Construction of a Self-Powered System for Simultaneous In Situ Remediation of Nitrate and Cr(VI) Contaminated Synthetic Groundwater and River Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Groundwater and Sediment
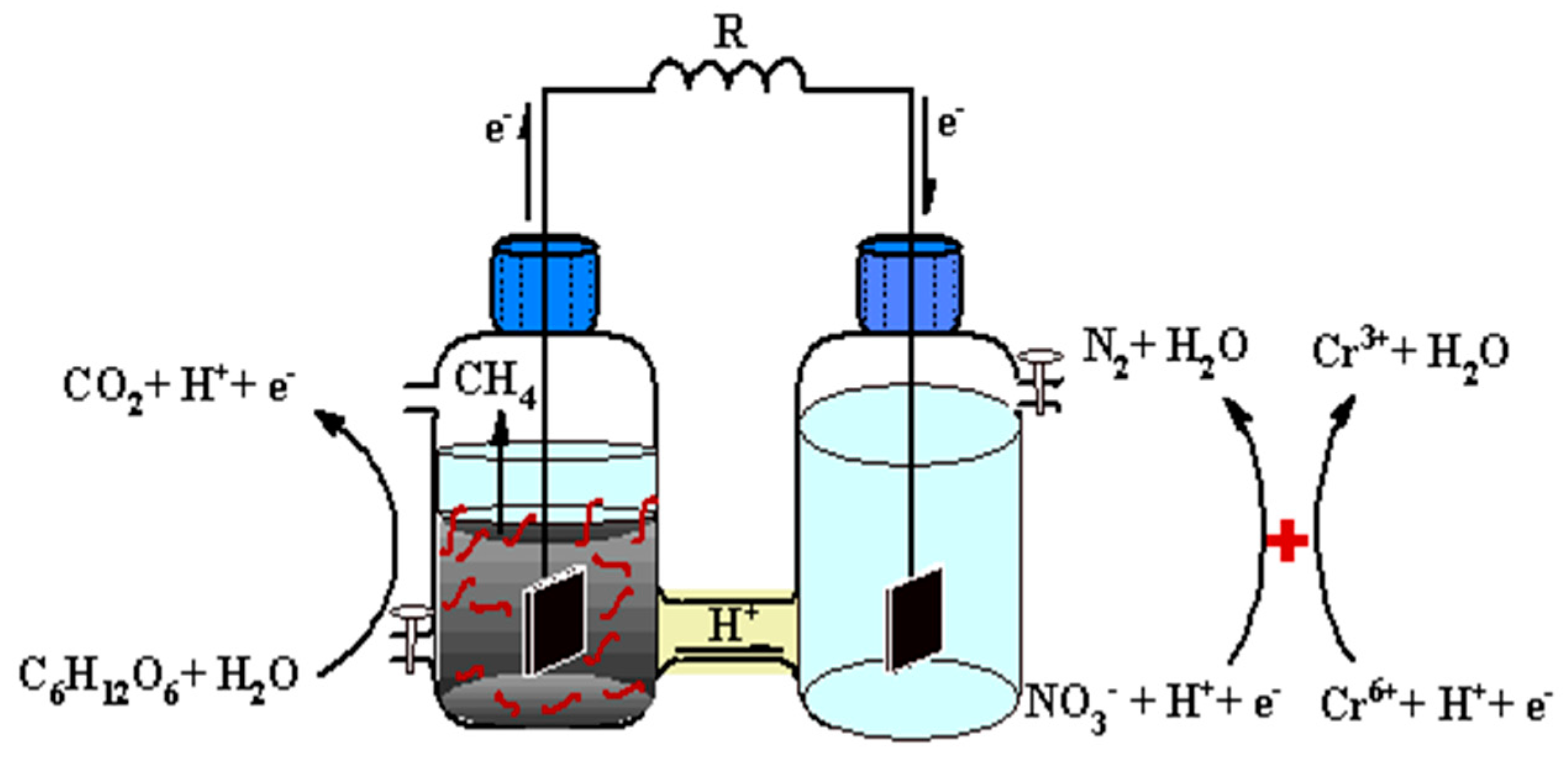
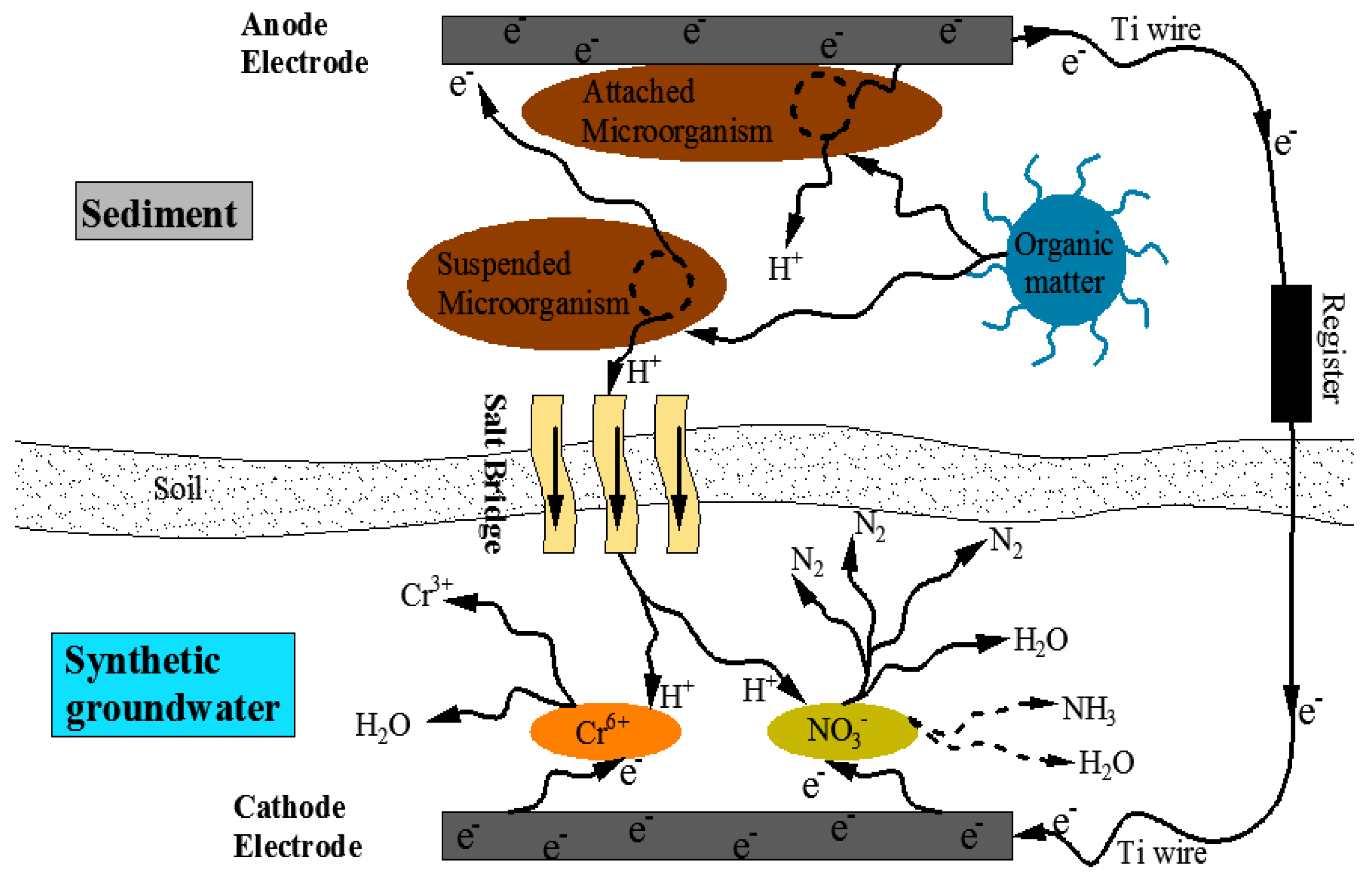
2.2. Self-Powered System Construction
2.3. Analysis and Calculation
3. Results and Discussions
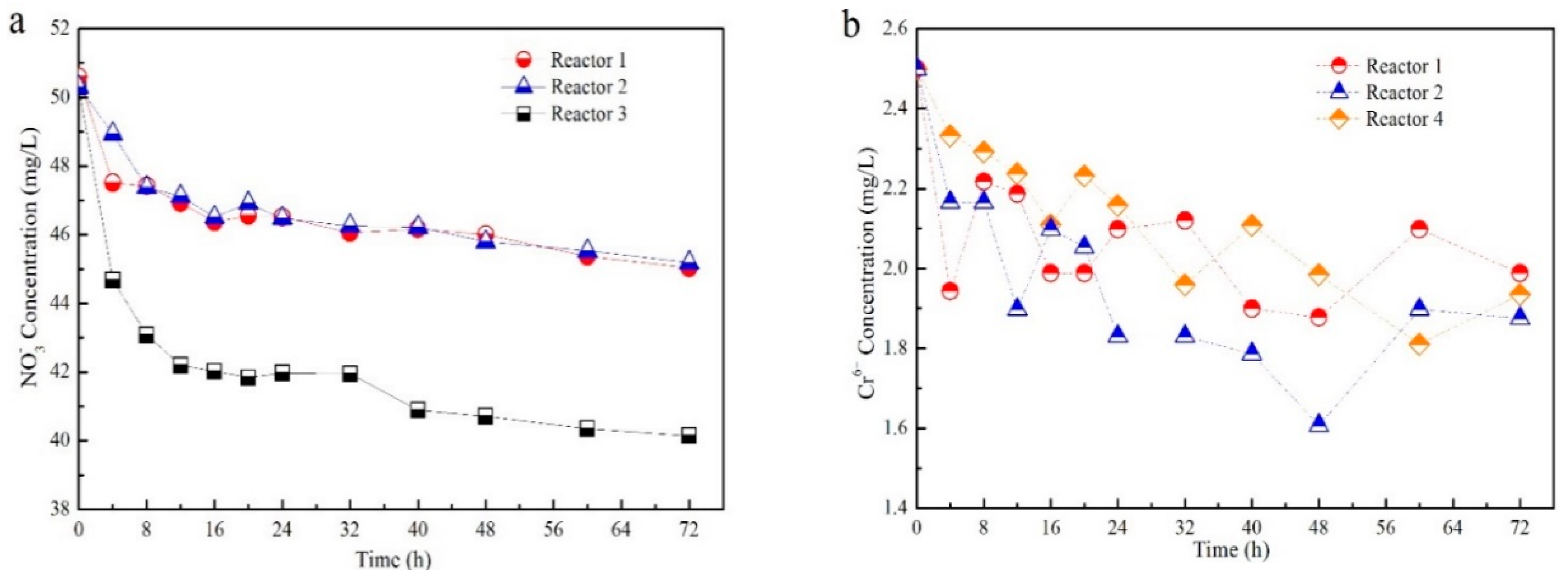
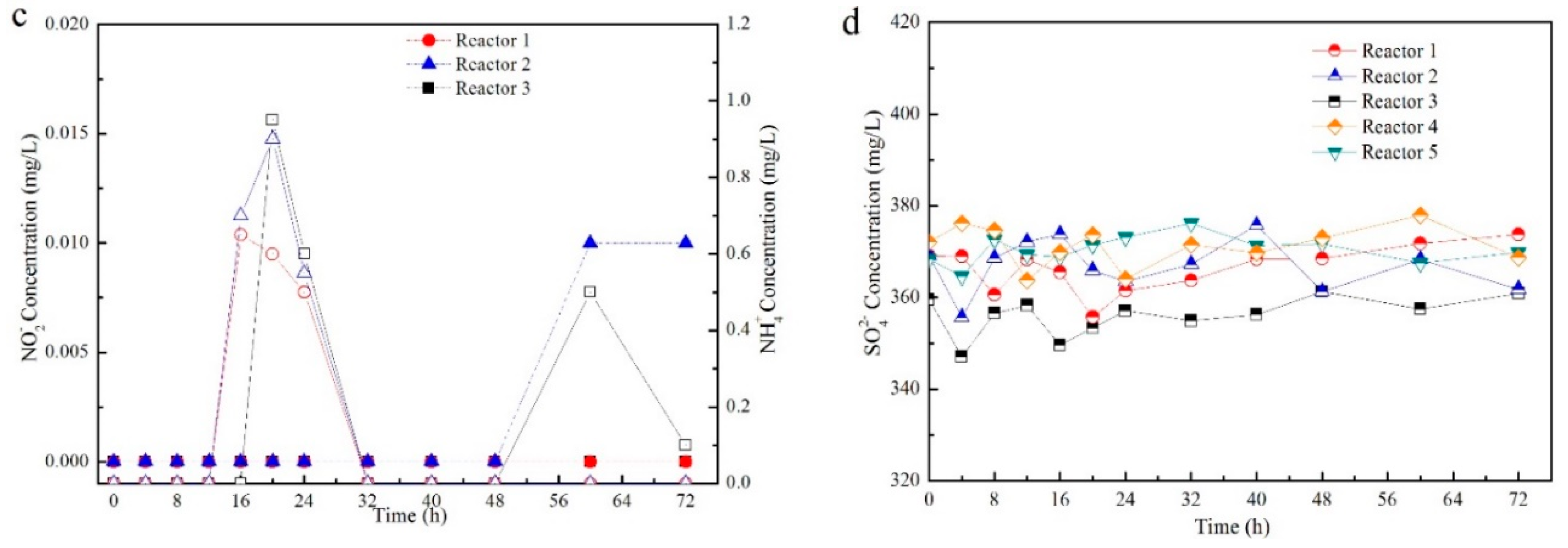
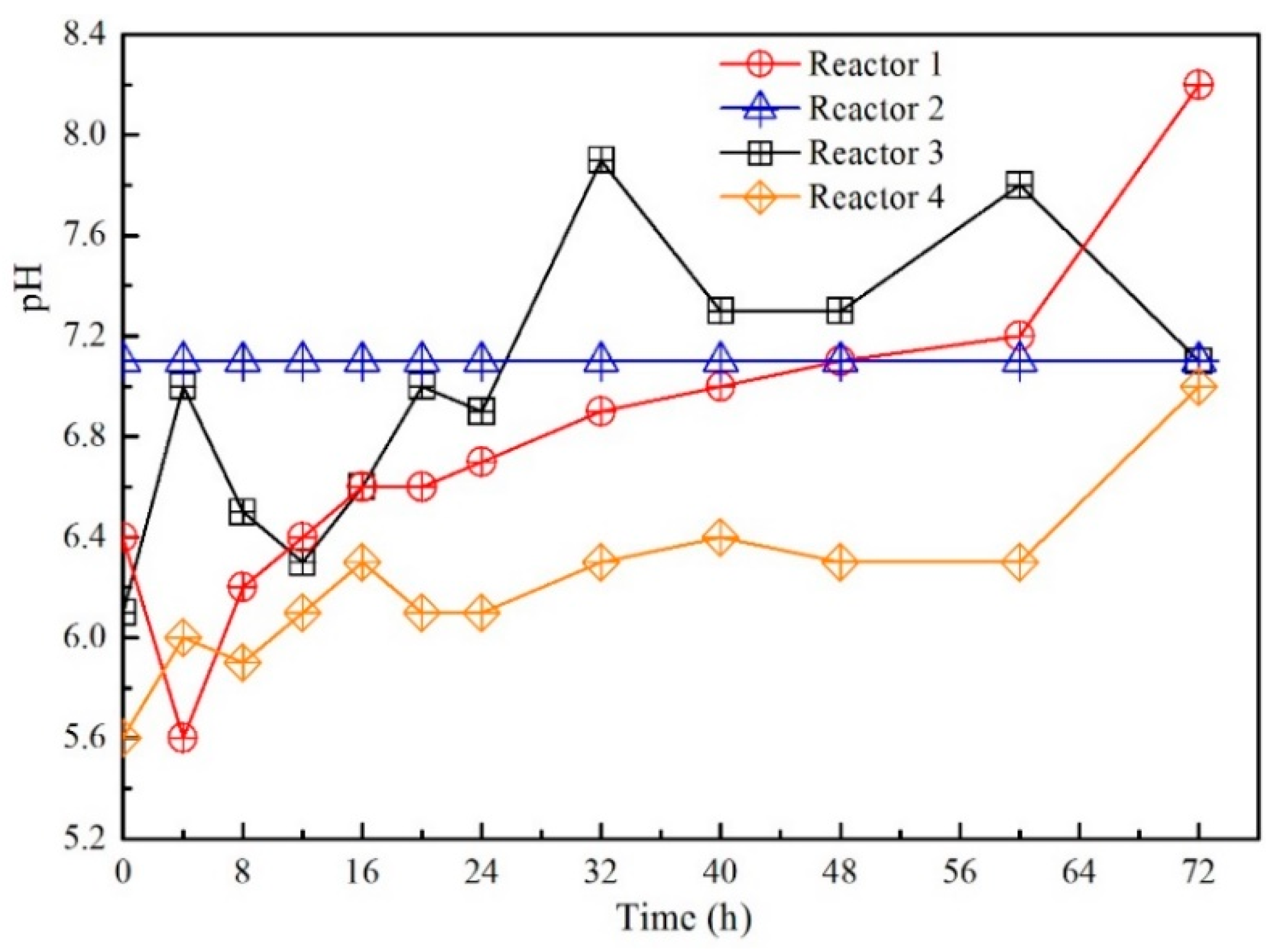
3.1. Contaminants Removal
3.2. Electrochemical Analysis
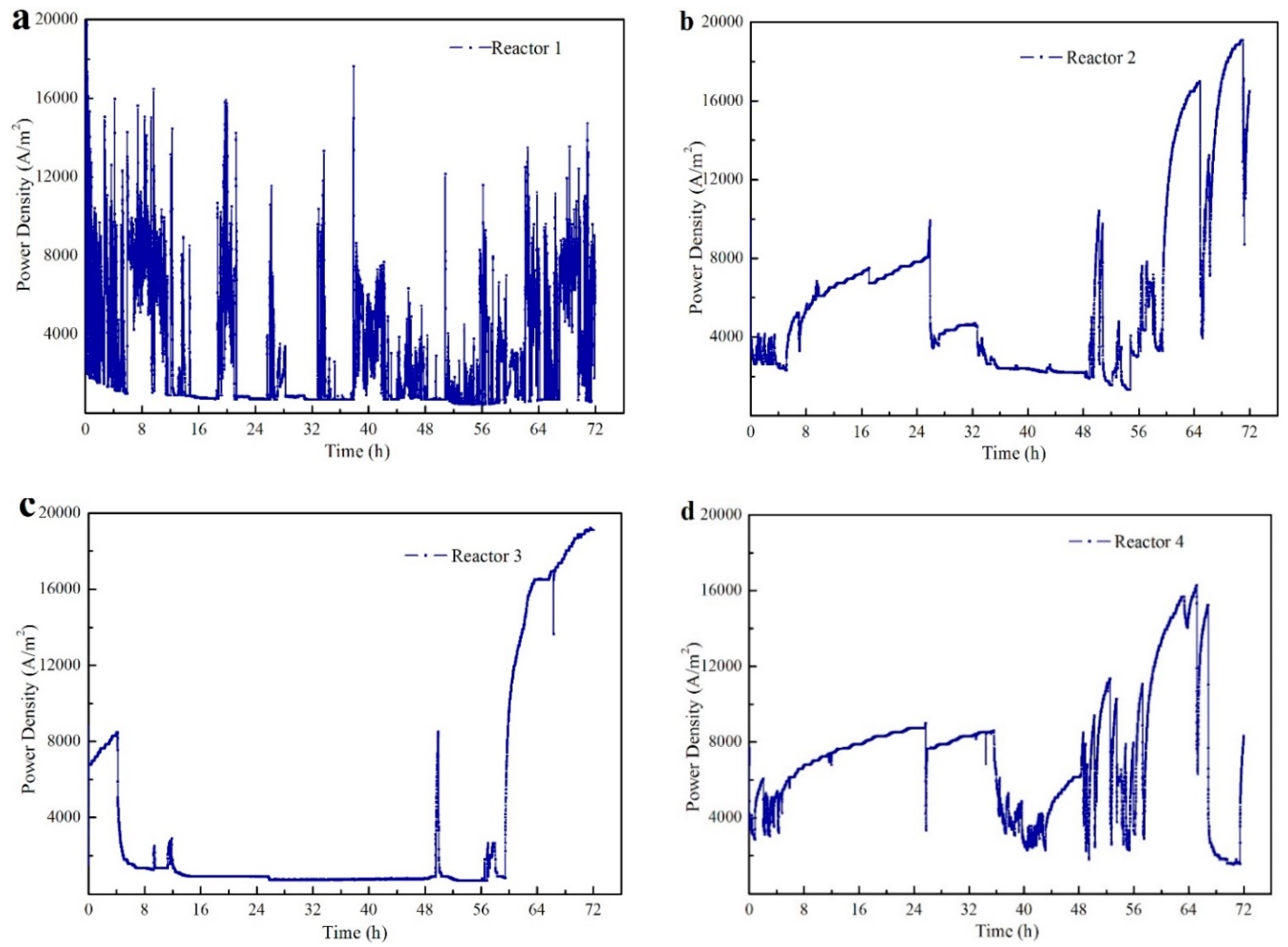
3.2.1. Energy Generation
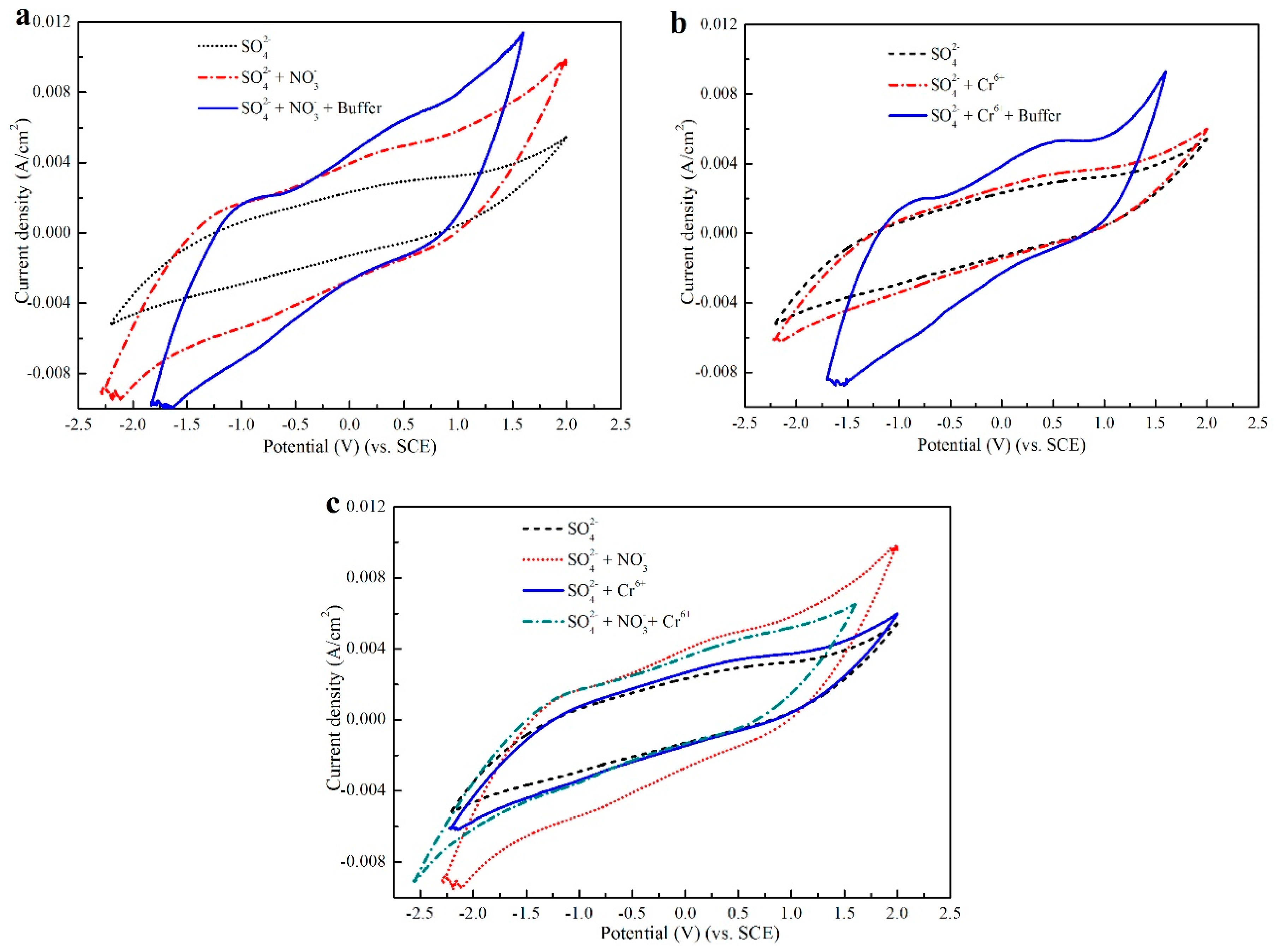
3.2.2. Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhai, Y.; Zhao, X.; Teng, Y.; Li, X.; Zhang, J.; Wu, J.; Zuo, R. Groundwater nitrate pollution and human health risk assessment by using hhra model in an agricultural area, NE China. Ecotoxicol. Environ. Saf. 2017, 137, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mao, G.; Crittenden, J.; Liu, X.; Du, H. Groundwater remediation from the past to the future: A bibliometric analysis. Water Res. 2017, 119, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Anornu, G.; Gibrilla, A.; Adomako, D. Tracking nitrate sources in groundwater and associated health risk for rural communities in the white volta river basin of ghana using isotopic approach (δ15N, δ18ONO3 and 3H). Sci. Total Environ. 