Sorption Characteristics and Fraction Distribution Changes of Selenite in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples
2.2. Sorption Experiments
2.3. Sorption Models
2.3.1. Sorption Isotherm
2.3.2. Sorption Kinetics
2.4. Sequential Extraction
3. Results and Discussion
3.1. Soil Characterization
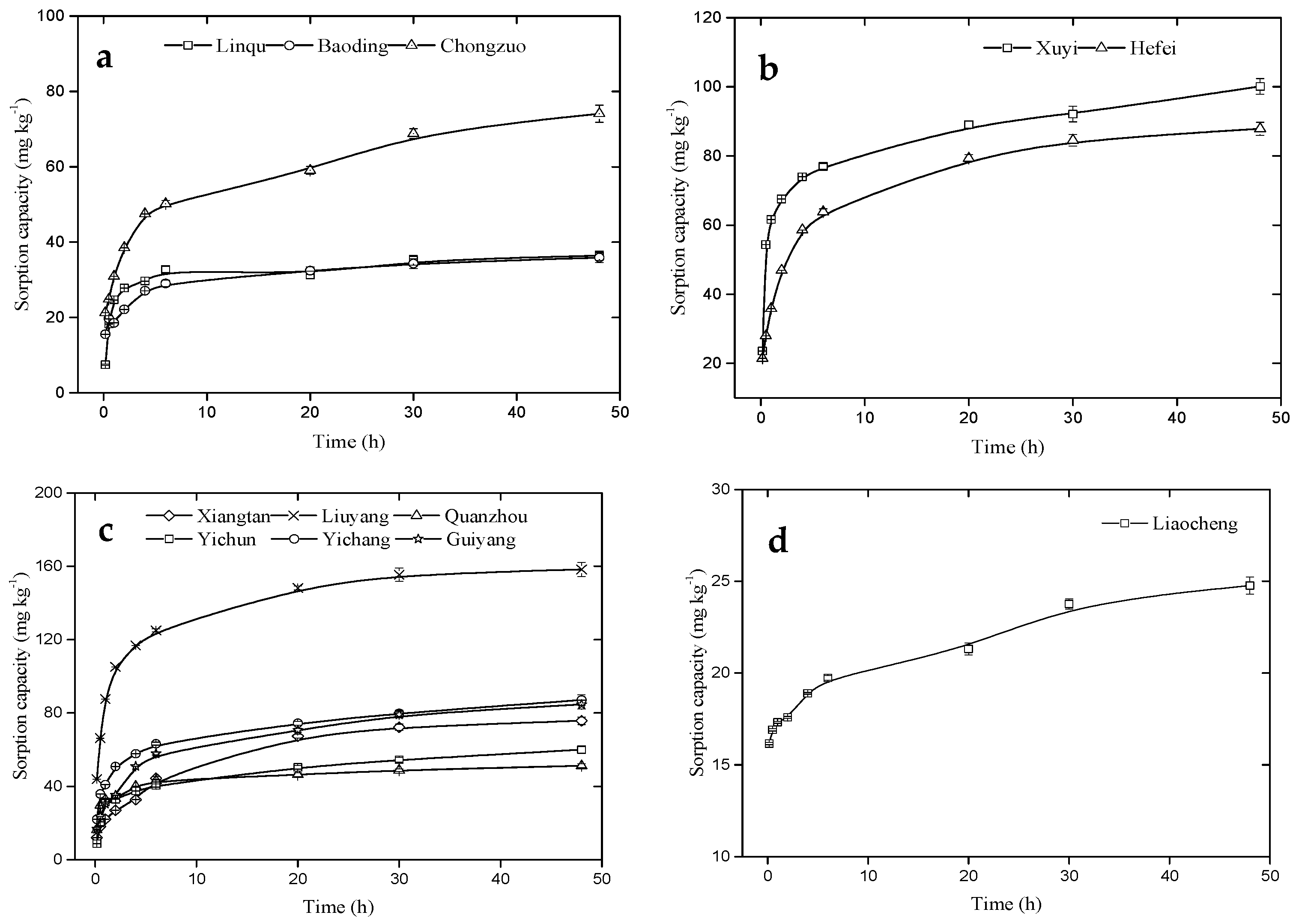
3.2. Selenite Kinetic Sorption in Soil
3.3. Batch Sorption of Selenite in Soils
3.4. Selenite Desorption
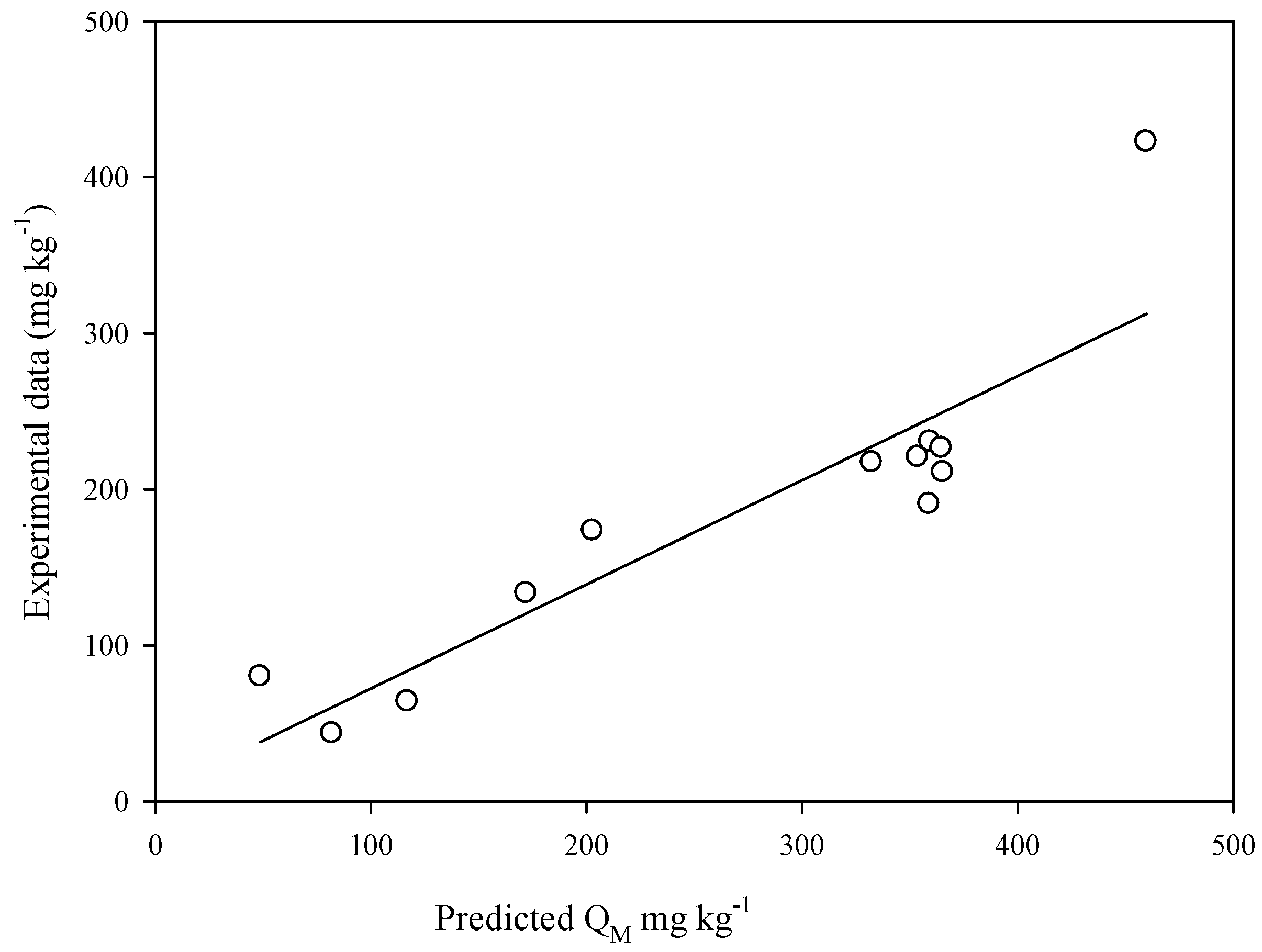
3.5. Relationship between Sorption Parameters and Soil Properties
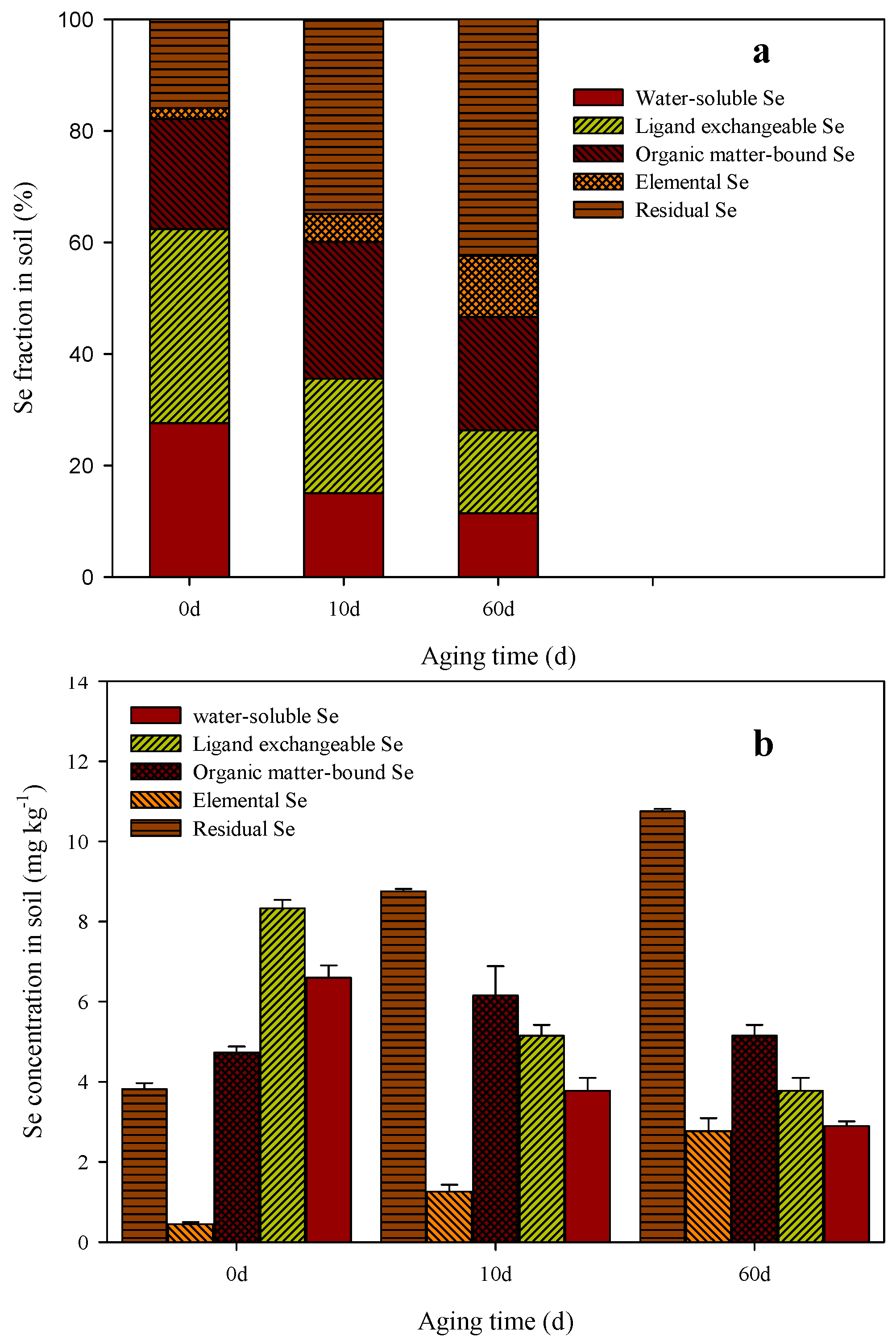
3.6. Se Fraction Distribution Change with Age
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F. Selenium in global food systems. Brit. J. Nutr. 2007, 85, 517–547. [Google Scholar] [CrossRef]
- Sathe, S.K.; Mason, A.C.; Rodibaugh, R.; Weaver, C.M. Chemical form of selenium in soybean (Glycine max L.) lectin. J. Agric. Food Chem. 1992, 40, 2084–2091. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2010; p. 505. [Google Scholar]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Q.; Wang, S.Z.; Zhou, R.H.; Sun, S.Z. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Winkel, L.; Vriens, B.; Jones, G.; Schneider, L.; Pilon-Smits, E.; Bañuelos, G. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 41–99. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Q.; Liang, D.L.; Liang, S.J.; Chen, J.; Sun, H.; Li, S.Q.; Lei, P.H. Effects of aging on the fraction distribution and bioavailability of selenium in three different soils. Chemosphere 2016, 144, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.; Ritter, A.; Scheinost, A.C.; Weiss, S.; Schild, D.; Hubner, R. Selenium(IV) Uptake by Maghemite (γ-Fe2O3). Environ. Sci. Technol. 2014, 48, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Kamei-Ishikawa, N.; Nakamaru, Y.; Tagami, K.; Uchida, S. Sorption behavior of selenium on humic acid under increasing selenium concentration or increasing solid/liquid ratio. J. Environ. Radioactiv. 2008, 99, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Charlet, L. The Mechanism of Selenate Adsorption on Goethite and Hydrous Ferric Oxide. J. Colloid Interface Sci. 1994, 168, 87–93. [Google Scholar] [CrossRef]
