The Prospective Approach for the Reduction of Fluoride Ions Mobility in Industrial Waste by Creating Products of Commercial Value
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Methodology
2.2.1. The Chemical Analysis
2.2.2. The Leaching of Fluoride Ions
2.2.3. The Application of Silica Gel Waste
2.3. Instrumental Analysis
3. Results and Discussion
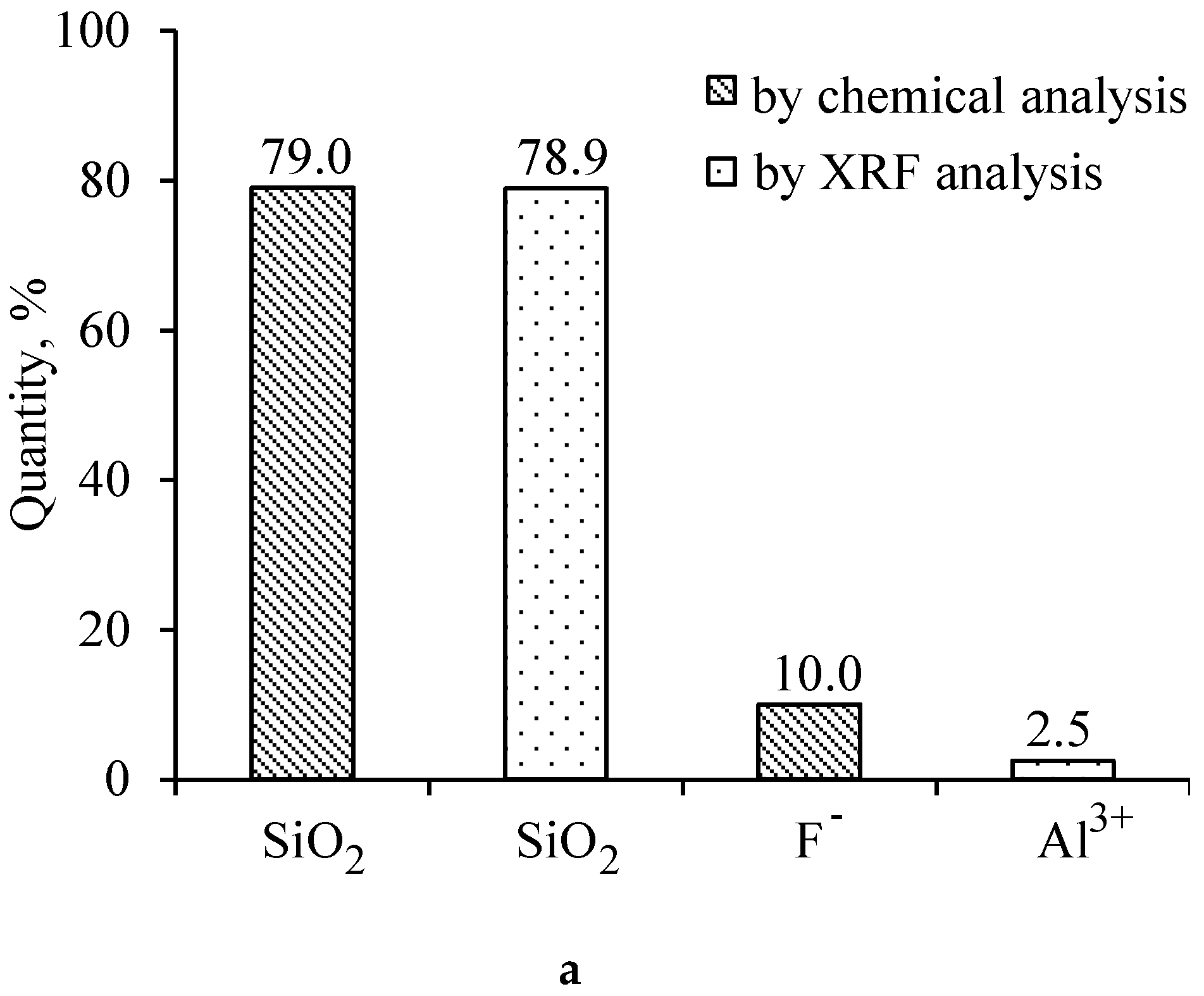
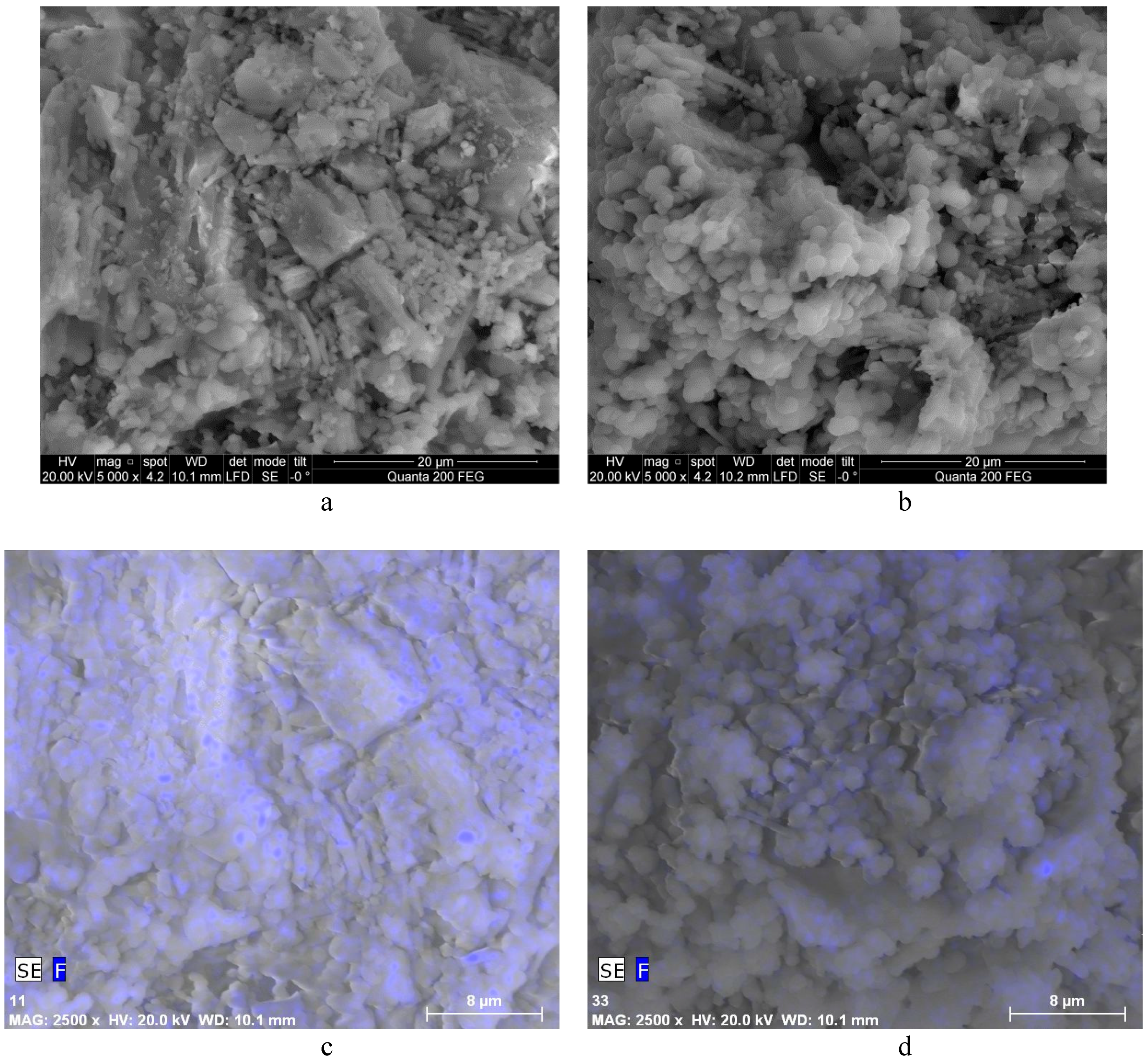
3.1. The Properties of Silica Gel Waste (SGW)
3.2. The Leaching Peculiarities of F− Ions under Static and Dynamic Conditions
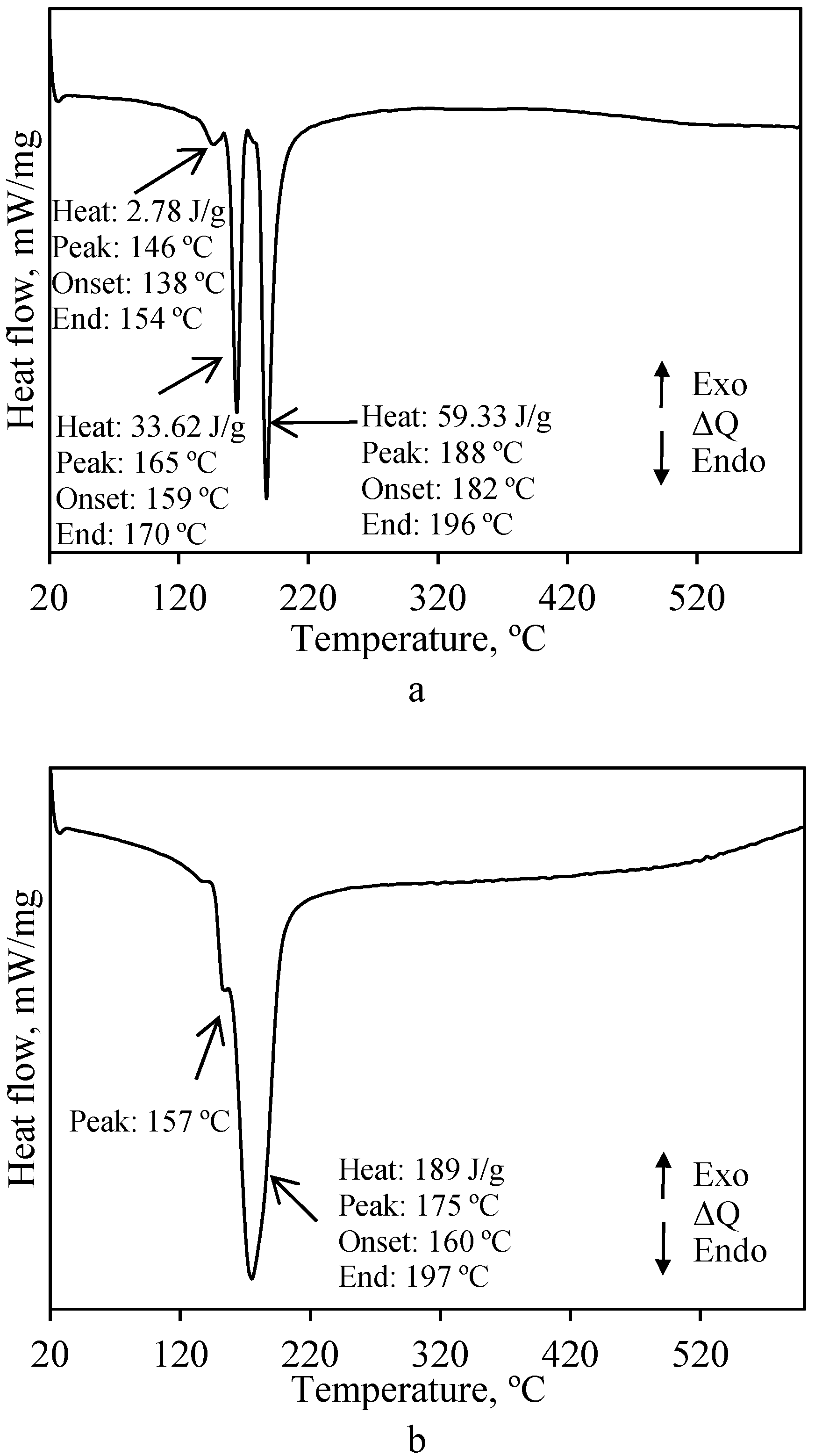
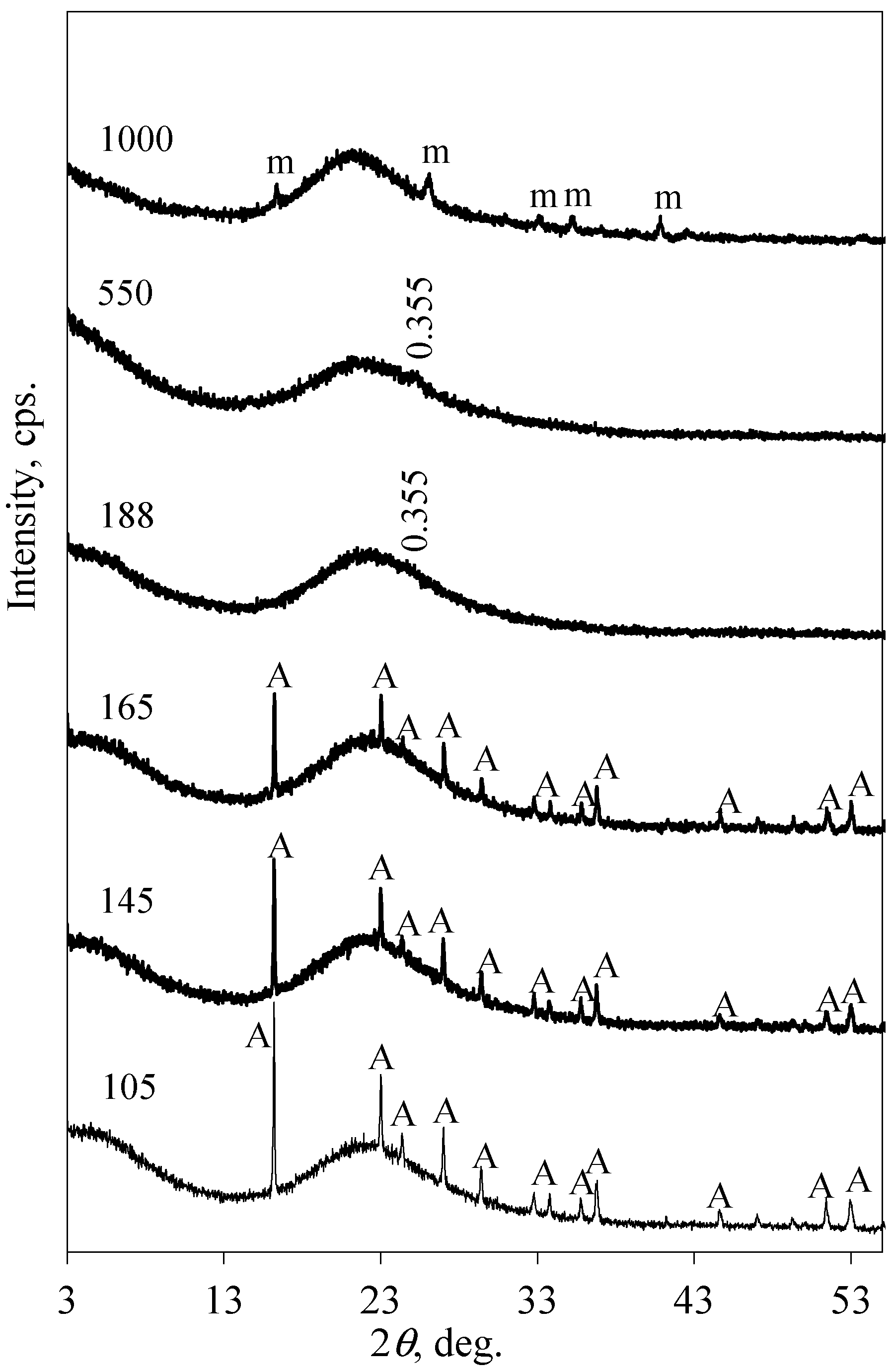
3.3. Application of SGW for the Direct Hydrothermal Synthesis of Cuspidine
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angin, D. Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.W. Thermal utilization (treatment) of plastic waste. Energy 2015, 90, 1468–1477. [Google Scholar] [CrossRef]
- Moh, Y.C.; Abd Manaf, L. Solid waste management transformation and future challenges of source separation and recycling practice in Malaysia. Resour. Conserv. Recy. 2017, 116, 1–14. [Google Scholar] [CrossRef]
- Shen, C.; Tran, P.P.; Ly, P.T.M. Chemical waste management in the U.S. Semiconductor industry. Sustainability 2018, 10, 1545. [Google Scholar] [CrossRef]
- Stonys, R.; Kuznetsov, D.; Krasnikovs, A.; Škamat, J.; Baltakys, K.; Antonovič, V.; Černašėjus, O. Reuse of ultrafine mineral wool production waste in the manufacture of refractory concrete. J. Environ. Manag. 2016, 176, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Kara, B.Y.; Yetis, U. Hazardous waste management system design under population and environmental impact considerations. J. Environ. Manag. 