Evaluating Sampling Designs for Demersal Fish Communities
Abstract
:1. Introduction
2. Methods
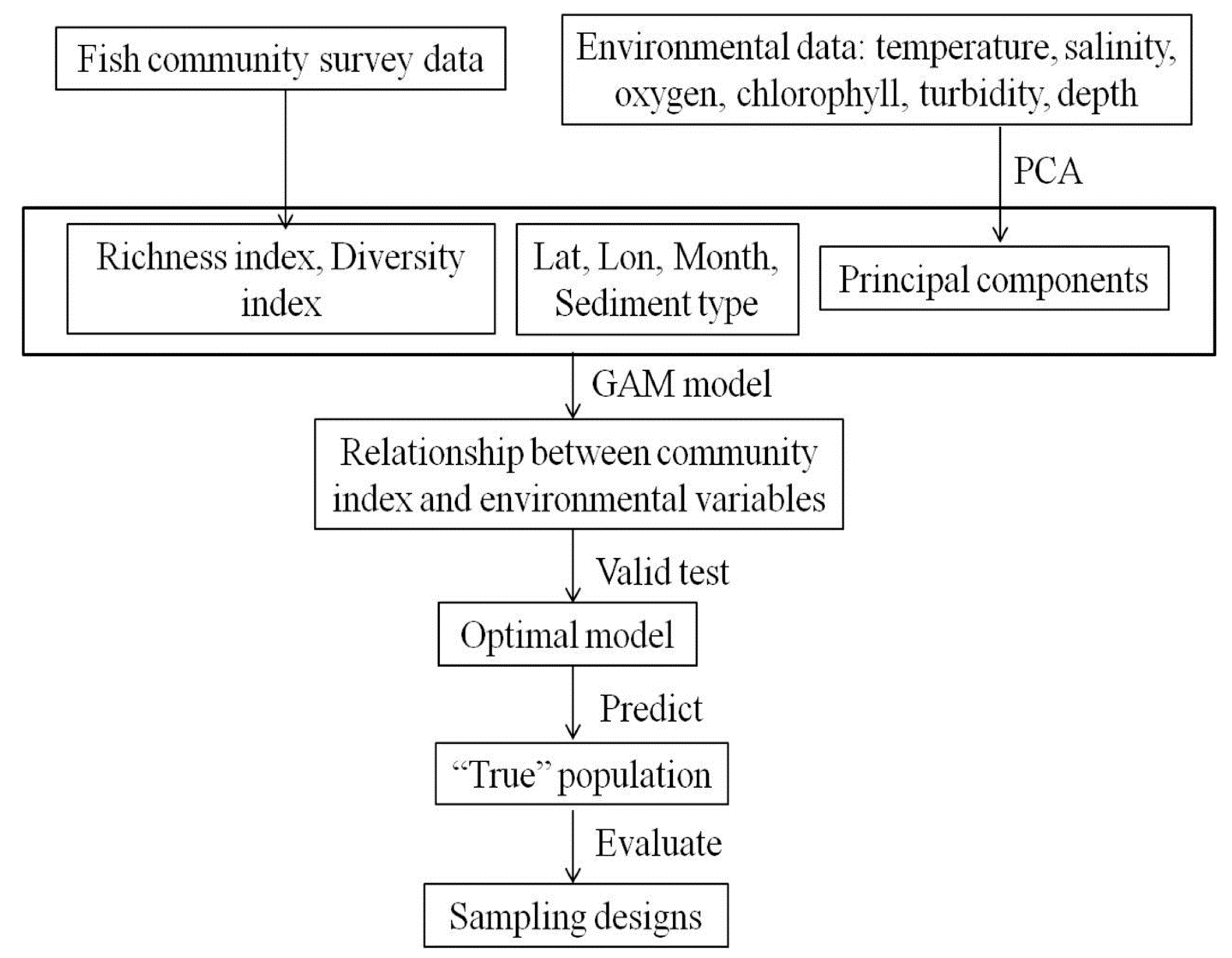
2.1. Approach
2.2. Study Area and Fish Species Composition
2.3. Data Collection
2.4. Treatment Sampling Designs
- (1)
- Allocate samples based on the habitat area proportion of each stratum (referred to as Design III)
- (2)
- Allocate the first half of samples evenly among the strata first, then allocate the remaining samples in a way that is inversely proportional to the variances of strata (Neyman allocation referred to as Design IV).