- Zhang, P.; Sparks, D.L. Kinetics of selenate and selenite adsorption/desorption at the goethite/water interface. Environ. Sci. Technol. 1990, 24, 1848–1856. [Google Scholar] [CrossRef]
- Dhillon, K.S.; Dhillon, S.K. Adsorption-desorption reactions of selenium in some soils of India. Geoderma 1999, 93, 19–31. [Google Scholar] [CrossRef]
- Lessa, J.H.L.; Araujo, A.M.; Silva, G.N.T.; Guilherme, L.R.G.; Lopes, G. Adsorption-desorption reactions of selenium (VI) in tropical cultivated and uncultivated soils under Cerrado biome. Chemosphere 2016, 164, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Man, N.; Wang, S.S.; Liang, D.L.; Liu, J.J. Selenite adsorption and desorption in main Chinese soils with their characteristics and physicochemical properties. J. Soil Sediments 2015, 15, 1150–1158. [Google Scholar] [CrossRef]
- Söderlund, M.; Virkanen, J.; Holgersson, S.; Lehto, J. Sorption and speciation of selenium in boreal forest soil. J. Environ. Radioactiv. 2016, 164, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Chen, H.M. Forms and distribution of selenium at different depths and among particle size fractions of three Taiwan soils. Chemosphere 2003, 52, 585–593. [Google Scholar] [CrossRef]
- Alexander, M. Aging, Bioavailability, and Overestimation of Risk from Environmental Pollutants. Environ. Sci. Technol. 2000, 34, 4259–4265. [Google Scholar] [CrossRef]
- Tom-Petersen, A.; Hansen, H.C.B.; Nybroe, O. Time and Moisture Effects on Total and Bioavailable Copper in Soil Water Extracts. J. Environ. Qual. 2004, 33, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Settimio, L.; McLaughlin, M.J.; Kirby, J.K.; Langdon, K.A.; Lombi, E.; Donner, E.; Scheckel, K.G. Fate and lability of silver in soils: Effect of ageing. Environ. Pollut. 