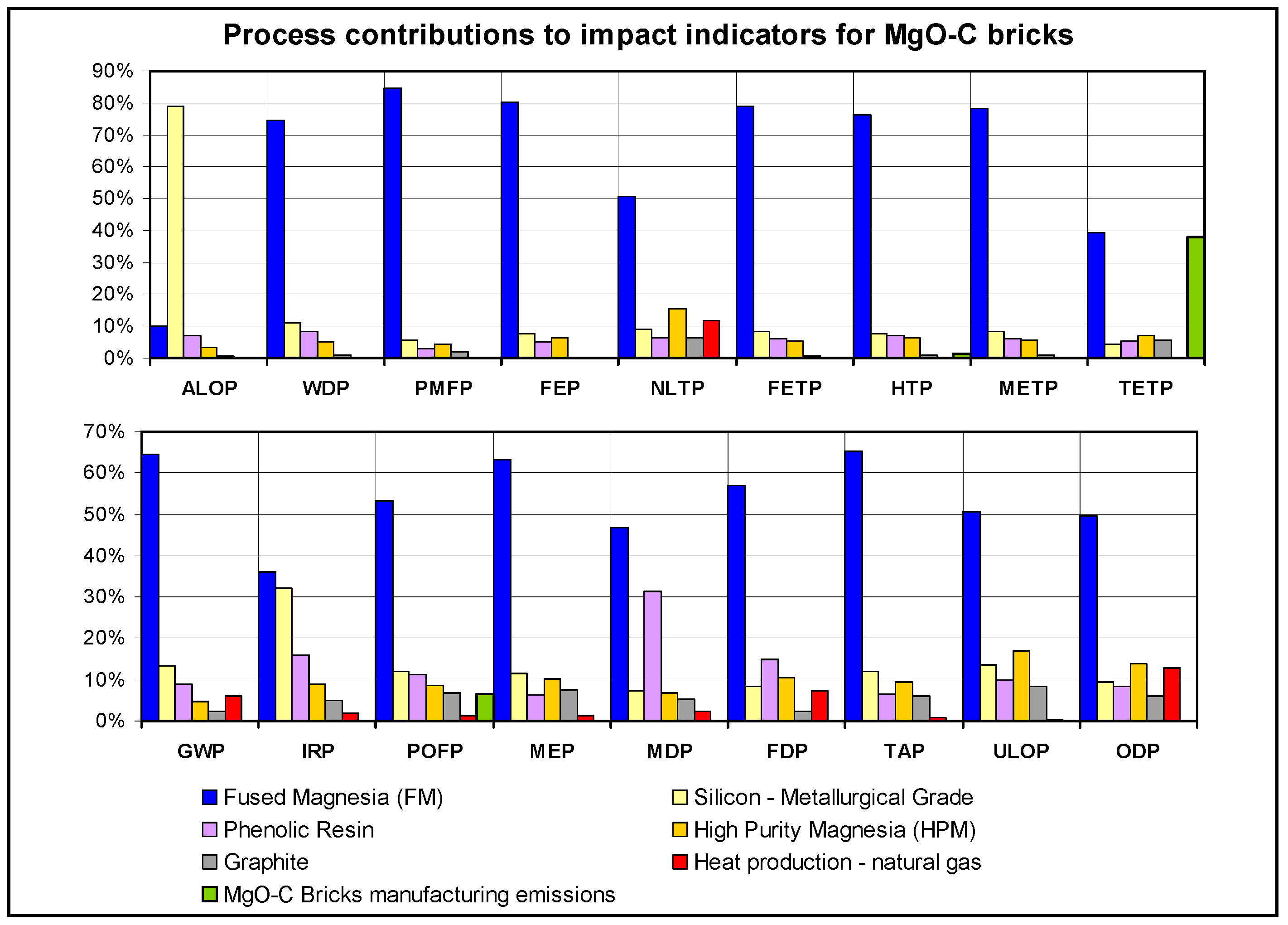
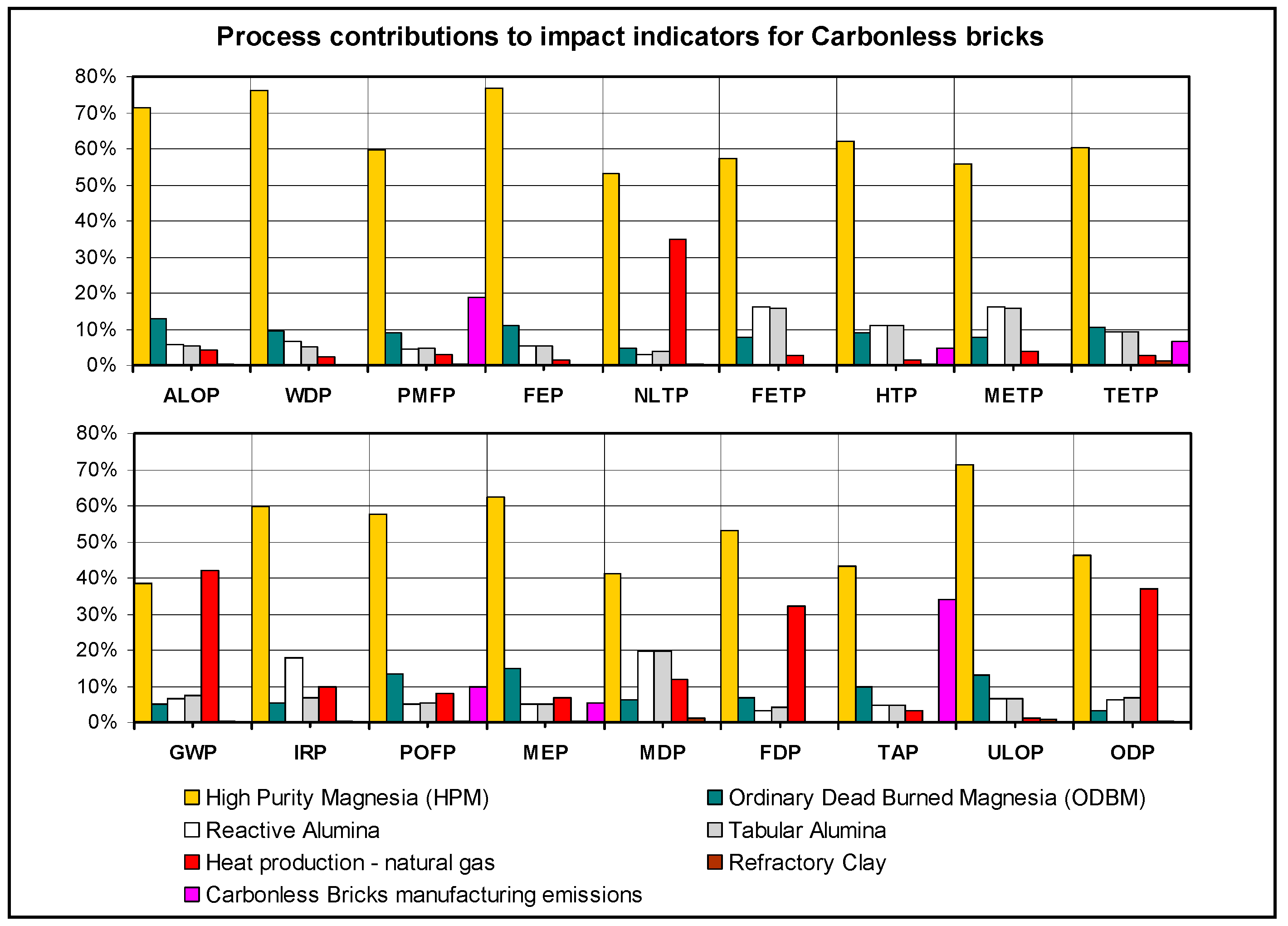
3.2. Analysis of the Major Process Contributors to Impacts
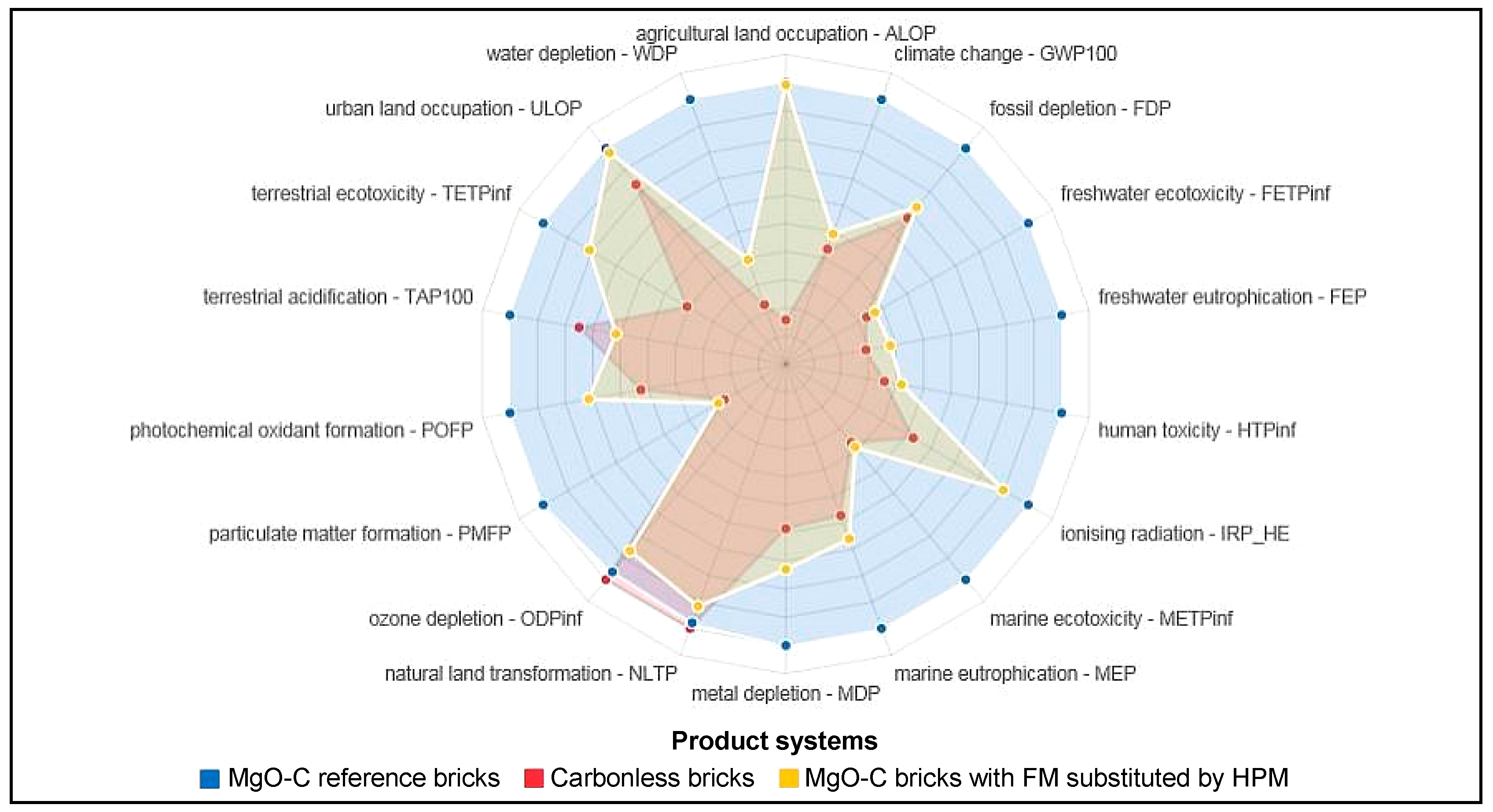
3.3. Results of the Comparison Substituting Fused Magnesia with High Purity Magnesia in the Reference Bricks’ Recipe
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Input 1/Output | Flow | Value |
|---|---|---|
| Input materials (t) | DBM97 | 0.2154 |
| FM97 | 0.6154 | |
| Graphite | 0.1231 | |
| Phenolic resin | 0.05128 | |
| Antioxidants (silicon) | 0.0205 | |
| Energy consumption (MJ) | Heat (from gas) | 1800 |
| Emissions to air (kg) | Water | 5.878 |
| CO | 2.148 | |
| CO2 | 0.982 | |
| CH4 | 2.237 | |
| Phenol | 9.318 | |
| Xylenol 2 | 3.066 | |
| Benzene | 0.218 | |
| Toluene | 0.385 | |
| H2 | 1.408 | |
| Output wanted (t) | MgO-C bricks | 1 |
| Input 1/ Output | Flow | Value |
|---|---|---|
| Input materials [t] | DBM97 | 0.7488 |