2.5. Simulation Procedure and Measure Indices
2.6. The Influence of Temperature Change
3. Results
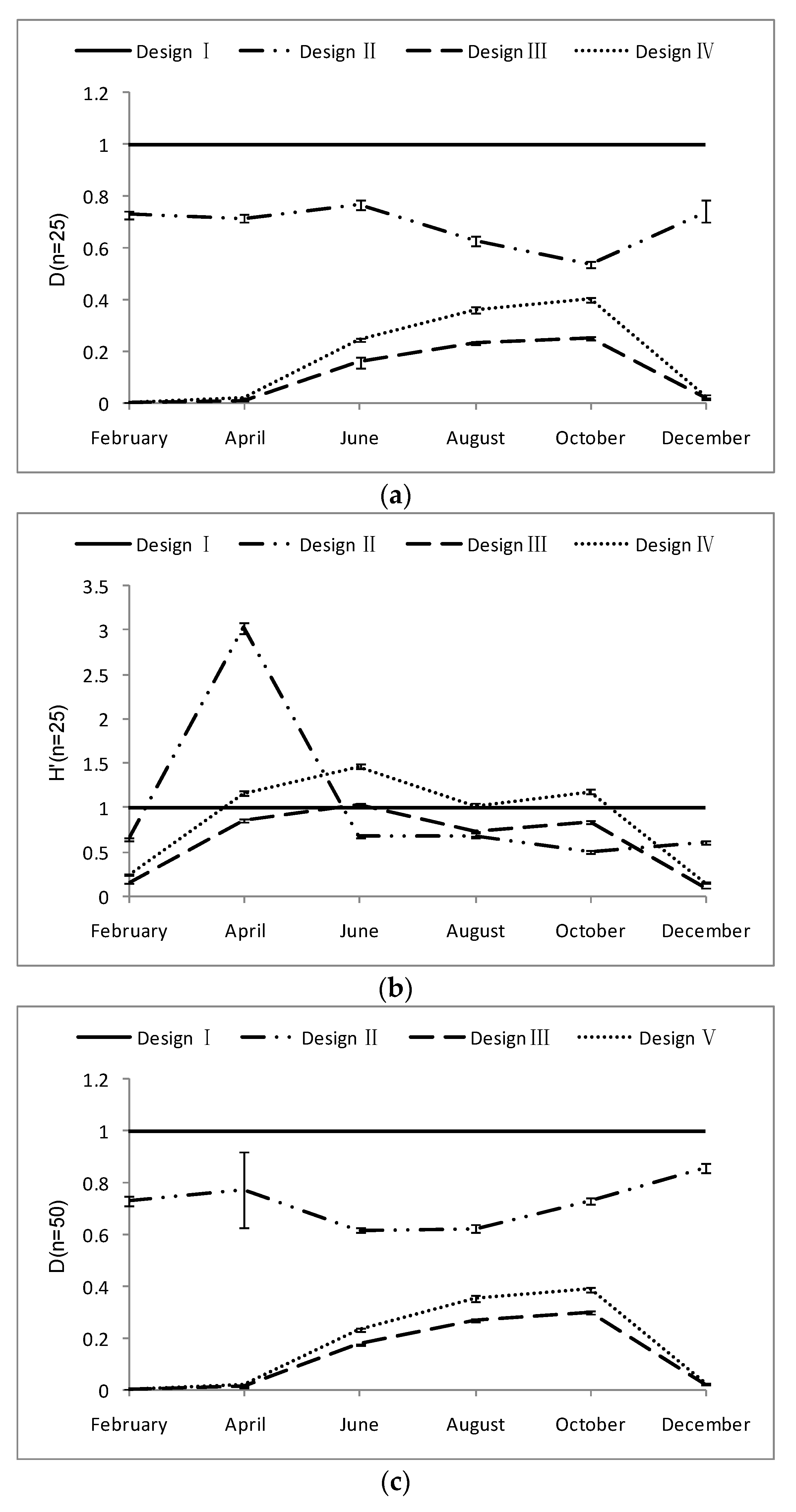
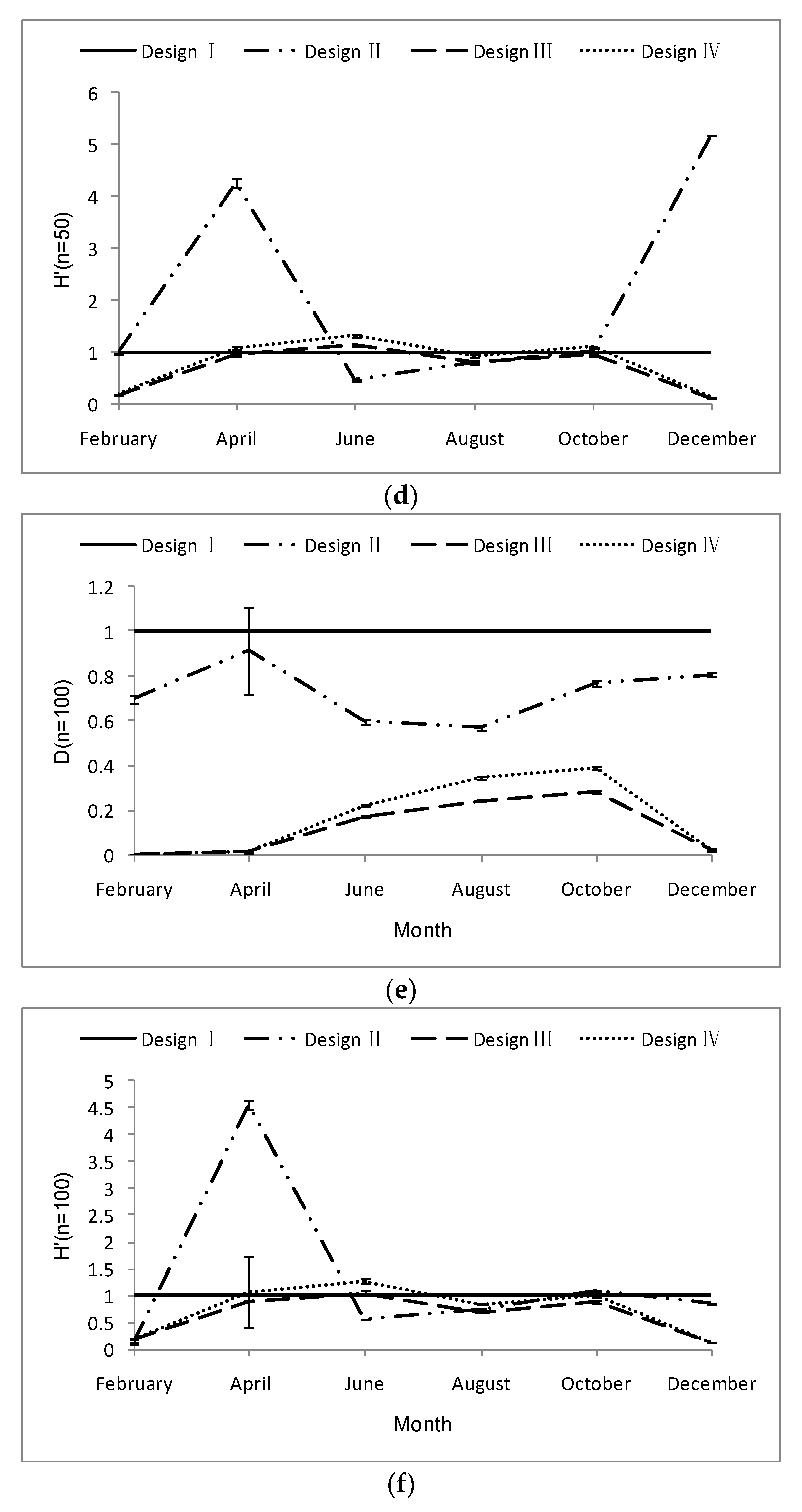
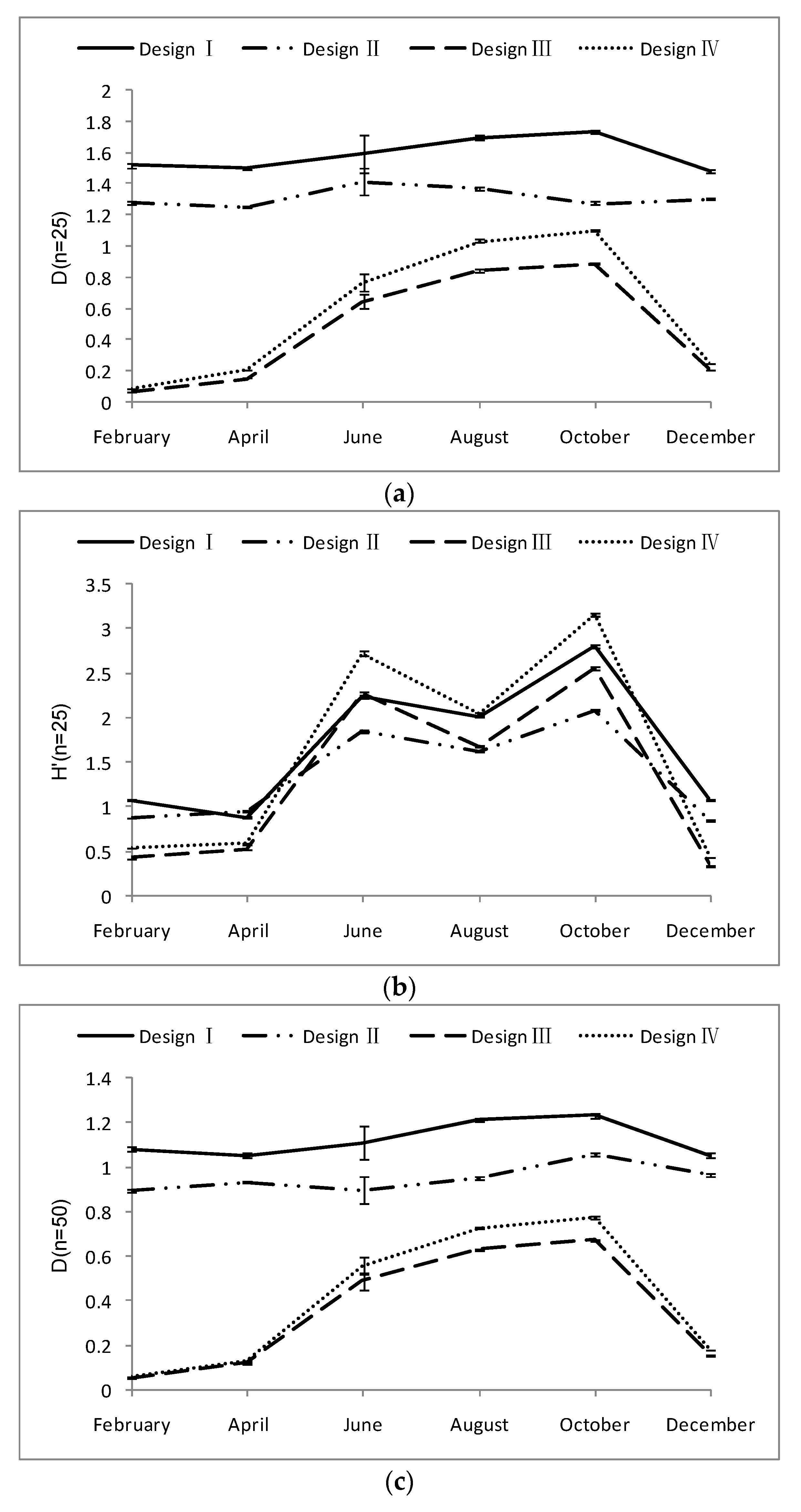
3.1. Design Effect (DEFF)
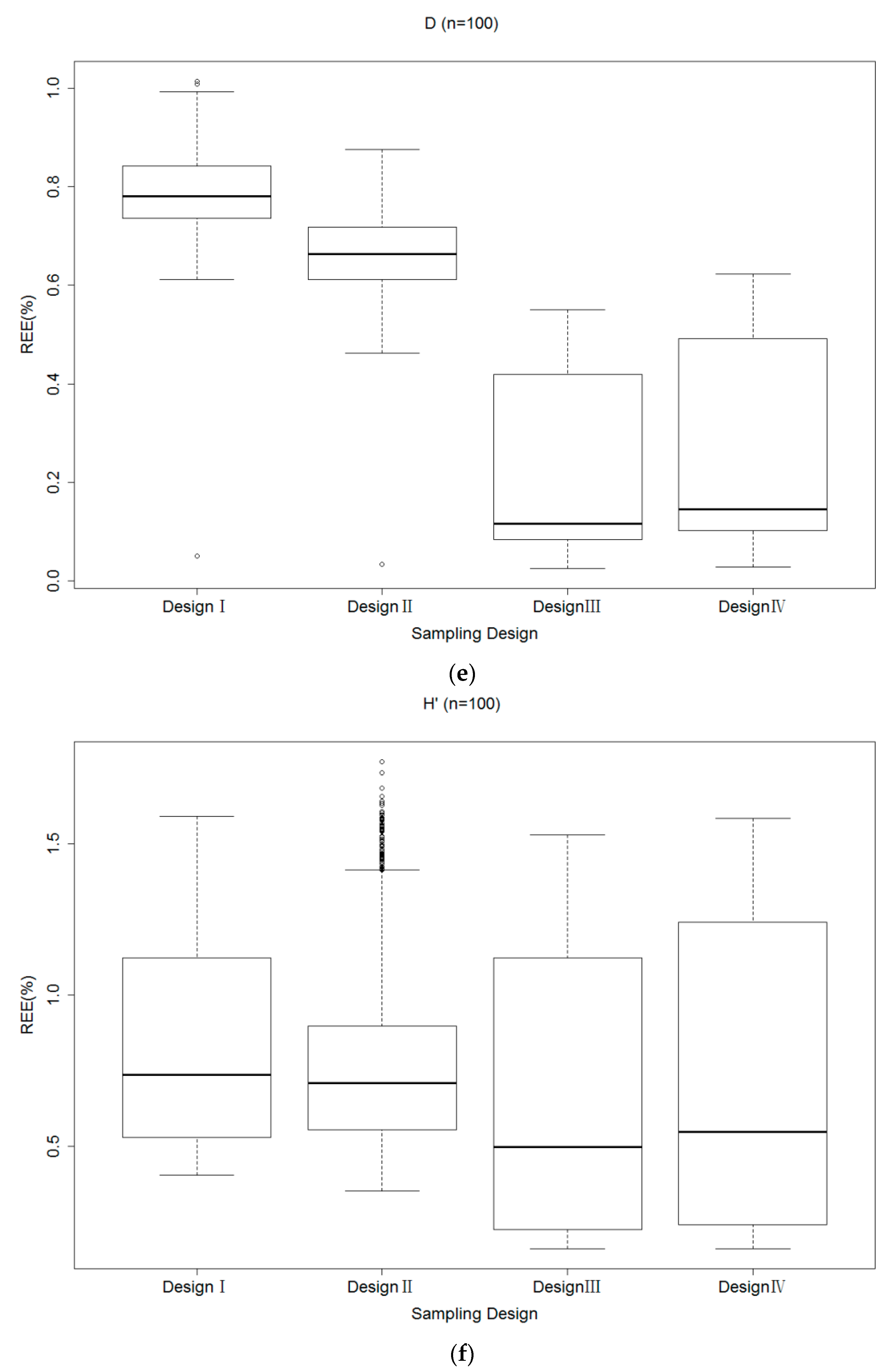
3.2. Relative Estimation Error (REE)
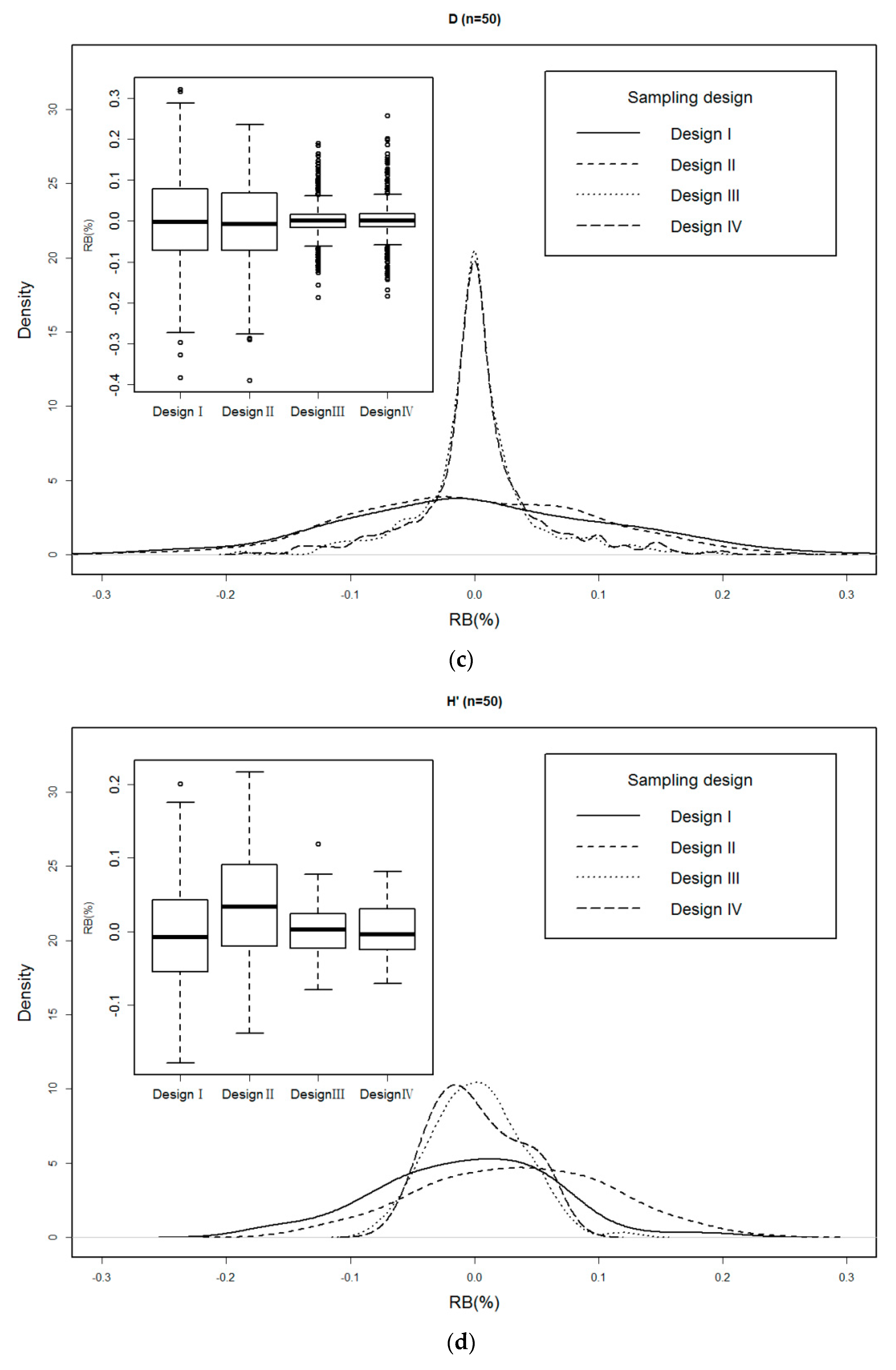
3.3. Relative Bias (RB) of Each Sampling Design
3.4. Possible Influence of Temperaturechanges
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agboola, J.I.; Uchimiya, M.; Kudo, I.; Osawa, M.; Kido, K. Seasonality and environmental drivers of biological productivity on the western Hokkaido coast, Ishikari Bay, Japan. Estuar. Coast. Shelf Sci. 2013, 127, 12–13. [Google Scholar] [CrossRef]
- Ault, J.S.; Diaz, G.A.; Smith, S.G.; Luo, J.G. An efficient Sampling survey design to estimate pink shrimp population abundance in Biscayne Bay, Florida. N. Am. J. Fish. Manag. 1999, 19, 696–712. [Google Scholar] [CrossRef]
- Barbraud, C.; Rolland, V.; Jenouvrier, S.; Nevoux, M.; Delord, K.; Weimerskirch, H. Effects of climate change and fisheries bycatch on Southern Ocean seabirds: A review. Mar. Ecol. Prog. Ser. 2012, 454, 285–307. [Google Scholar] [CrossRef]
- Bazigos, G.; Kavadas, S. Optimal sampling designs for large-scale fishery sample surveys in Greece. Mediterr. Mar. Sci. 2007, 8, 65–82. [Google Scholar] [CrossRef]
- Bonvechio, K.I. Determining electric fishing sample size for monitoring fish communities in three Florida lakes. Fish. Manag. Ecol. 2009, 16, 409–412. [Google Scholar] [CrossRef]
