Towards the Development of Perennial Barley for Cold Temperate Climates—Evaluation of Wild Barley Relatives as Genetic Resources
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Pre-Cultivation in Climate Chamber and Greenhouse, and Trait Evaluation in Field
2.3. Data Analysis
3. Results
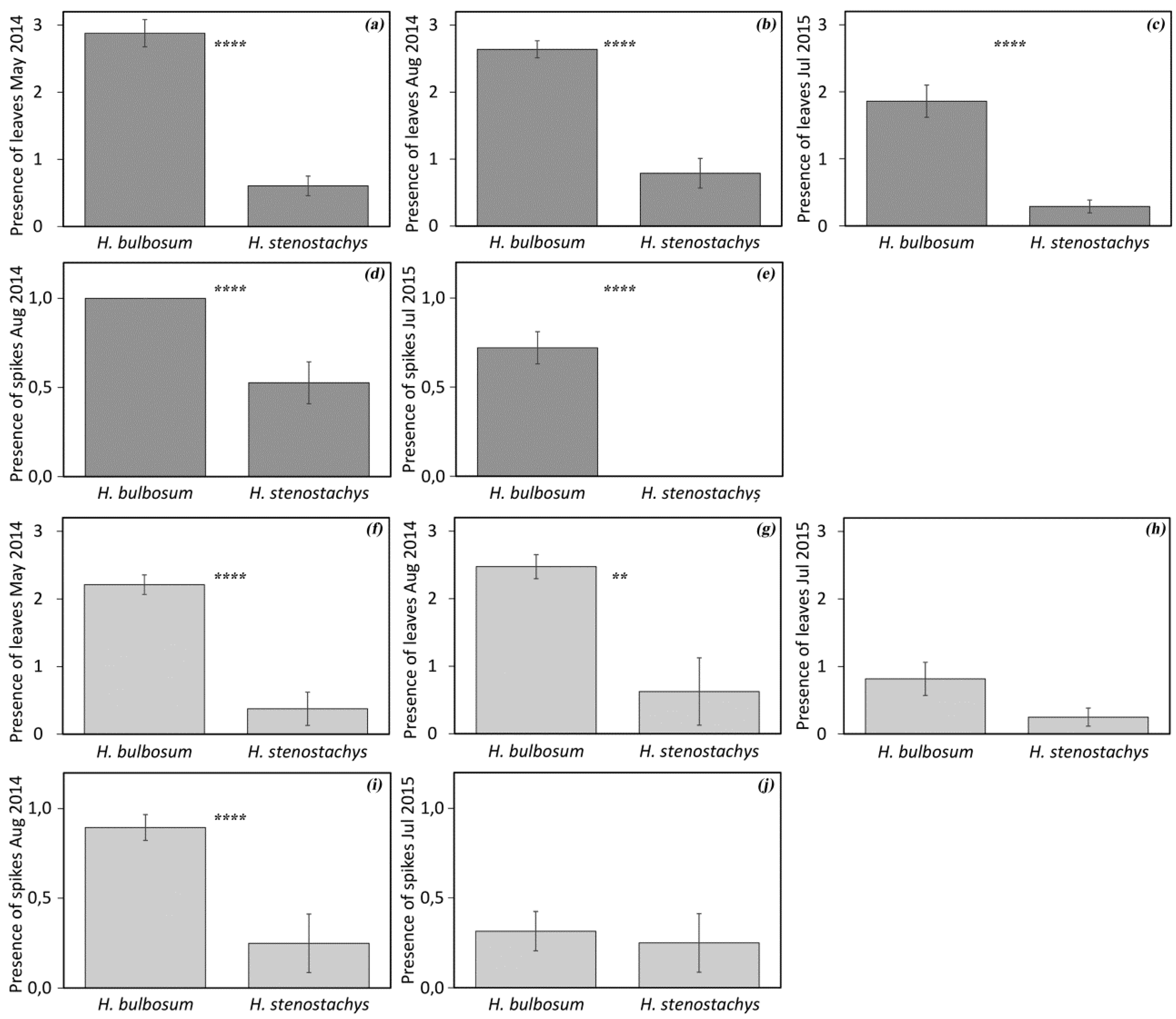
3.1. Diversity in Regrowth Ability
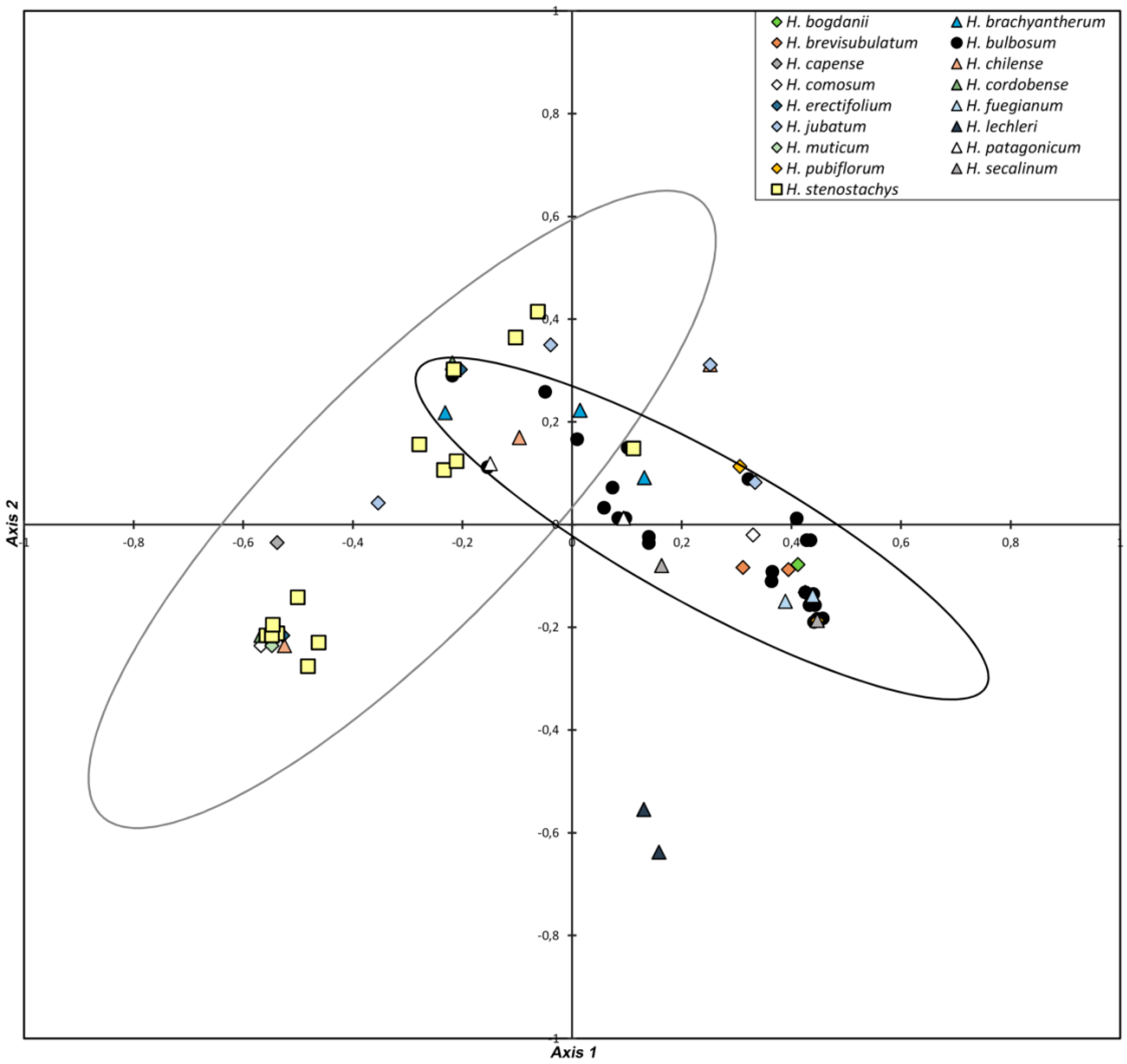
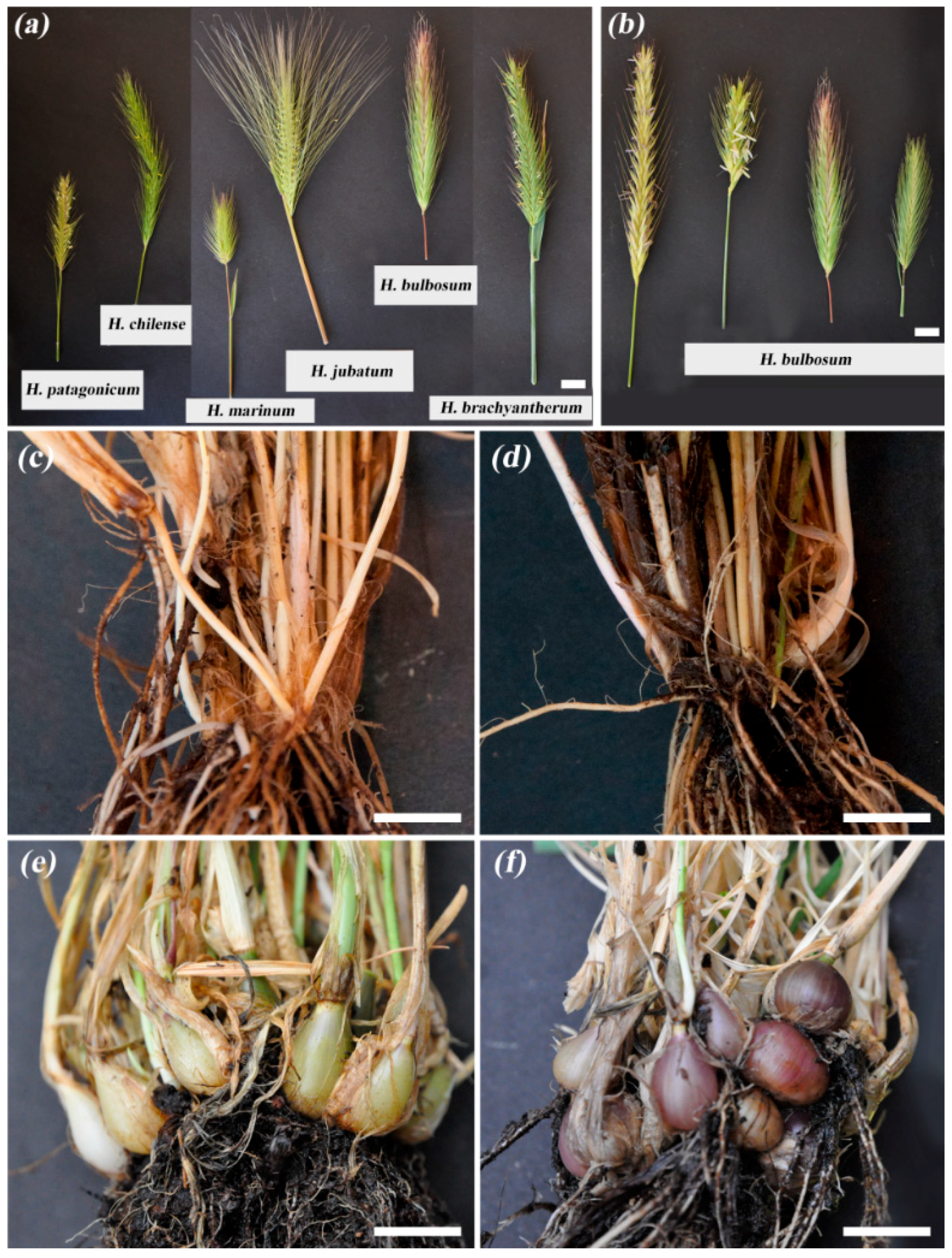
3.2. Diversity in Spike Morphology and Storage Bulbs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crews, T.E.; Blesh, J.; Culman, S.W.; Hayes, R.C.; Jensen, E.S.; Mack, M.C.; Peoples, M.B.; Schipanski, M.E. Going where no grains have gone before: From early to mid-succession. Agric. Ecosyst. Environ. 2016, 223, 223–238. [Google Scholar] [CrossRef]
- Crews, T.; Rumsey, B. What agriculture can learn from native ecosystems in building soil organic matter: A review. Sustainability 2017, 9, 578. [Google Scholar] [CrossRef]
- Glover, J.D.; Culman, S.W.; DuPont, S.T.; Broussard, W.; Young, L.; Mangan, M.E.; Mai, J.G.; Crews, T.E.; DeHaan, L.R.; Buckley, D.H. Harvested perennial grasslands provide ecological benchmarks for agricultural sustainability. Agric. Ecosyst. Environ. 2010, 137, 3–12. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased food and ecosystem security via perennial grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Nabukalu, P.; Paterson, A.; Kong, W.; Nakasagga, S. Development of perennial grain sorghum. Sustainability 2018, 10, 172. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Yang, C.; Liu, H.; Yang, F.; Zhou, J.; Samson, B.K.; Boualaphanh, C.; Huang, L.; Huang, G.; et al. Genotype by environment interactions for grain yield of perennial rice derivatives (Oryza sativa L./Oryza longistaminata) in southern China and Laos. Field Crops Res. 2017, 207, 62–70. [Google Scholar] [CrossRef]