| DBM90 | 0.1497 | |
| Tabular alumina | 0.04 | |
| Reactive alumina | 0.04 | |
| Clay | 0.02 | |
| Carboxylic acid salt of Aluminum | 0.0015 | |
| Water | sensitive information | |
| Energy consumption [MJ] | Heat (from gas) | 5430 |
| Emissions to air [g] (original values as reported in [1]) | CO | 3237 |
| CO2 | 425.785 | |
| SO2 | 2465 | |
| NOx | 183 | |
| Particulate matter | 30.2 | |
| Hydrogen Fluoride | 38.25 | |
| Hydrogen Chloride | 23.44 | |
| 4-chlorotoluene 2 | 0.921 | |
| 2-chlorotoluene 3 | 0.914 | |
| 1,1,2,2-tetrachloroethane | 20.37 | |
| Benzene | 5.149 | |
| Toluene | 7.916 | |
| p+m Xylene 4 | 1.504 | |
| Ethylbenzene 5 | 1.496 | |
| Styrene | 0.698 | |
| O-xylene | 0.954 | |
| Isopropyl-Benzene 6 | 0.083 | |
| n-propyl-Benzene 7 | 0.215 | |
| Trimethyl-Benzene | 0.895 | |
| Butylbenzenes 8 | 0.936 | |
| 4-Isopropyltoluen 9 | 0.264 | |
| Naphthalene | 0.123 | |