2014, 191, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tolu, J.; Thiry, Y.; Bueno, M.; Jolivet, C.; Potin-Gautier, M.; Le-Hécho, I. Distribution and speciation of ambient selenium in contrasted soils, from mineral to organic rich. Sci. Total Environ. 2014, 479–480, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.X.; Wang, Y.J.; Cui, X.D.; Zhou, D.M. Sorption isotherms and kinetics of Sb(V) on several Chinese soils with different physicochemical properties. J. Soil Sediments 2013, 13, 344–353. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis Part 3—Chemical Methods, (SSSA Book Series); Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total Environ. 2015, 532, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Kulp, T.R.; Pratt, L.M. Speciation and weathering of selenium in Upper Cretaceous chalk and shale from South Dakota and Wyoming, USA. Geochim. Cosmochim. 2004, 68, 3687–3701. [Google Scholar] [CrossRef]
- Wang, S.S.; Liang, D.L.; Wang, D.; Wei, W.; Fu, D.D.; Lin, Z.Q. Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ. 2012, 427, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M.; Zeng, X.C.; Yu, H.F. Study on crack density of concrete exposed to stress corrosion. Constr. Build. Mater. 2015, 82, 264–273. [Google Scholar] [CrossRef]
- Yasar, U.; Ozyigit, I.I.; Serin, M. Judas tree (Cercis siliquastrum L. subsp siliquastrum) as a possible biomonitor for Cr, Fe and Ni in Istanbul (Turkey). Rom. Biotech. Lett. 2010, 15, 4979–4989. [Google Scholar]
- Jiang, W.; Zhang, S.; Shan, X.Q.; Feng, M.; Zhu, Y.G.; McLaren, R.G. Adsorption of arsenate on soils. Part 2: Modeling the relationship between adsorption capacity and soil physiochemical properties using 16 Chinese soils. Environ. Pollut. 2005, 138, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.X.; Wang, Y.J.; Liu, C.; Wang, L.H.; Yang, K.; Zhou, D.M.; Li, W.; Sparks, D.L. Effect of iron oxide reductive dissolution on the transformation and immobilization of arsenic in soils: New insights from X-ray photoelectron and X-ray absorption spectroscopy. J. Hazard. Mater. 