- Cabral, H.N.; Murta, A.G. Effect of sampling design on abundance estimates of benthic invertebrates in environmental monitoring studies. Mar. Ecol. Prog. Ser. 2004, 276, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Chen, Y.; Chang, J.H.; Chen, X.J. An evaluation of an inshore bottom trawl survey design for American lobster (Homarusamericanus) using computer simulations. J. Northwest Atl. Fish. Sci. 2014, 46, 27–39. [Google Scholar] [CrossRef]
- Chen, Y. A Monte Carlo study on impacts of the size of subsample catch on estimation of fish stock parameters. Fish. Res. 1996, 26, 207–223. [Google Scholar] [CrossRef]
- Cochran, W.G. Sampling Techniques, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1977; 900p. [Google Scholar]
- Dorner, B.; Holt, K.R.; Peterman, R.M.; Jordan, C.; Larsen, D.P.; Olsen, A.R.; Abdul-Aziz, O.I. Evaluating alternative methods for monitoring and estimating responses of salmon productivity in the North Pacific to future climate change and other processes: A simulation study. Fish. Res. 2013, 147, 10–23. [Google Scholar] [CrossRef]
- Folmer, O.; Pennington, M. A statistical evaluation of the design and precision of the shrimp trawl survey off West Greenland. Fish. Res. 2000, 49, 165–178. [Google Scholar] [CrossRef]
- Gavaris, S.; Smith, S.J. Effect of allocation and stratification strategies on precision of survey abundance estimates for Atlantic Cod (Gadusmorhua) on the eastern Scotian shelf. J. Northwest Atl. Fish. Sci. 1987, 7, 137–144. [Google Scholar] [CrossRef]
- Giri, S.; Mukhopadhyay, A.; Hazra, S.; Roy, D.; Ghosh, S.; Ghosh, T.; Mitra, D. A study on abundance and distribution of mangrove species in Indian Sundarban using remote sensing technique. J. Northwest Atl. Fish. Sci. 2014, 4, 359–367. [Google Scholar] [CrossRef]