2017, 203, 720–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźniak, J.; Pactwa, K. Overview of polish mining wastes with circular economy model and its comparison with other wastes. Sustainability 2018, 10, 3994. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of industrial by-products in concrete. Procedia Eng. 2014, 95, 335–347. [Google Scholar] [CrossRef]
- Dyachenko, A.N.; Petlin, I.V.; Malyutin, L.N. The research of sulfuric acidic recycling of aluminum industry fluorine-containing waste products. Procedia Chem. 2014, 11, 10–14. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, Z.; Shao, L.; He, P. Treatment and resource recovery from inorganic fluoride-containing waste produced by the pesticide industry. J. Environ. Sci. 2015, 31, 21–29. [Google Scholar] [CrossRef]
- De Beer, M.; Doucet, F.J.; Maree, J.P.; Liebenberg, L. Synthesis of high-purity precipitated calcium carbonate during the process of recovery of elemental sulphur from gypsum waste. Waste Manag. 2015, 46, 619–627. [Google Scholar] [CrossRef]
- Beak, C.; Seo, J.; Choi, M.; Cho, J.; Ahn, J.; Cho, K. Utilization of CFBC fly ash as a binder to produce in-furnace desulfurization sorbent. Sustainability 2018, 10, 4854. [Google Scholar] [CrossRef]
- Krysztafkiewicz, A.; Rager, B.; Maik, M. Silica recovery from waste obtained in hydrofluoric acid and aluminum fluoride production from fluosilicic acid. J. Hazard. Mater. 1996, 48, 31–49. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Vaitkevičius, V.; Kantautas, A.; Sasnauskas, V. Utilization of by-product waste silica in concrete-based materials. Mat. Res. 2012, 15, 561–567. [Google Scholar] [CrossRef]