2017, 603, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; Mishra, B.K.; Gupta, S.K. Human health risk assessment of chromium in drinking water: A case study of sukinda chromite mine, Odisha, India. Expo. Health 2016, 8, 253–264. [Google Scholar] [CrossRef]
- Elisante, E.; Muzuka, A.N.N. Assessment of sources and transformation of nitrate in groundwater on the slopes of Mount Meru, Tanzania. Environ. Earth Sci. 2016, 75, 277. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Zhang, F.S.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazakis, N.; Kantiranis, N.; Kalaitzidou, K.; Kaprara, E.; Mitrakas, M.; Frei, R.; V-Argemezis, G.; Tsourlos, P.; Zouboulis, A.; Filippidis, A. Origin of hexavalent chromium in groundwater: The example of Sarigkiol Basin, Northern Greece. Sci. Total Environ. 2017, 593, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Shabir, G.; Iqbal, S.; Mustafa, T.; Khan, Q.M.; Khalid, Z.M. Assessment of heavy metal contamination in soil and groundwater at leather industrial area of Kasur, Pakistan. Clean-Soil Air Water 2014, 42, 1133–1139. [Google Scholar] [CrossRef]
- Lelli, M.; Grassi, S.; Amadori, M.; Franceschini, F. Natural Cr(VI) contamination of groundwater in the cecina coastal area and its inner sectors (Tuscany, Italy). Environ. Earth Sci. 2014, 71, 3907–3919. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Ji, L.; Zhang, X.; Yang, F.; Wang, J.; Kang, W. Highly sensitive detection of Cr(VI) in groundwater by bimetallic NiFe nanoparticles. Anal. Methods 2017, 9, 1031–1037. [Google Scholar] [CrossRef]
- Zhai, S.; Zhao, Y.; Ji, M.; Oi, W. Simultaneous removal of nitrate and chromate in groundwater by a spiral fiber based biofilm reactor. Bioresour. Technol. 2017, 232, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Sahinkaya, E.; Yurtsever, A.; Ucar, D. A novel elemental sulfur-based mixotrophic denitrifying membrane bioreactor for simultaneous Cr(VI) and nitrate reduction. J. Hazard. Mater. 2017, 324, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Fratino, U.; Petrella, A.; Torretta, V.; Rada, E.C. Ailanthus Altissima and Phragmites Australis for chromium removal from a contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 15983–15989. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, G.; Qin, Y.H.; Cao, M.J.; Liu, Y.; Li, J.H.; Qu, J.H.; Liu, H.J. Redox conversion of chromium(VI) and arsenic(III) with the intermediates of chromium(V) and arsenic(IV) via AuPd/CNTs electrocatalysis in acid aqueous solution. Environ. Sci. Technol. 