| Formaldehyde | 1.99 | |
| Arsenic | 0.0729 | |
| Chromium | 0.1102 | |
| Cadmium | 0.0044 | |
| Copper | 0.1248 | |
| Manganese | 0.0189 | |
| Nickel | 0.0038 | |
| Lead | 0.097 | |
| Titanium | 0.2011 | |
| Water vapour | same as input (sensitive information) | |
| Output wanted [t] | Carbonless bricks | 1 |
References
- Ozkan, A.; Günkaya, Z.; Tok, G.; Karacasulu, L.; Metesoy, M.; Banar, M.; Kara, A. Life cycle assessment and life cycle cost analysis of magnesia spinel brick production. Sustainability 2016, 8, 662. [Google Scholar] [CrossRef]
- Lachmund, H. Demands on refractory material for secondary metallurgy. In Proceedings of the International Seminars Refractories Technology—Steel Ladle Lining, Ijmuiden, The Netherlands, 20–22 June 2002. [Google Scholar]
- Buhr, A. Trends in Clean Steel Technology and Refractories Engineering. In Proceedings of the UNITECR 2015, Wein, Austria, 15–18 September 2015. [Google Scholar]
- Roberts, J. Outlook for Refractory End Market to 2020. In Proceedings of the 57th International Colloquium on Refractories, Aachen, Germany, 24–25 September 2014. [Google Scholar]
- Lange, K.W. Thermodynamic and kinetic aspects of secondary steelmaking processes. Int. Mater. Rev. 1988, 33, 53–89. [Google Scholar] [CrossRef]
- Hansén, T.; Jönsson, P. Some ideas of determining the macro inclusion characteristics during steelmaking. In Proceedings of the 24th Electric Furnace Conference, Phoenix, AZ, USA, 11–14 November 2004. [Google Scholar]
- Wang, H.; White, J.F.; Sichen, D. A New Experimental Design to Study the Kinetics of Solid Dissolution into Liquids at Elevated Temperature. Metall. Mater. Trans. B 2018, 49, 688–698. [Google Scholar] [CrossRef] [Green Version]
- openLCA—The Life Cycle and Sustainability Modeling Suite. Available online: http://www.openlca.org/openlca (accessed on 13 January 2019).