- Joint-Stock “Lifosa” Official Website. Available online: https://www.alufluor.com/alusilica/ (accessed on 1 February 2018).
- Sener, S.; Sener, E.; Karagüzel, R. Solid waste disposal site selection with GIS and AHP methodology: A case study in Senirkent–Uluborlu (Isparta) Basin, Turkey. Environ. Monit. Assess. 2011, 173, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Iljina, A.; Baltakys, K.; Baltakys, M.; Siauciunas, R. Neutralization and removal of compounds containing fluoride ions from waste silica gel. Rev. Rom. Mater. 2014, 44, 265–271. [Google Scholar]
- Iljina, A.; Baltakys, K.; Bankauskaite, A.; Eisinas, A.; Kitrys, S. The stability of formed CaF2 and its influence on the thermal behavior of C–S–H in CaO–silica gel waste-H2O system. J. Therm. Anal. Calorim. 2017, 127, 221–228. [Google Scholar] [CrossRef]
- Kaminskas, R.; Kubiliute, R. Artificial pozzolana from silica gel waste-clay-limestone composite. Adv. Cem. Res. 2014, 26, 155–168. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Feng, C.; Zhu, Y.; Pang, Y.; Hou, J.; Luo, K.; Liao, X. Non-competitive and competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto SDS in process of micellar-enhanced ultrafiltration. Sustainability 2018, 10, 92. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, X.; Gao, C.; Yu, Z. Adsorption behavior of inorganic and organic phosphate by iron manganese plaques on reed roots in wetlands. Sustainability 2018, 10, 4578. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Q.; Rao, P.; Yan, L.; Shakib, A.; Shen, G. Formulating and Optimizing a Novel Biochar-Based Fertilizer for Simultaneous Slow-Release of Nitrogen and Immobilization of Cadmium. Sustainability 2018, 10, 2740. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, D. immobilization of heavy metals in sewage sludge during land application process in China: A review. Sustainability 2017, 9, 2020. [Google Scholar] [CrossRef]