2015, 49, 9289–9297. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, H.; Yang, K. Effective biodegradation of nitrate, Cr(VI) and p-fluoronitrobenzene by a novel three dimensional bioelectrochemical system. Bioresour. Technol. 2016, 203, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Salomons, W.; Derooij, N.M.; Kerdijk, H.; Bril, J. Sediments as a source for contaminants. Hydrobiologia 1987, 149, 13–30. [Google Scholar] [CrossRef]
- Kilunga, P.I.; Sivalingam, P.; Laffite, A.; Grandjean, D.; Mulaji, C.K.; de Alencastro, L.F.; Mpiana, P.T.; Pote, J. Accumulation of toxic metals and organic micro-pollutants in sediments from tropical urban rivers, Kinshasa, Democratic Republic of the Congo. Chemosphere 2017, 179, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Perelo, L.W. Review: In Situ and bioremediation of organic pollutants in aquatic sediments. J. Hazard. Mater. 2010, 177, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.S.S.; Chu, L.M. Contaminant release from sediments in a coastal wetland. Water Res. 1999, 33, 909–918. [Google Scholar] [CrossRef]
- Rekha, P.; Suman Raj, D.S.; Aparna, C.; Hima Bindu, V.; Anjaneyulu, Y. Bioremediation of contaminated lake sediments and evaluation of maturity indicies as indicators of compost stability. Int. J. Environ. Res. Public Health 