- International Organization for Standardization (ISO). ISO 14044:2006 Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO Central Secretariat: Geneva, Switzerland, 2006. [Google Scholar]
- Glossary Page on the Ecoinvent Database Official Site. Available online: https://www.ecoinvent.org/support/glossary/glossary-detail.html (accessed on 13 January 2019).
- Margni, M.; Curran, M.A. Chapter 4—Life Cycle Impact Assessment. In Life Cycle Assessment Handbook: A Guide for Environmentally Sustainable Products; Curran, M.A., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2012; pp. 67–104. ISBN 978-1-118-09972-8. [Google Scholar]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; van Zelm, R. ReCiPe 2008—A Life Cycle Impact Assessment Method Which Comprises Harmonised Category Indicators at the Midpoint and the Endpoint Level—Report I: Characterisation, First edition (version 1.08)—May 2013 Amendment; Ministerie van VROM: Den Haag, The Netherlands, 2013. [Google Scholar]
- About RIVM. Available online: https://www.rivm.nl/en/about-rivm (accessed on 13 January 2019).
- European Commission—Joint Research Center (JRC)—Institute for Environment and Sustainability (IES). ILCD Handbook: Recommendations for Life Cycle Impact Assessment in the European Context—Based on Existing Environmental Impact Assessment Models and Factors, 1st ed.; Publications Office of the European Union: Luxemburg, 2011; ISBN 978-92-79-17451-3. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Kaya, S.; Mançuhan, E.; Küçükada, K. Modelling and optimization of the firing zone of a tunnel kiln to predict the optimal feed locations and mass fluxes of the fuel and secondary air. Appl. Energy 2009, 86, 325–332. [Google Scholar] [CrossRef]
- European Commission—Joint Research Center (JRC). Reference Document on Best Available Techniques in the Ceramic Manufacturing Industry; European Commission: Luxemburg, 2007. [Google Scholar]
- Prasertsan, S.; Theppaya, T.; Prateepchaikul, G.; Kirirat, P. Development of an energy-efficient brick kiln. Int. J. Energy Res. 1997, 21, 1363–1383. [Google Scholar] [CrossRef]
- Irie, S.; Rappolt, J. Chapter 19—Phenolic Resin for Refractories. In Phenolic Resins: A Century of Progress; Pilato, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 503–516. ISBN 978-3-642-04713-8, e-ISBN 978-3-642-04714-5. [Google Scholar]
- Funabiki, K.; Nakamura, M.; Tsuriya, M. Carbonization of Phenolic Resins. Jpn. Thermosetting Plast. Ind. Assoc. 1981, 2, 220–235. [Google Scholar] [CrossRef]
- Ebner, C.; Skala, K.; Rechberger, L.; Neubauer, B. Avoidance of Hazardous Substances via Low Emission MgO-C Technology Shown with the Example of a Ladle Lining Refractory. RHI Bull. J. Refract. Innov. 2017, 22–27. Available online: http://digital.library.aist.org/pages/PR-372-292.htm (accessed on 13 January 2019).