2014, 279, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.X.; Wang, Y.J.; Fan, T.T.; Dang, F.; Zhou, D.M. Effect of aqueous Fe(II) on Sb(V) sorption on soil and goethite. Chemosphere 2016, 147, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gabos, M.B.; Goldberg, S.; Alleoni, L.R.F. Modeling selenium (IV and VI) adsorption envelopes in selected tropical soils using the constant capacitance model. Environ. Toxicol. Chem. 2014, 33, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Doolittle, J.J.; Woodard, H.J. Selenite Adsorption and Desorption in Selected South Dakota Soils as a Function of pH and Other Oxyanions. Soil Sci. 2011, 176, 73–79. [Google Scholar]
- Wang, Q.; Zhang, J.; Zhao, B.; Xin, X.; Deng, X.; Zhang, H. Influence of Long-Term Fertilization on Selenium Accumulation in Soil and Uptake by Crops. Pedosphere 2016, 26, 120–129. [Google Scholar] [CrossRef]
- Sharma, P.; Ofner, J.; Kappler, A. Formation of Binary and Ternary Colloids and Dissolved Complexes of Organic Matter, Fe and As. Environ. Sci. Technol. 2010, 44, 4479–4485. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tejada, M.M.; Ontiveros, A.; Viota, J.L.; Durán, J.D.G. Interfacial and rheological properties of humic acid/hematite suspensions. J. Colloid Interfaces Sci. 2003, 268, 85–95. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zhu, Y.G.; Cui, Y.S.; Duan, J.; Tang, L. The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ. Int. 2006, 32, 682–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayen, S.; Mallet, J.; Guillon, E. Aging effect on the copper sorption on a vineyard soil: Column studies and SEM–EDS analysis. J. Colloid Interfaces Sci. 2009, 331, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Vlčková, K.; Hofman, J. A comparison of POPs bioaccumulation in Eisenia fetida in natural and artificial soils and the effects of aging. Environ. Pollut. 