- Gray, C.A. Evaluation of fishery-dependent sampling strategies for monitoring a small-scale beach clam fishery. Fish. Res. 2016, 177, 24–30. [Google Scholar] [CrossRef]
- Irvine, G.V.; Shelly, A. Sampling design for long-term regional trends in marine rocky intertidal communities. Environ. Monit. Assess. 2013, 185, 6963–6987. [Google Scholar] [CrossRef] [PubMed]
- Jaureguizar, A.J.; Menni, R.; Guerrero, R.; Lastan, C. Environmental factors structuring fish communities of the Río de la Plata estuary. Fish. Res. 2004, 66, 195–211. [Google Scholar] [CrossRef]
- Jenkins, G.P.; Wheatley, M.J. The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: Comparison of shallow seagrass, reef-algal and unvegetated sand habitats, with emphasis on their importance to recruitment. J. Exp. Mar. Biol. Ecol. 1998, 221, 147–172. [Google Scholar] [CrossRef]
- Johnston, R.; Sheaves, M.; Molony, B. Are distributions of fishes in tropical estuaries influenced by turbidity over small spatial scales? J. Fish Biol. 2007, 71, 657–671. [Google Scholar] [CrossRef]
- Kodama, K.; Aoki, I.; Shimizu, M.; Taniuchi, T. Long-term changes in the assemblage of demersal fishes and invertebrates in relation to environmental variations in Tokyo Bay, Japan. Fish. Manag. Ecol. 2002, 9, 303–313. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Cheng, J. A comparative study of optimization methods and conventional methods for sampling design in fishery-independent surveys. ICES J. Mar. Sci. 2009, 66, 1873–1882. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; White, M.; Newell, G.; Griffioen, P. Species distribution modeling for conservation planning in Victoria, Australia. Ecol. Model. 2013, 249, 68–74. [Google Scholar] [CrossRef]