- DeHaan, L.R.; Van Tassel, D.L.; Anderson, J.A.; Asselin, S.R.; Barnes, R.; Baute, G.J.; Cattani, D.J.; Culman, S.W.; Dorn, K.M.; Hulke, B.S.; et al. A pipeline strategy for grain crop domestication. Crop Sci. 2016, 56, 917–930. [Google Scholar] [CrossRef]
- Jaikumar, N.S.; Snapp, S.S.; Murphy, K.; Jones, S.S. Agronomic assessment of perennial wheat and perennial rye as cereal crops. Agron. J. 2012, 104, 1716. [Google Scholar] [CrossRef]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.A.; Timmerman-Vaughan, G.M.; Farnden, K.J.F.; Pickering, R. Marker development and characterisation of Hordeum bulbosum introgression lines: A resource for barley improvement. Theor. Appl. Genet. 2009, 118, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Blattner, F.R. Progress in phylogenetic analysis and a new infrageneric classification of the barley genus Hordeum (Poaceae: Triticeae). Breed. Sci. 2009, 59, 471–480. [Google Scholar] [CrossRef]
- von Bothmer, R.; Jacobsen, N.; Baden, C.; Jørgensen, R.B.; Linde-Laursen, I. An Ecogeographical Study of the Genus Hordeum, 2nd ed.; Systematic and ecogeographic studies on crop genepools 7; International Plant Genetic Resources Institute: Rome, Italy, 1995. [Google Scholar]
- Blattner, F.R.; Pleines, T.; Jakob, S.S. Rapid radiation in the barley genus Hordeum (poaceae) during the pleistocene in the americas. In Evolution in Action: Case Studies in Adaptive Radiation, Speciation and the Origin of Biodiversity; Glaubrecht, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–33. [Google Scholar]