2012, 160, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kausch, M.; Ng, P.; Ha, J.; Pallud, C. Soil-Aggregate-Scale Heterogeneity in Microbial Selenium Reduction. Vadose Zone J. 2012. [Google Scholar] [CrossRef]
- Sun, J.; Quicksall, A.N.; Chillrud, S.N.; Mailloux, B.J.; Bostick, B.C. Arsenic mobilization from sediments in microcosms under sulfate reduction. Chemosphere 2016, 153, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Classification | a pH | a OM | a Fetotal | a Altotal | a Mntotal | a FeOX | a AlOX | a MnOX | a FeDCB | a AlDCB | a MnDCB | Se | Texture (v/v) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay (%) | Silt (%) | Sand (%) | |||||||||||||
| Inceptisol | |||||||||||||||
| Baoding | 6.9 ± 0.2 | 0.059 ± 0.0031 | 2.8 ± 0.12 | 5.4 ± 0.24 | 318 ± 10.1 | 494 ± 21.1 | 392 ± 17.1 | 113 ± 8.45 | 546 ± 21.1 | 86.0 ± 6.85 | 148 ± 9.46 | 0.40 ± 0.008 | 5.50 | 39.9 | 54.6 |
| Linqu | 6.2 ± 0.1 | 0.60 ± 0.025 | 2.3 ± 0.13 | 4.9 ± 0.16 | 236 ± 9.56 | 471 ± 15.9 | 417 ± 31.1 | 141 ± 11.2 | 486 ± 15.9 | 133 ± 11.1 | 135 ± 11.7 | 0.43 ± 0.009 | 4.41 | 33.1 | 62.5 |
| Chongzuo | 6.3 ± 0.2 | 2.3 ± 0.14 | 3.9 ± 0.21 | 9.3 ± 0.45 | 113 ± 8.23 | 975 ± 36.7 | 603 ± 19.8 | 52.0 ± 3.1 | 1118 ± 87.6 | 205 ± 9.87 | 33.0 ± 1.98 | 0.57 ± 0.023 | 10.6 | 53.6 | 35.8 |
| Alfisols | |||||||||||||||
| Hefei | 6.4 ± 0.2 | 0.088 ± 0.0022 | 3.3 ± 0.18 | 2.5 ± 0.12 | 321 ± 15.2 | 354 ± 17.5 | 2340 ± 80.9 | 655 ± 31.2 | 617 ± 21.3 | 279 ± 18.8 | 209 ± 17.8 | 0.39 ± 0.014 | 21.9 | 45.0 | 33.1 |
| Xuyi | 5.50 ± 0.1 | 1.9 ± 0.11 | 2.4 ± 0.15 | 6.0 ± 0.20 | 295 ± 14.1 | 203 ± 14.7 | 794 ± 65.2 | 191 ± 9.12 | 1673 ± 92.1 | 264 ± 19.7 | 229 ± 18.4 | 0.47 ± 0.029 | 36.9 | 53.8 | 39.3 |
| Ultisols | |||||||||||||||
| Yichun | 5.9 ± 0.2 | 2.5 ± 0.13 | 1.32 ± 0.085 | 7.7 ± 0.46 | 104 ± 7.35 | 485 ± 21.8 | 452 ± 36.4 | 25.0 ± 2.42 | 1178 ± 13.6 | 229 ± 13.5 | 26.0 ± 1.74 | 0.69 ± 0.007 | 20.1 | 66.6 | 13.3 |