- Li, J.; Zhang, Y.; Shao, S.; Zhang, S. Comparative life cycle assessment of conventional and new fused magnesia production. J. Clean. Prod. 2015, 91, 170–179, Supplementary data in Appendix A. [Google Scholar] [CrossRef]
- Mikami, H.M. Chapter 10—Refractory MgO. In Proceedings of the Raw Materials for Refractories Conference; Smothers, W.J., Ed.; The American Ceramic Society Inc.: Columbus, OH, USA, 1983; pp. 97–118. ISBN 9780470374009. [Google Scholar]
- Li, J.; Zhang, Y.; Shao, S.; Zhang, S.; Ma, S. Application of cleaner production in a Chinese magnesia refractory material plant. J. Clean. Prod. 2016, 113, 1015–1023. [Google Scholar] [CrossRef]
- Büchel, G.; Liu, X.; Buhr, A.; Dutton, J. Review of tabular alumina as high performance refractory material. InterCeram Refract. Man. 2007, 6–12. Available online: https://www.researchgate.net/publication/288399513_Review_of_tabular_alumina_as_high_performance_refractory_material (accessed on 13 January 2019).
- Gürel, S.B.; Altun, A. Reactive alumina production for the refractory industry. Powder Technol. 2009, 196, 115–121. [Google Scholar] [CrossRef]
- Bond, F.C. Crushing and Grinding Calculations—Parts I and II. Br. Chem. Eng. 1961, 6, 378–385 & 543–548. [Google Scholar]
- 911Metallurgist Site—Table of Bond Work Index by Minerals. Available online: https://www.911metallurgist.com/blog/table-of-bond-work-index-by-minerals (accessed on 13 January 2019).
- Weiss, N.L. SME Mineral Processing Handbook; Society of Mining Engineers of the American Institute of Mining, Metallurgical, and Petroleum Engineers: New York, NY, USA, 1985. [Google Scholar]
- Li, W.; Liu, J.; Yin, Z.; Liu, W.; Zhang, Z.; Hu, Y. Technology improvement of sandy alumina production from diasporic bauxite in China. In Proceedings of the 7th International Alumina Quality Workshop, Perth, Western Australia, Australia, 16–21 October 2005. [Google Scholar]
- Alteo Alumina. Specialty Aluminas for high Performance Refractories, 2018 ed. Available online: https://www.alteo-alumina.com/IMG/pdf/alteo_2018_refractory_brochure_web.pdf (accessed on 13 January 2019).
- Vidal de Almeida, B.; Marinho de Faria, R.; Tarcizo de Oliveira Vieira, A.; Nascimento Silva, S.; Vernilli, F. Recycling of steelworks refractories: Processing and properties. Ironmak. Steelmak. 2016, 43, 775–779. [Google Scholar] [CrossRef]
- Plešingerová, B.; Vadasz, P.; Kameský, R.; Derďak, J.; Bounziova, J.; Dedinska, E.; Vojtko, M. Post-mortem characterization of alumina-c refractory bricks with organic bond from steel production: Potentially of Al2O3-material recovery. Ceramics–Silikáty 2017, 61, 223–230. [Google Scholar] [CrossRef]




| Raw Material | MgO-C Mass % | Carbonless Mass % |
|---|---|---|
| DBM97 | 21 | 74.88 |
| DBM90 | / | 14.97 |
| FM97 | 60 | / |
| Graphite | 12 | / |
| Phenolic resin | 5 | / |
| Antioxidants (silicon) | 2 | / |