- von Bothmer, R.; Jacobsen, N. Interspecific crosses in Hordeum (Poaceae). Plant Syst. Evol. 1986, 153, 49–64. [Google Scholar] [CrossRef]
- Walther, U.; Rapke, H.; Proeseler, G.; Szigat, G. Hordeum bulbosum-a new source of disease resistance-transfer of resistance to leaf rust and mosaic viruses from H. bulbosum into winter barley. Plant Breed. 2000, 119, 215–218. [Google Scholar] [CrossRef]
- Toubia-Rahme, H.; Johnston, P.A.; Pickering, R.A.; Steffenson, B.J.; Graner, A. Inheritance and chromosomal location of Septoria passerinii resistance introgressed from Hordeum bulbosum into Hordeum vulgare. Plant Breed. 2003, 122, 405–409. [Google Scholar] [CrossRef]
- Fetch, T.; Johnston, P.A.; Pickering, R. Chromosomal location and inheritance of stem rust resistance transferred from Hordeum bulbosum into cultivated barley (H. vulgare). Phytopathology 2009, 99, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Brassac, J.; Blattner, F.R. Species-level phylogeny and polyploid relationships in Hordeum (poaceae) inferred by next-generation sequencing and in silico cloning of multiple nuclear loci. Syst. Biol. 2015, 64, 792–808. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.S.; Meister, A.; Blattner, F.R. The considerable genome size variation of Hordeum species (poaceae) is linked to phylogeny, life form, ecology, and speciation rates. Mol. Biol. Evol. 2004, 21, 860–869. [Google Scholar] [CrossRef] [PubMed]
- von Bothmer, R.; Komatsuda, T. Barley origin and related species. In Barley; Ullrich, S.E., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 14–62. [Google Scholar]