- Loeng, H. The influence of temperature on some fish population parameters in the Barents Sea. J. Northwest Atl. Fish. Sci. 1989, 9, 103–113. [Google Scholar] [CrossRef]
- Lohr, S.L. Sampling: Design and Analysis; Duxbury: Pacific Grove, CA, USA, 1999. [Google Scholar]
- Lopez-Lopez, L.; Preciado, I.; Velasco, F.; Olaso, I.; Gutiérrez-Zabala, J.L. Resource partitioning amongst five coexisting species of gurnards (Scorpaeniforme: Triglidae): Role of trophic and habitat segregation. J. Sea Res. 2011, 66, 58–68. [Google Scholar] [CrossRef]
- Maes, J.; Stevens, M.; Breine, J. Modelling the migration opportunities of diadromous fish species along a gradient of dissolved oxygen concentration in a European tidal watershed. Estuar. Coast. Shelf Sci. 2007, 75, 1–12. [Google Scholar] [CrossRef]
- Manly, B.F.J.; Akroyd, J.M.; Walshe, K.A.R. Two-phase stratified random surveys on multiple populations at multiple locations. N. Z. J. Mar. Freshw. Res. 2002, 36, 581–591. [Google Scholar] [CrossRef]
- Masia, A.; Iskander, M.; Negm, A. Coastal protection measures, case study (Mediterranean zone, Egypt). J. Coast. Conserv. 2015, 3, 281–294. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Pennington, M.; Vølstad, J.H. Optimum size of sampling unit for estimating the density of marine populations. Biometrics 1991, 47, 717–723. [Google Scholar] [CrossRef]
- Pinheiro, H.T.; Martins, A.S.; Joyeux, J.C. The importance of small-scale environment factors to community structure patterns of tropical rocky reef fish. J. Mar. Biol. Assoc. UK 2013, 93, 1175–1185. [Google Scholar] [CrossRef]