- Kim, J.; Tae, S.; Kim, R. Theoretical study on the production of environment-friendly recycled cement using inorganic construction wastes as secondary materials in South Korea. Sustainability 2018, 10, 4449. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Rat, A.I.; Azarova, T.A.; Azarov, S.M.; Al-Khowaiter, S.H.; Al-Harbi, O.; Shemchonok, S.V.; Dobysh, V.A.; Tarasevich, V.A.; Agabekov, V.E.; et al. Preparation and properties of microfiltration membranes based on natural crystalline SiO2. Ceram. Int. 2014, 40, 12343–12351. [Google Scholar] [CrossRef]
- Koner, S.; Pal, A.; Adak, A. Utilization of silica gel waste for adsorption of cationic surfactant and adsolubilization of organics from textile wastewater: A case study. Desalination 2011, 276, 142–147. [Google Scholar] [CrossRef]
- Rat’ko, A.I.; Ivanets, A.I.; Azarov, S.M. Effect of additives on the pore structure of ceramics based on crystalline SiO2. Inorg Mater. 2008, 44, 778–784. [Google Scholar] [CrossRef]
- Unob, F.; Wongsiri, B.; Phaeon, N.; Puanngam, M.; Shiowatana, J. Reuse of waste silica as adsorbent for metal removal by iron oxide modification. J. Hazard. Mater. 2007, 142, 455–462. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Kantautas, A.; Vaitkevičius, V.; Jakevičius, L.; Rudžionis, Ž.; Paškevičius, M. Effects of ultrasonic treatment on zeolite NaA synthesized from by-product silica. Ultrason. Sonochem. 2015, 27, 515–521. [Google Scholar] [CrossRef]
- LTD “Alufluor AB” Official Website. Available online: http://www.lifosa.com/en/products-and-services (accessed on 10 February 2018).