- Kakeda, K.; Ibuki, T.; Suzuki, J.; Tadano, H.; Kurita, Y.; Hanai, Y.; Kowyama, Y. Molecular and genetic characterization of the s locus in Hordeum bulbosum L., a wild self-incompatible species related to cultivated barley. Mol. Genet. Genomics 2008, 280, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Xia, Z.R.; Wu, G.Q.; Yuan, H.J.; Wang, X.R.; Li, J.H.; Tian, F.P.; Zhang, Q.; Zhu, X.Q.; He, J.J.; et al. The coordinated regulation of Na(+) and K(+) in Hordeum brevisubulatum responding to time of salt stress. Plant Sci. 2016, 252, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, A.J.; von Bothmer, R.; Colmer, T.D. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl− into the shoots. J. Exp. Bot. 2005, 56, 2365–2378. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Wheat and barley dehydrins under cold, drought, and salinity—What can LEA-II proteins tell us about plant stress response? Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, G. Micrornas contribute to enhanced salt adaptation of the autopolyploid Hordeum bulbosum compared with its diploid ancestor. Plant J. 2017, 91, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Aliyeva-Schnorr, L.; Stein, N.; Houben, A. Collinearity of homoeologous group 3 chromosomes in the genus Hordeum and Secale cereale as revealed by 3H-derived FISH analysis. Chromosome Res. 2016, 24, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Mascher, M.; Richmond, T.A.; Gerhardt, D.J.; Himmelbach, A.; Clissold, L.; Sampath, D.; Ayling, S.; Steuernagel, B.; Pfeifer, M.; D’ascenzo, M. Barley whole exome capture: A tool for genomic