- Pooler, P.S.; Smith, D.R. Optimal sampling design for estimating spatial distribution and abundance of a freshwater mussel population. J. N. Am. Benthol. Soc. 2005, 24, 525–537. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Ripley, B.D. Spatial Statistics; John Wiley and Sons: New York, NY, USA, 1981; 252p. [Google Scholar]
- Skibo, K.M.; Schwarz, C.J.; Peterman, R.M. Evaluation of sampling designs for red sea urchins Strongylocentrotus franciscanus in British Columbia. N. Am. J. Fish. Manag. 2008, 28, 219–230. [Google Scholar] [CrossRef]
- Simmonds, E.J.; Fryer, R.J. Which are better, random or systematic acoustic surveys? A simulation using North Sea herring as an example. ICES J. Mar. Sci. 1996, 53, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Simth, S.G.; Ault, J.S.; Bohnsack, J.A.; Harper, D.E.; Luo, J.G.; McClellan, D.B. Multispecies survey design for assessing reef-fish stocks, spatially explicit management performance, and ecosystem condition. Fish. Res. 2011, 109, 25–41. [Google Scholar] [CrossRef]
- Sobocinski, K.L.; Orth, R.J.; Fabrizio, M.C.; Latour, R.J. Historical comparison of fish community structure in lower Chesapeake bay seagrass habitats. Estuar. Coast. 2013, 36, 775–794. [Google Scholar] [CrossRef]
- Somerton, D.A.; McConnaughey, R.A.; Intelmann, S.S. Evaluating the use of acoustic bottom typing to inform models of bottom trawl sampling efficiency. Fish. Res. 2017, 185, 14–16. [Google Scholar] [CrossRef]
- Stein, A.; Ettema, C. An overview of spatial sampling procedures and experimental design of spatial studies for ecosystem comparisons. Agric. Ecosyst. Environ. 2003, 94, 31–47. [Google Scholar] [CrossRef]