- Aldaco, R.; Garea, A.; Irabien, A. Calcium fluoride recovery from fluoride wastewater in a fluidized bed reactor. Water Res. 2007, 41, 810–818. [Google Scholar] [CrossRef]
- Gogoi, S.; Nath, S.K.; Bordoloi, S.; Dutta, R.K. Fluoride removal from groundwater by limestone treatment in presence of phosphoric acid. J. Environ. Manag. 2015, 152, 132–139. [Google Scholar] [CrossRef]
- Liu, W.T.; Li, K.C. Application of reutilization technology to calcium fluoride sludge from semiconductor manufacturers. J. Air Waste Manag. Assoc. 2011, 61, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Baltakys, K.; Iljina, A.; Bankauskaite, A. Thermal properties and application of silica gel waste contaminated with F− ions for C-S-H synthesis. J. Therm. Anal. Calorim. 2015, 121, 145–154. [Google Scholar] [CrossRef]
- Casasola, R.; Pérez, J.M.; Romero, M. Effect of fluorine content on glass stability and the crystallisation mechanism for glasses in the SiO2–CaO–K2O–F system. J. Non-Cryst. Solids 2013, 378, 25–33. [Google Scholar] [CrossRef]
- Seo, M.D.; Shi, C.B.; Wang, H.; Cho, J.W.; Kim, S.H. Non-isothermal melt crystallization of cuspidine in CaO–SiO2–CaF2 based glasses. J. Non-Cryst. Solids 2015, 412, 58–65. [Google Scholar] [CrossRef]