| Yichang | 5.9 ± 0.1 | 2.2 ± 0.085 | 2.44 ± 0.14 | 6.5 ± 0.54 | 147 ± 6.89 | 233 ± 15.6 | 854 ± 42.7 | 67 ± 4.19 | 1998 ± 27.1 | 312 ± 21.1 | 86.0 ± 2.81 | 0.53 ± 0.011 | 6.90 | 51.2 | 41.9 |
| Liuyang | 5.6 ± 0.2 | 2.9 ± 0.15 | 4.42 ± 0.25 | 5.9 ± 0.36 | 253 ± 12.1 | 278 ± 17.3 | 940 ± 59.6 | 127 ± 9.92 | 2104 ± 16.4 | 452 ± 16.9 | 84.0 ± 2.36 | 0.41 ± 0.018 | 5.80 | 56.9 | 37.3 |
| Xiangtan | 6.4 ± 0.1 | 4.6 ± 0.32 | 2.81 ± 0.18 | 7.0 ± 0.29 | 232 ± 13.6 | 225 ± 9.12 | 1080 ± 76.9 | 113 ± 7.89 | 1208 ± 10.6 | 192 ± 8.46 | 107 ± 1.91 | 0.46 ± 0.016 | 5.40 | 39 | 55.6 |
| Quanzhou | 5.9 ± 0.2 | 1.3 ± 0.052 | 2.77 ± 0.11 | 8.4 ± 0.37 | 287 ± 9.81 | 580 ± 24.1 | 1160 ± 87.1 | 203 ± 18.7 | 1146 ± 16.1 | 453 ± 23.8 | 138 ± 4.36 | 0.49 ± 0.019 | 5.00 | 39.4 | 55.6 |
| Guiyang | 6.2 ± 0.1 | 2.9 ± 0.13 | 5.74 ± 0.29 | 14 ± 0.85 | 185 ± 8.97 | 480 ± 31.0 | 3560 ± 101 | 113 ± 5.16 | 913 ± 18.6 | 459 ± 29.1 | 46.0 ± 2.75 | 0.72 ± 0.012 | 9.60 | 49.6 | 40.8 |
| Entisol | |||||||||||||||
| Liaocheng | 6.7 ± 0.2 | 1.4 ± 0.097 | 2.55 ± 0.17 | 4.6 ± 0.31 | 274 ± 15.1 | 351 ± 19.2 | 294 ± 19.2 | 106 ± 4.98 | 429 ± 17.4 | 74.0 ± 6.98 | 127 ± 3.57 | 0.38 ± 0.014 | 5.00 | 35.8 | 59.2 |
| Soil | Double Constant | Reversible Langmuir Kinetics | Pseudo-Second-Order Model | Elovich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a# | b# | R2 | a1# | b1# | R2 | Qe# | K2# | h# | R2 | α# | K# | R2 | |
| Baoding | 20.8 | 0.147 | 0.987 | 0.0287 | 0.0140 | 0.986 | 36.07 | 0.0259 | 33.7 | 0.998 | 1.01 × 103 | 0.264 | 0.969 |
| Liaocheng | 17.5 | 0.0810 | 0.988 | 0.0310 | 0.0125 | 0.894 | 24.67 | 0.0437 | 26.6 | 0.996 | 1.69 × 105 | 0.659 | 0.904 |
| Linqu | 21.6 | 0.151 | 0.888 | 0.0149 | 0.0174 | 0.922 | 36.19 | 0.0375 | 49.1 | 0.997 | 557 | 0.227 | 0.930 |
| Hefei | 38.7 | 0.228 | 0.979 | 0.0110 | 0.0052 | 0.965 | 89.76 | 0.00667 | 53.7 | 0.998 | 229 | 0.0745 | 0.992 |
| Xuyi | 55.7 | 0.158 | 0.947 | 0.0117 | 0.0164 | 0.964 | 99.61 | 0.00886 | 87.9 | 0.997 | 1.41 × 103 | 0.0858 | 0.942 |