- Stokesbury, K.D.E. Estimation of sea scallop abundance in closed areas of Georges Bank, USA. Trans. Am. Fish. Soc. 2002, 131, 1081–1092. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, S.Y.; Chen, Q.M.; Xu, M.; Wang, K. Fish community ecology in rocky reef habitat of Ma’an Archipelago. I. Species compositon and diversity. Biodivers. Sci. 2012, 20, 41–50. (In Chinese) [Google Scholar]
- Xu, B.D.; Ren, Y.P.; Chen, Y.; Xue, Y.; Zhang, C.L.; Wan, R. Optimization of stratification scheme for a fishery-independent survey with multiple objectives. Acta Oceanol. Sin. 2015, 34, 154–169. [Google Scholar] [CrossRef]
- Yu, H.; Jiao, Y.; Su, Z.; Reid, K. Performance comparison of traditional sampling designs and adaptive sampling designs for fishery-independent surveys: A simulation study. Fish. Res. 2012, 113, 173–181. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, J.; Tian, S.Q.; Chen, Y.; Zhang, S.Y.; Wang, Z.H.; Zhou, X.J. A comparison of two GAM models in quantifying relationships of environmental variables and fish richness and diversity indices. Aquat. Ecol. 2013, 48, 297–312. [Google Scholar] [CrossRef]








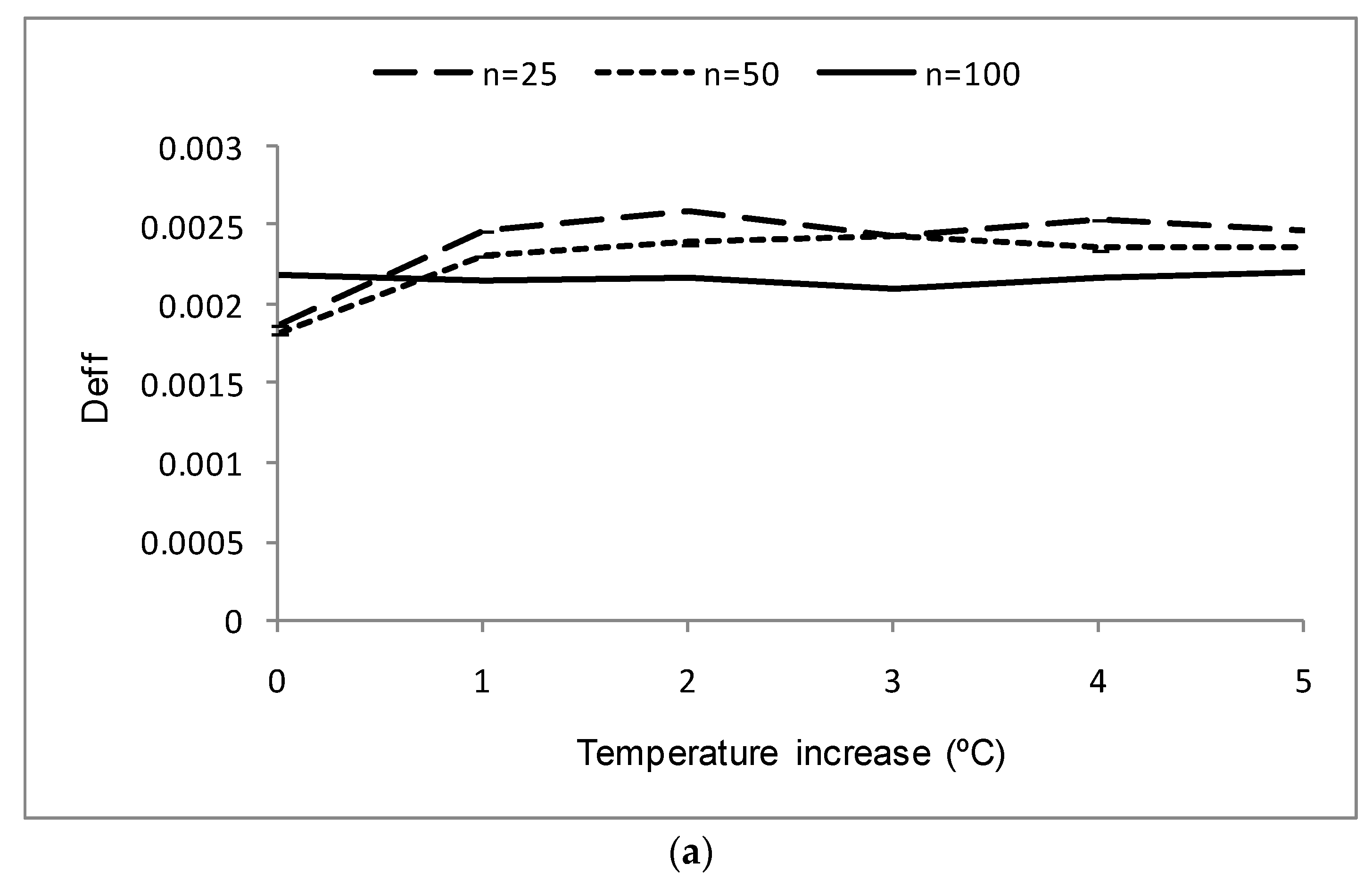

| Temperature Increase (°C) | Deff | REE (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | ||
| 0 | Average | 1 | 0.580 | 0.002 | 0.003 | 1.180 | 0.920 | 0.053 | 0.063 |
| Coefficient of Variation (CV) | 0 | 0.240 | 0.100 | 0.190 | 0.290 | 0.380 | 0.320 | 0.370 | |
| 1 | Average | 1 | 0.600 | 0.002 | 0.003 | 1.110 | 0.890 | 0.054 | 0.064 |
| Coefficient of Variation (CV) | 0 | 0.200 | 0.070 | 0.050 | 0.350 | 0.430 | 0.320 | 0.350 | |
| 2 | Average | 1 | 0.610 | 0.002 | 0.003 | 1.108 | 0.880 | 0.053 | 0.064 |
| Coefficient of Variation (CV) | 0 | 0.200 | 0.090 | 0.020 | 0.340 | 0.430 | 0.320 | 0.360 | |
| 3 | Average | 1 | 0.600 | 0.0023 | 0.003 | 1.110 | 0.890 | 0.053 | 0.064 |