- Jung, I.H.; Lehmann, J.; Jak, E. Treatise on Process Metallurgy. Volume 2: Process Phenomena. Chapter 5.3—Applications; Elsevier: Oxford, UK, 2014; pp. 675–798. [Google Scholar]
- Rungchet, A.; Chindaprasirt, P.; Wansom, S.; Pimraksa, K. Hydrothermal synthesis of calcium sulfoaluminate–belite cement from industrial waste materials. J. Clean. Prod. 2016, 115, 273–283. [Google Scholar] [CrossRef]
- Dambrauskas, T.; Baltakys, K.; Eisinas, A.; Siauciunas, R. A study on the thermal stability of kilchoanite synthesized under hydrothermal conditions. J. Therm. Anal. Calorim. 2017, 127, 229–238. [Google Scholar] [CrossRef]
- Delong, X.; Yongqin, L.; Ying, J.; Longbao, Z.; Wenkui, G. Thermal behavior of aluminum fluoride trihydrate. Thermochim. Acta 2000, 352–353, 47–52. [Google Scholar] [CrossRef]
- Yang, G.Y.; Shi, Y.C.; Liu, X.D.; Mujumdar, A.S. TG-DTG analysis of chemically bound moisture removal of AlF3·3H2O. Dry Technol. 2007, 25, 675–680. [Google Scholar] [CrossRef]
- Krysztafkiewicz, A.; Świt, Z.; Jesionowski, T. Evaluation of waste silica precipitated in the process of hydrofluoric acid production from fluosilicic acid. Physicochem. Probl. Miner. Process. 2005, 39, 165–176. [Google Scholar]