2005, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Xu, S.; Zhang, D.; Zeng, L.; He, F.; Zhou, Q.; Wu, Z. Spatial and temporal variations of water-soluble organic carbon in surface sediments of west lake, Hangzhou. J. Hydroecol. 2015, 36, 57–62. [Google Scholar]
- Feng, Y.; Lin, Y.; Zhang, X.; Xu, Y.; Yu, F.; Xu, J. Organic contamination in river sediment and its distribution characteristics in southern Jiangsu. J. Agro-Environ. Sci. 2007, 26, 1240–1244. [Google Scholar]
- Sherafatmand, M.; Ng, H.Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015, 195, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting energy from the marine sediment-water interface. Environ. Sci. Technol. 2001, 35, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q. Recent advances in the separators for microbial fuel cells. Bioresour. Technol. 2011, 102, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, J.S.; Prasana, K.; Nithiyanantham, S.; Renganathan, K. Comparative study of electricity production and treatment of different wastewater using microbial fuel cell (MFC). Environ. Earth Sci. 2015, 73, 2409–2413. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, Y.; Yu, J.; Liu, J.; Fu, J. Performance of chemically modified carbon felt air cathode with HNO_3. China Water Wastewater 2014, 30, 10–14. [Google Scholar]
- Sophia, A.C.; Saikant, S. Reduction of chromium(VI) with energy recovery using microbial fuel cell technology. J. Water Process Eng. 2016, 11, 39–45. [Google Scholar] [CrossRef]
- Jia, Y.-H.; Tran, H.-T.; Kim, D.-H.; Oh, S.-J.; Park, D.-H.; Zhang, R.-H.; Ahn, D.-H. Simultaneous organics removal and bio-electrochemical denitrification in microbial fuel cells. Bioprocess Biosyst. Eng. 2008, 31, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.D.; Blowes, D.W.; Ptacek, C.J.; Cherry, J.A. Long-term performance of in situ reactive barriers for nitrate remediation. Ground Water 2000, 38, 689–695. [Google Scholar] [CrossRef]
- Dima, G.E.; de Vooys, A.C.A.; Koper, M.T.M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J. Electroanal. Chem. 2003, 554, 15–23. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.; Wu, M.; Ding, J.; Zhang, W. Biodegradation of organic matter and anodic microbial communities analysis in sediment microbial fuel cells with/without Fe(III) oxide addition. Bioresour. Technol. 2017, 225, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, R.Y.; Ji, M.; Ren, Z.J. Organic content influences sediment microbial fuel cell performance and community structure. Bioresour. Technol. 2016, 220, 549–556. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-R.; Xiao, X.; Li, W.