| Coefficient of Variation (CV) | 0 | 0.190 | 0.080 | 0.010 | 0.340 | 0.430 | 0.320 | 0.350 | |
| 4 | Average | 1 | 0.560 | 0.002 | 0.003 | 1.110 | 0.890 | 0.053 | 0.064 |
| Coefficient of Variation (CV) | 0 | 0.020 | 0.080 | 0.040 | 0.350 | 0.430 | 0.320 | 0.360 | |
| 5 | Average | 1 | 0.610 | 0.0024 | 0.003 | 1.110 | 0.880 | 0.053 | 0.064 |
| Coefficient of Variation (CV) | 0 | 0.180 | 0.060 | 0.030 | 0.340 | 0.430 | 0.320 | 0.350 | |
| Temperature Scenario (°C) | Deff | REE (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | ||
| 02–04 | Average | 1 | 0.730 | 0.002 | 0.002 | 1.230 | 1.050 | 1.140 | 1.140 |
| Cofficient of Variation (CV) | 0 | 0.290 | 0.240 | 0.200 | 0.280 | 0.330 | 0.004 | 0.004 | |
| 04–06 | Average | 1 | 0.730 | 0.011 | 0.015 | 1.240 | 1.041 | 1.180 | 1.180 |
| Cofficient of Variation (CV) | 0 | 0.290 | 0.270 | 0.200 | 0.300 | 0.340 | 0.013 | 0.015 | |
| 06–08 | Average | 1 | 0.680 | 0.160 | 0.190 | 1.290 | 1.060 | 1.300 | 1.310 |
| Cofficient of Variation (CV) | 0 | 0.290 | 0.270 | 0.200 | 0.300 | 0.350 | 0.070 | 0.067 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Cao, J.; Tian, S.; Chen, Y.; Zhang, S. Evaluating Sampling Designs for Demersal Fish Communities. Sustainability 2018, 10, 2585. https://doi.org/10.3390/su10082585
Zhao J, Cao J, Tian S, Chen Y, Zhang S. Evaluating Sampling Designs for Demersal Fish Communities. Sustainability. 2018; 10(8):2585. https://doi.org/10.3390/su10082585
Chicago/Turabian StyleZhao, Jing, Jie Cao, Siquan Tian, Yong Chen, and Shouyu Zhang. 2018. "Evaluating Sampling Designs for Demersal Fish Communities" Sustainability 10, no. 8: 2585. https://doi.org/10.3390/su10082585