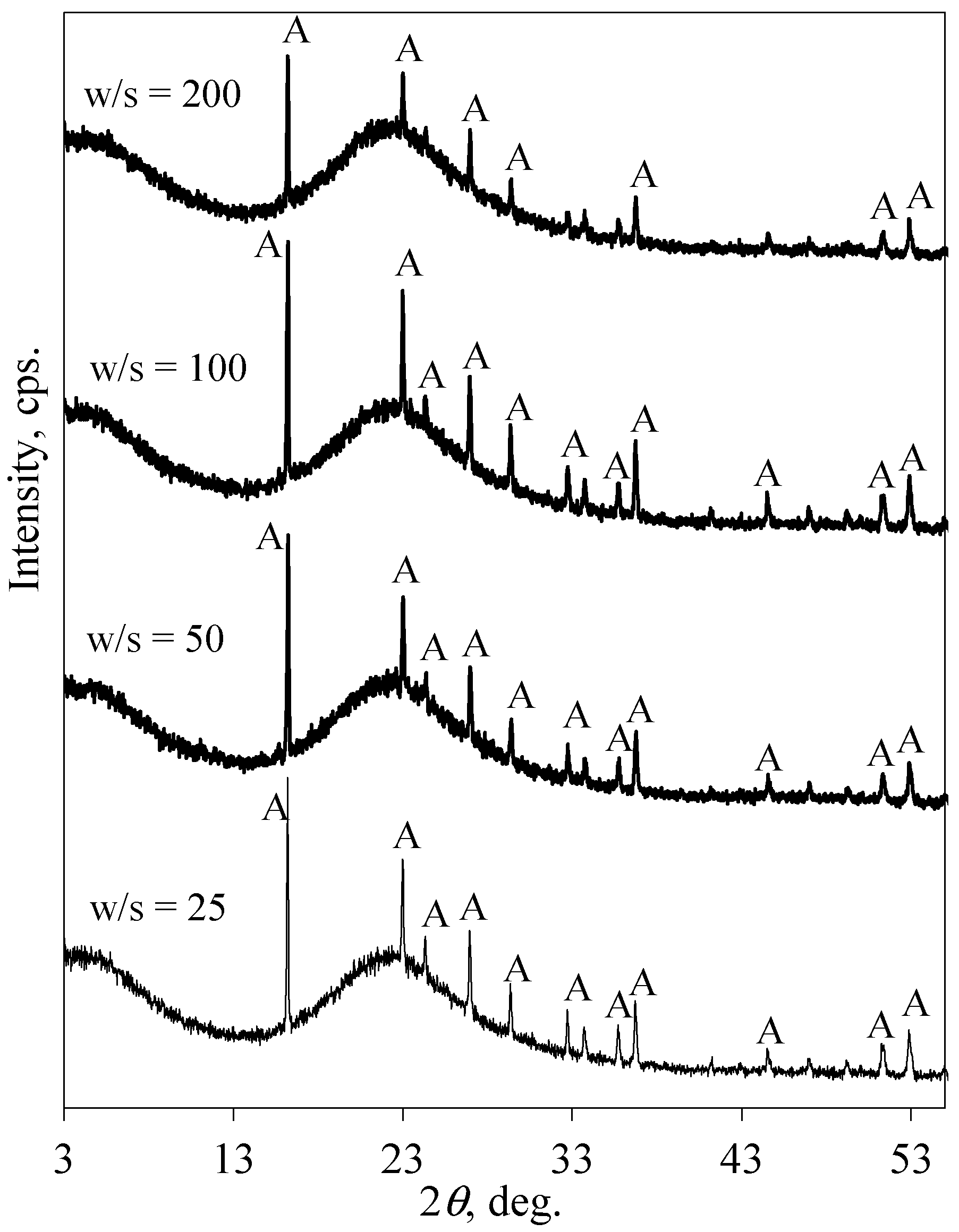
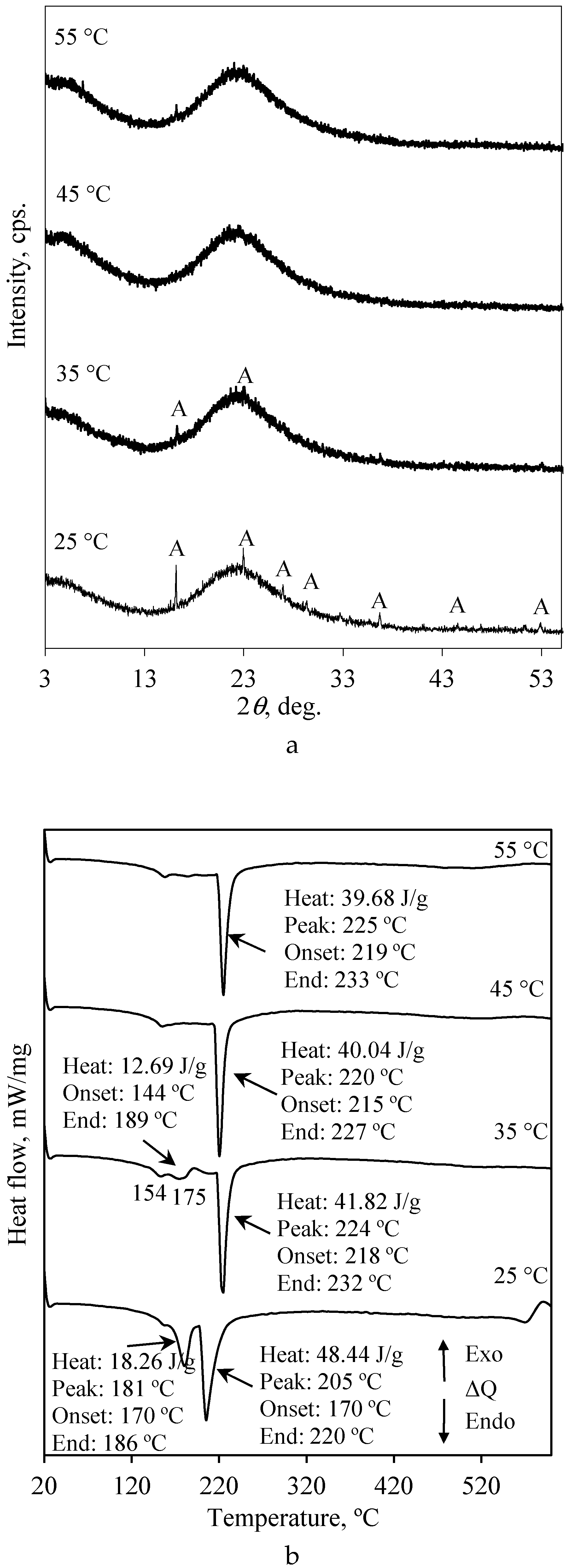
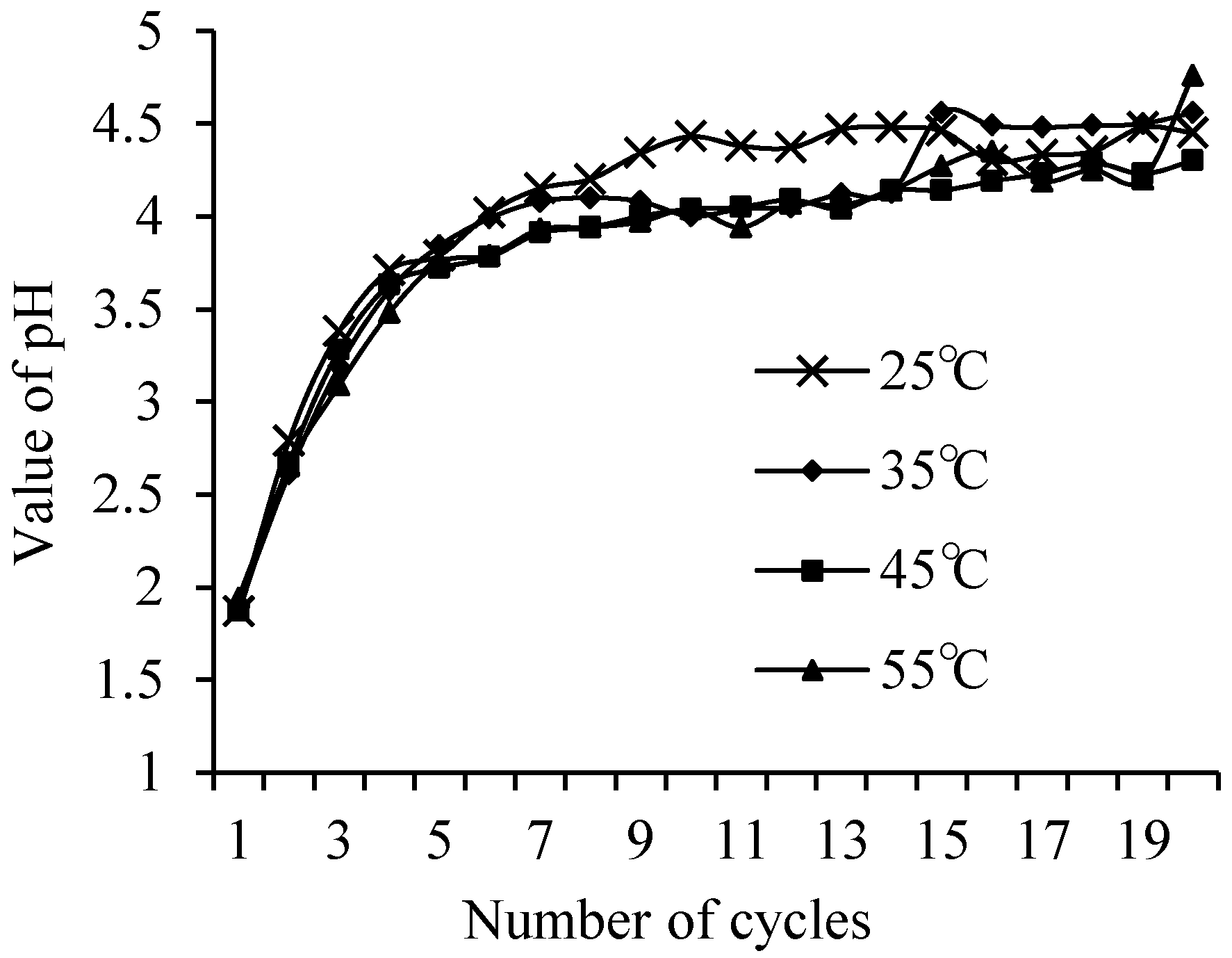


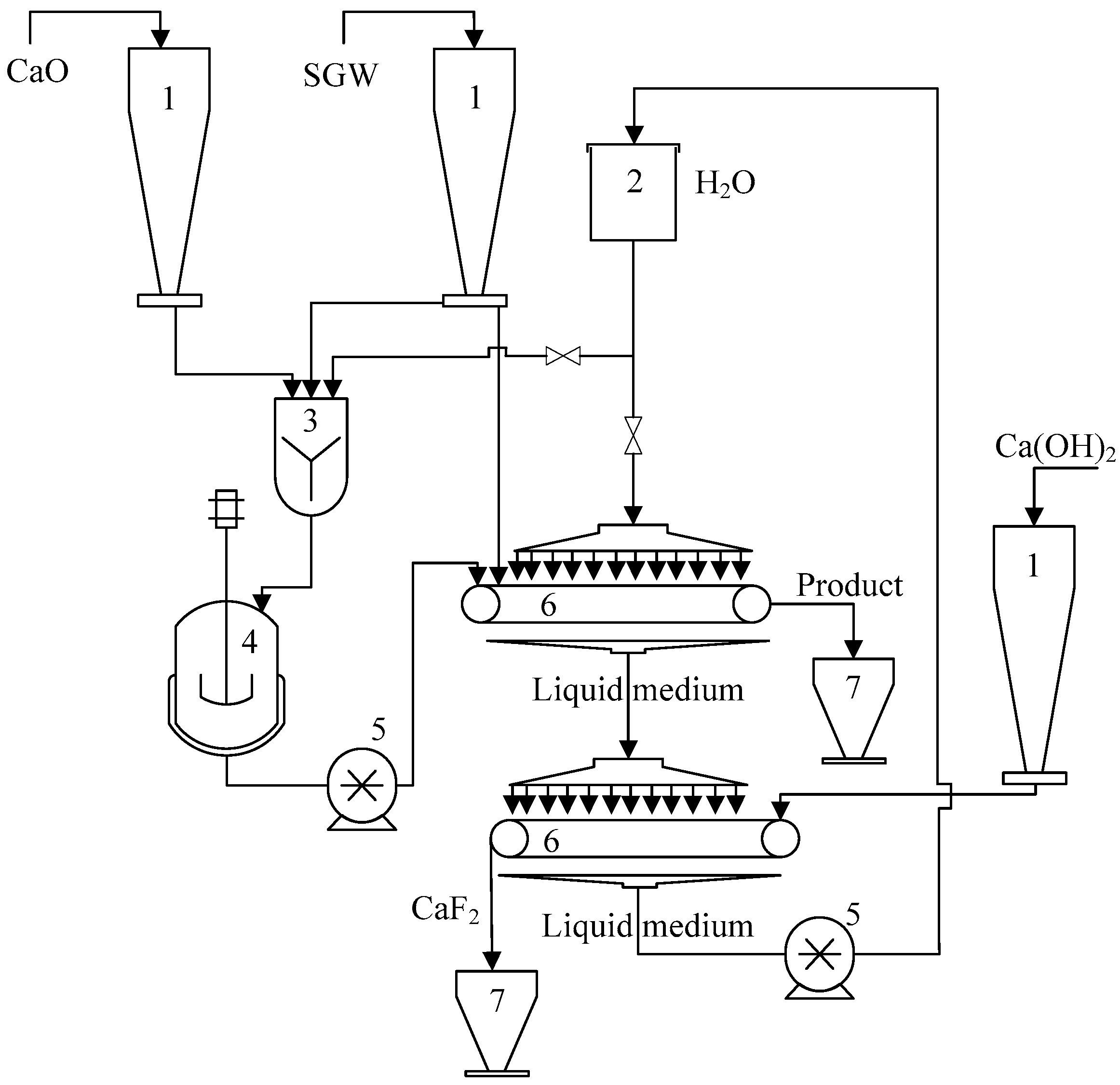
| Sample Name * | The Amount of F− Ions in Solid, % | The Amount of Released F− Ions, % | pH of Liquid Medium |
|---|---|---|---|
| SGW-25 °C-2 | - | 1.53 | |
| SGW-25 °C-4 | - | 1.76 | |
| SGW-25 °C-6 | - | 1.95 | |
| SGW-25 °C-8 | - | 2.06 | |
| SGW-25 °C-100 | 5.08 | 49.3 | 2.94 |
| SGW-25 °C-200 | 4.71 | 53.1 | 3.02 |
| SGW-35 °C-100 | 4.92 | 50.9 | 2.94 |
| SGW-45 °C-100 | 4.68 | 53.3 | 2.92 |
| SGW-55 °C-100 | 4.21 | 58.0 | 2.90 |
| Sample Name * | Density, kg/m3 | Loss on Ignition, % | |
|---|---|---|---|
| Temperature, °C | |||
| 50 | 188 | ||
| Untreated SGW | 2141 | 50.81 | 53.39 |
| SGW-25 °C-2 | 2263 | 44.33 | 49.70 |
| SGW-25 °C-100 | 2457 | 35.16 | 39.23 |
| SGW-55 °C-100 | 2708 | 32.28 | 37.55 |
| Sample Name * | The Amount of F− Ions in Solid, % | The Amount of Released F− Ions, % | pH of Liquid Medium | The Duration of Interaction, s |
|---|---|---|---|---|
| SGW-25 | 9.60 | 4.2 | 2.45 | 40 |