-W.; Cai, P.-J.; Yuan, S.-J.; Yan, F.-F.; He, M.-X.; Sheng, G.-P.; Tong, Z.-H.; Yu, H.-Q. Electricity generation from dissolved organic matter in polluted lake water using a microbial fuel cell (MFC). Biochem. Eng. J. 2013, 71, 57–61. [Google Scholar] [CrossRef]
- Xia, C.; Xu, M.; Liu, J.; Guo, J.; Yang, Y. Sediment microbial fuel cell prefers to degrade organic chemicals with higher polarity. Bioresour. Technol. 2015, 190, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Sevda, S.; Sreekrishnan, T.R. Effect of salt concentration and mediators in salt bridge microbial fuel cell for electricity generation from synthetic wastewater. J. Environ. Sci. Health Part A 2012, 47, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-D.; Li, H.-R. Effects of bio- and abio-factors on electricity production in a mediatorless microbial fuel cell. Biochem. Eng. J. 2007, 36, 209–214. [Google Scholar] [CrossRef]
- Scott, K.; Cotlarciuc, I.; Hall, D.; Lakeman, J.B.; Browning, D. Power from marine sediment fuel cells: The influence of anode material. J. Appl. Electrochem. 2008, 38, 1313–1319. [Google Scholar] [CrossRef]
- Zhao, Q.; Ji, M.; Li, R.; Ren, Z.J. Long-term performance of sediment microbial fuel cells with multiple anodes. Bioresour. Technol. 