| Yichun | 26.1 | 0.221 | 0.963 | 0.0124 | 0.0506 | 0.958 | 60.17 | 0.00880 | 31.8 | 0.995 | 177 | 0.117 | 0.978 |
| Yichang | 41.6 | 0.197 | 0.986 | 0.0066 | 0.0047 | 0.972 | 87.09 | 0.00729 | 55.3 | 0.996 | 449 | 0.0878 | 0.997 |
| Liuyang | 84.4 | 0.180 | 0.969 | 0.0476 | 0.0038 | 0.898 | 160.7 | 0.00572 | 148 | 0.999 | 1.19 × 103 | 0.0479 | 0.988 |
| Xiangtan | 23.1 | 0.325 | 0.984 | 0.0212 | 0.0083 | 0.961 | 80.06 | 0.00381 | 24.4 | 0.992 | 44.6 | 0.0637 | 0.973 |
| Quanzhou | 30.6 | 0.144 | 0.961 | 0.0184 | 0.0193 | 0.953 | 51.25 | 0.0213 | 55.9 | 0.998 | 1.33 × 103 | 0.178 | 0.953 |
| Guiyang | 32.0 | 0.263 | 0.984 | 0.0127 | 0.0109 | 0.949 | 86.41 | 0.00502 | 37.5 | 0.995 | 120 | 0.0713 | 0.988 |
| Chongzuo | 32.4 | 0.217 | 0.991 | 0.0122 | 0.0250 | 0.960 | 74.43 | 0.00683 | 37.8 | 0.992 | 257 | 0.0993 | 0.981 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Soil | K (L·mg−1) | QM (mg·kg−1) | R2 | 1/n | Kf ((mg·kg−1)(mg·L−1))−1/n | R2 |
| Baoding | 0.460 | 50.7 | 0.993 | 0.351 | 18.3 | 0.973 |
| Liaocheng | 0.0805 | 118 | 0.997 | 0.657 | 11.3 | 0.992 |
| Linqu | 0.244 | 98.1 | 0.980 | 0.475 | 23.1 | 0.990 |
| Hefei | 0.126 | 383 | 0.996 | 0.653 | 48.8 | 0.982 |
| Xuyi | 0.177 | 361 | 0.997 | 0.591 | 61.3 | 0.982 |
| Yichun | 0.311 | 216 | 0.991 | 0.471 | 56.8 | 0.990 |
| Yichang | 0.392 | 276 | 0.993 | 0.456 | 81.8 | 0.988 |
| Liuyang | 0.503 | 567 | 0.990 | 0.534 | 178 | 0.984 |
| Xiangtan | 0.109 | 391 | 0.994 | 0.657 | 45.6 | 0.990 |
| Quanzhou | 0.276 | 175 | 0.995 | 0.475 | 43.2 | 0.967 |
| Guiyang | 0.216 | 320 | 0.998 | 0.553 | 63.5 | 0.982 |
| Chongzuo | 0.263 | 261 | 0.993 | 0.507 | 60.3 | 0.965 |
| QMAX | Kf | h | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient | p | Correlation Coefficient | p | Correlation Coefficient | p | |
| pH | −0.563 | 0.057 | −0.675 * | 0.016 | −0.700 * | 0.011 |
| OM | 0.598 * | 0.04 | 0.079 | 0.541 | 0.074 | 0.819 |