| SGW-50 | - | - | 2.67 | 69 |
| SGW-100 | 8.33 | 16.9 | 2.90 | 108 |
| SGW-200 | 6.78 | 32.3 | 3.11 | 215 |
| Sample Name * | The Amount of F− Ions in Solid, % | The Amount of Released F− Ions, % |
|---|---|---|
| SGW-25 °C | 5.45 | 45.6 |
| SGW-35 °C | 4.92 | 50.9 |
| SGW-45 °C | 4.68 | 53.3 |
| SGW-55 °C | 4.44 | 55.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudelis, V.; Dambrauskas, T.; Grineviciene, A.; Baltakys, K. The Prospective Approach for the Reduction of Fluoride Ions Mobility in Industrial Waste by Creating Products of Commercial Value. Sustainability 2019, 11, 634. https://doi.org/10.3390/su11030634
Rudelis V, Dambrauskas T, Grineviciene A, Baltakys K. The Prospective Approach for the Reduction of Fluoride Ions Mobility in Industrial Waste by Creating Products of Commercial Value. Sustainability. 2019; 11(3):634. https://doi.org/10.3390/su11030634
Chicago/Turabian StyleRudelis, Valdas, Tadas Dambrauskas, Agne Grineviciene, and Kestutis Baltakys. 2019. "The Prospective Approach for the Reduction of Fluoride Ions Mobility in Industrial Waste by Creating Products of Commercial Value" Sustainability 11, no. 3: 634. https://doi.org/10.3390/su11030634