2017, 237, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the internal resistance distribution of microbial fuel cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Roman, O.B.; Angelidaki, I. Importance of temperature and anodic medium composition on microbial fuel cell (MFC) performance. Biotechnol. Lett. 2008, 30, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, H.J.; Liu, Y.; Qu, J.H.; Li, J.H. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale 2015, 7, 1250–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Liu, R.; Li, M.; Zhang, N.; Zhang, F.; Liu, X. Construction of a Self-Powered System for Simultaneous In Situ Remediation of Nitrate and Cr(VI) Contaminated Synthetic Groundwater and River Sediment. Sustainability 2018, 10, 2806. https://doi.org/10.3390/su10082806
Han L, Liu R, Li M, Zhang N, Zhang F, Liu X. Construction of a Self-Powered System for Simultaneous In Situ Remediation of Nitrate and Cr(VI) Contaminated Synthetic Groundwater and River Sediment. Sustainability. 2018; 10(8):2806. https://doi.org/10.3390/su10082806
Chicago/Turabian StyleHan, Limei, Rui Liu, Miao Li, Ning Zhang, Fang Zhang, and Xiang Liu. 2018. "Construction of a Self-Powered System for Simultaneous In Situ Remediation of Nitrate and Cr(VI) Contaminated Synthetic Groundwater and River Sediment" Sustainability 10, no. 8: 2806. https://doi.org/10.3390/su10082806