research in the genus Hordeum and beyond. Plant J. 2013, 76, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Wendler, N.; Mascher, M.; Himmelbach, A.; Bini, F.; Kumlehn, J.; Stein, N. A high-density, sequence-enriched genetic map of Hordeum bulbosum and its collinearity to H. vulgare. Plant Genome 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Pickering, R. The production of fertile triploid hybrids from crosses between Hordeum vulgare L. (2n = 4x = 28) and H. bulbosum L. (2n = 2x = 14). Hereditas 1991, 114, 227–236. [Google Scholar] [CrossRef]



| Plant Material/ Species | Ploidy Level | Growth Habit a | Accession ID b | No. of Accessions per Species | Mean no. of Plants per Accession | No. of Accessions and ID with Regrowth c (no. of Accessions Evaluated) d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | ID | Year | ID | Year | ID | ||||||
| 2014 | 2015 | 2016 | |||||||||
| Two-year-old plants | |||||||||||
| H. bogdanii | 2x | P | 1 | 1 | 2 | 1 (1) | 1 | 1 (1) | 1 | - | |
| H. brachyantherum | 4x | P | 2,3 | 2 | 2 | 2 (2) | 2,3 | 1 (2) | 3 | - | |
| H. brevisubulatum | 4x | P | 4 | 1 | 2 | 1 (1) | 4 | 1 (1) | 4 | 1 (1) | 4 |
| H. bulbosum | 4x | P | 5–27 | 23 | 1.1 | 23 (23) | 5–27 | 20 (20) | 5–10,12–19,22–27 | 8 (8) | 5,12,15–17,19,22,24 |
| H. capense | 4x | P | 34 | 1 | 2 | 1 (1) | 34 | 0 (1) | 0 (1) | ||
| H. chilense | 2x | P | 35,36 | 2 | 2 | 2 (2) | 35,36 | 2 (2) | 35,36 | - | |
| H. comosum | 2x | P | 38 | 1 | 2 | 1 (1) | 38 | 1 (1) | 38 | - | |
| H. cordobense | 2x | P | 39 | 1 | 2 | 1 (1) | 39 | 0 (1) | 0 (1) | ||
| H. depressum | 4x | A | 40 | 1 | 2 | 0 (1) | 0 (1) | 0 (1) | |||