| Tabular alumina | / | 4 |
| Reactive alumina | / | 4 |
| Clay | / | 2 |
| Carboxylic acid salt of Aluminum | / | 0.15 |
| Total | 100 | 100 |
| Phase & Equipment | |||
|---|---|---|---|
| Molding Phase in a Hydraulic Press | Baking/Sintering Phase in a Gas Combustion Tunnel Kiln | ||
| Type of Pressing | Temperature and Duration | Energy Consumption (Thermal Energy) | |
| MgO-C Reference Bricks | Single press cycle at 2000 t | 200 °C for 24 h | 1800 MJ/t |
| Carbonless Bricks | Single press cycle at 1600 t | Drying at 100 °C for several hours – Sintering at around 1600 °C for around one hour | 5430 MJ/t |
| Raw Material | Before Heating | After Heating | Re-calculated Mass % |
|---|---|---|---|
| DBM97 | 21 | 21 | 21.54 |
| FM97 | 60 | 60 | 61.54 |
| Graphite | 12 | 12 | 12.31 |
| Phenolic resin (in structure) | 5 | 2.5 | 2.56 |
| Phenolic resin (volatile) | / | 2.5 | 2.56 |
| Antioxidants (silicon) | 2 | 2 | 2.05 |
| Total (Solid Mass fraction only) | 100 | 97.5 | 100 |
| Substance | Water | CO | CO2 | CH4 | Phenol | Xylenol | Benzene | Toluene | H2 |
|---|---|---|---|---|---|---|---|---|---|
| mol% (at 1000 °C) | 23.4 | 5.5 | 1.6 | 10 | 7.1 | 1.8 | 0.2 | 0.3 | 50.1 |
| Molecular weight (g/mol) | 18.0153 | 28.0101 | 44.0095 | 16.0425 | 94.1112 | 122.1600 | 78.1118 | 92.1384 | 2.0159 |
| Weight% | 22.93 | 8.38 | 3.83 | 8.72 | 36.34 | 11.96 | 0.85 | 1.50 | 5.49 |
| Mass (kg) | 5.878 | 2.148 | 0.982 | 2.237 | 9.318 | 3.066 | 0.218 | 0.385 | 1.408 |
| Input/Output | Flow | Value in [22] | Adjusted Value for Yield = 0.5 |
|---|---|---|---|
| Materials consumption | Strip mine (kg) | 1087 1 | 2000 |
| Water (m3) | 0.0065 | 0.011960 | |
| Energy consumption | Electricity (kWh) | 0.327 | 0.601656 |
| Diesel (kg) | 0.268 | 0.493100 | |
| Emissions to air | CO2 (kg) | 0.069215 | 0.127351 |
| CO (kg) | 0.010648 | 0.019592 | |
| NOx (kg) | 0.005686 | 0.010462 | |
| Dust (kg) | 0.01076 | 0.019798 | |
| Water vapour (m3) | 0.0065 | 0.011960 | |
| Waste | Magnesite tailings (kg) | 51 (60%) | 600 |
| Valuable materials | Tailings powder from open magnesite mining (kg) | 34 (40%) | 400 |
| Output wanted | Magnesite Extracted (kg) | 1000 | 1000 |
| Impact Category | MgO-C Ref. Bricks | Carbonless Bricks |
|---|---|---|
| Agricultural Land Occupation (m2a) | 74.27 | 11.63 (−84.3%) |
| Water Depletion (m3) | 6.67 | 1.49 (−77.6%) |
| Particulate Matter Form. (kg PM10-Eq) | 11.14 | 2.83 (−74.6%) |
| Freshwater Eutrophication (kg P-Eq) | 1.41 | 0.41 (−71%) |
| Freshwater Ecotoxicity (kg 1.4-DCB-Eq) | 30.61 | 10.21 (−66.6%) |
| Human Toxicity (kg 1.4-DCB-Eq) | 985.37 | 352.45 (−64.2%) |
| Marine Ecotoxicity (kg 1.4-DCB-Eq) | 28.16 | 10.26 (−63.6%) |
| Terrestrial Ecotoxicity (kg 1.4-DCB-Eq) | 1.81E-1 | 7.38E-2 (−59.2%) |
| Climate Change—GWP100 (kg CO2-Eq) | 2059.04 | 894.86 (−56.5%) |