| Fetotal | 0.455 | 0.137 | 0.422 | 0.172 | 0.261 | 0.412 |
| Altotal | 0.256 | 0.422 | 0.08 | 0.805 | −0.195 | 0.544 |
| Mntotal | −0.048 | 0.882 | −0.187 | 0.561 | 0.237 | 0.458 |
| FeDCB | 0.678 * | 0.015 | 0.815 ** | 0.001 | 0.714 ** | 0.009 |
| AlDCB | 0.601 * | 0.039 | 0.664 * | 0.018 | −0.012 | 0.970 |
| MnDCB | −0.018 | 0.955 | −0.268 | 0.400 | 0.329 | 0.296 |
| FeOX | 0.663 * | 0.019 | 0.804 ** | 0.002 | 0.676 | 0.016 |
| AlOX | 0.317 | 0.315 | 0.188 | 0.559 | 0.543 | 0.068 |
| MnOX | 0.148 | 0.645 | −0.073 | 0.822 | 0.187 | 0.561 |
| Sand | −0.663 * | 0.019 | −0.498 | 0.100 | −0.442 | 0.150 |
| Slit | 0.708 * | 0.01 | 0.560 | 0.058 | 0.522 | 0.082 |
| Clay | 0.373 | 0.145 | 0.230 | 0.275 | 0.017 | 0.958 |
| Model | Multiple Linear Equation | R2 | p | S.E. |
|---|---|---|---|---|
| The stepwise regression for QM | ||||
| 1 | QM = 0.176[FeDCB] + 71.8 | 0.459 | 0.0154 | 113 |
| 2 | QM = 0.130[FeDCB] + 37.5[OM] + 50.5 | 0.535 | 0.0319 | 111 |
| 3 | QM = 0.0338[FeDCB] + 9.30[silt] + 58.8[OM] − 311 | 0.846 | 0.00129 | 67.8 |
| 4 | QM = 0.0240[FeDCB] + 0.105[AlDCB] + 8.87[silt] + 57.5[OM] − 305 | 0.852 | 0.00400 | 71.2 |
| The stepwise regression for Kf | ||||
| 1 | Kf = 0.0616[FeDCB] − 11.3 | 0.667 | 0.00123 | 26.1 |
| 2 | Kf = 0.0533[FeDCB] + 1.11[FeOX] − 52.9 | 0.721 | 0.00321 | 25.1 |
| 3 | Kf = 0.0454[FeDCB] + 0.847[FeOX] + 0.0709[AlDCB] − 50.6 | 0.752 | 0.00834 | 25.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Zhao, G. Sorption Characteristics and Fraction Distribution Changes of Selenite in Soil. Sustainability 2018, 10, 2491. https://doi.org/10.3390/su10072491
Fan J, Zhao G. Sorption Characteristics and Fraction Distribution Changes of Selenite in Soil. Sustainability. 2018; 10(7):2491. https://doi.org/10.3390/su10072491
Chicago/Turabian StyleFan, Jianxin, and Guoliang Zhao. 2018. "Sorption Characteristics and Fraction Distribution Changes of Selenite in Soil" Sustainability 10, no. 7: 2491. https://doi.org/10.3390/su10072491