| Ionizing Radiation (kg U235-Eq) | 78.74 | 41.37 (−47.5%) |
| Photochem. Oxidant Form. (kg NMVOC) | 7.68 | 4.04 (−47.5%) |
| Marine Eutrophication (kg N-Eq) | 2.28 | 1.31 (−42.7%) |
| Metal Depletion (kg Fe-Eq) | 26.31 | 15.38 (−41.5%) |
| Fossil Depletion (kg oil-Eq) | 744.43 | 504.07 (−32.3%) |
| Terrestrial Acidification (kg SO2-Eq) | 10.08 | 7.56 (−25%) |
| Urban Land Occupation (m2a) | 17.03 | 14.18 (−16.7%) |
| Natural Land Transformation (m2) | 2.61E-1 | 2.67E-1 (+2.2%) |
| Ozone Depletion (kg CFC-11-Eq) | 1.23E-4 | 1.27E-4 (+3.7%) |
| Impact Category | MgO-C Ref. Bricks | Carbonless Bricks | MgO-C Reference Bricks with FM Substituted by HPM |
|---|---|---|---|
| Particulate Matter Form. (kg PM10-Eq) | 11.14 | 2.83 (−74.6%) | 3.095 (−72.2%) |
| Marine Ecotoxicity (kg 1.4-DCB-Eq) | 28.16 | 10.26 (−63.6%) | 10.85 (−61.5%) |
| Freshwater Ecotoxicity (kg 1.4-DCB-Eq) | 30.61 | 10.21 (−66.6%) | 11.24 (−63.3%) |
| Human Toxicity (kg 1.4-DCB-Eq) | 985.37 | 352.45 (−64.2%) | 413.48 (−58%) |
| Climate Change—GWP100 (kg CO2-Eq) | 2059.04 | 894.86 (−56.5%) | 1011.3 (−50.9%) |
| Freshwater Eutrophication (kg P-Eq) | 1.41 | 0.41 (−71%) | 0.53 (−62.1%) |
| Fossil Depletion (kg oil-Eq) | 744.43 | 504.07 (−32.3%) | 541.66 (−27.2%) |
| Marine Eutrophication (kg N-Eq) | 2.28 | 1.31 (−42.7%) | 1.51 (−33.9%) |
| Water Depletion (m3) | 6.67 | 1.49 (−77.6%) | 2.63 (−60.6%) |
| Metal Depletion (kg Fe-Eq) | 26.31 | 15.38 (−41.5%) | 19.19 (−27%) |
| Photochem. Oxidant Form. (kg NMVOC) | 7.68 | 4.04 (−47.5%) | 5.49 (−28.5%) |
| Terrestrial Ecotoxicity (kg 1.4-DCB-Eq) | 1.81E-1 | 7.38E-2 (−59.2%) | 1.46E-1 (−19.1%) |
| Ionising Radiation (kg U235-Eq) | 78.74 | 41.37 (−47.5%) | 70.57 (−10.4%) |
| Urban Land Occupation (m2a) | 17.03 | 14.18 (−16.7%) | 16.69 (−2%) |
| Agricultural Land Occupation (m2a) | 74.27 | 11.63 (−84.3%) | 73.64 (−0.9%) |
| Terrestrial Acidification (kg SO2-Eq) | 10.08 | 7.56 (−25%) | 6.2 (−38.5%) |
| Ozone Depletion (kg CFC-11-Eq) | 1.23E-4 | 1.27E-4 (+3.7%) | 1.1E-4 (−10.2%) |
| Natural Land Transformation (m2) | 2.61E-1 | 2.67E-1 (+2.2%) | 2.45E-1 (−6.4%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boenzi, F.; Ordieres-Meré, J.; Iavagnilio, R. Life Cycle Assessment Comparison of Two Refractory Brick Product Systems for Ladle Lining in Secondary Steelmaking. Sustainability 2019, 11, 1295. https://doi.org/10.3390/su11051295
Boenzi F, Ordieres-Meré J, Iavagnilio R. Life Cycle Assessment Comparison of Two Refractory Brick Product Systems for Ladle Lining in Secondary Steelmaking. Sustainability. 2019; 11(5):1295. https://doi.org/10.3390/su11051295
Chicago/Turabian StyleBoenzi, Francesco, Joaquín Ordieres-Meré, and Raffaello Iavagnilio. 2019. "Life Cycle Assessment Comparison of Two Refractory Brick Product Systems for Ladle Lining in Secondary Steelmaking" Sustainability 11, no. 5: 1295. https://doi.org/10.3390/su11051295