| H. erectifolium | 2x | P | 41 | 1 | 2 | 1 (1) | 41 | 0 (1) | 0 (1) | ||
| H. euclaston | 2x | A | 42 | 1 | 1 | 0 (1) | 0 (1) | 0 (1) | |||
| H. fuegianum | 4x | P | 44 | 1 | 2 | 1 (1) | 44 | 1 (1) | 44 | 1 (1) | 44 |
| H. intercedens | 2x | A | 45–47 | 3 | 1.7 | 0 (3) | 0 (3) | 0 (3) | |||
| H. jubatum | 4x | P | 48,49 | 2 | 2 | 2 (2) | 48,49 | 2 (2) | 48,49 | 2 (2) | 48,49 |
| H. lechleri | 6x | P | 50 | 1 | 2 | 1 (1) | 50 | 1 (1) | 50 | 1 (1) | 50 |
| H. muticum | 2x | P | 51 | 1 | 1 | 0 (1) | 0 (1) | 0 (1) | |||
| H. patagonicum | 2x | P | 53 | 1 | 2 | 1 (1) | 53 | 1 (1) | 53 | - | |
| H. pubiflorum | 2x | P | 54 | 1 | 2 | 1 (1) | 54 | 1 (1) | 54 | - | |
| H. secalinum | 4x | P | 58 | 1 | 2 | 1 (1) | 58 | 1 (1) | 58 | 1 (1) | 58 |
| H. stenostachys | 2x | P | 59–67 | 9 | 1.7 | 7 (9) | 59,60,62–65,67 | 3 (9) | 62,63,67 | - | |
| Young plants, vernalized | |||||||||||
| H. bulbosum | 2x | P | 29,30 | 2 | 2.5 | 2 (2) | 29,30 | 1 (2) | 29 | - | |
| H. bulbosum | 4x | P | 10,13,28,31–33 | 6 | 2.7 | 6 (6) | 10,13,28,31–33 | 3 (6) | 10,31,32 | 1 (1) | 10 |
| H. chilense | 2x | P | 37 | 1 | 3 | 1 (1) | 37 | 0 (1) | 0 (1) | ||
| H. euclaston | 2x | A | 42,43 | 2 | 2 | 0 (2) | 0 (2) | 0 (2) | |||
| H. pusillum | 2x | A | 55–57 | 3 | 2.7 | 0 (3) | 0 (3) | 0 (3) | |||
| H. stenostachys | 2x | P | 59–61 | 3 | 2.7 | 2 (3) | 59,60 | 1 (3) | 59 | - | |
| H. vulgare | 2x | A | 68 | 1 | 2 | 0 (1) | 0 (1) | 0 (1) | |||
| Young plants, not vernalized | |||||||||||
| H. brevisubulatum | 2x | P | 4 | 1 | 2 | 1 (1) | 4 | 1 (1) | 4 | 1 (1) | 4 |
| H. muticum | 2x | P | 52 | 1 | 2 | 1 (1) | 52 | 0 (1) | 0 (1) | ||
| H. pubiflorum | 2x | P | 54 | 1 | 1 | 0 (1) | 0 (1) | 0 (1) | |||
| Seed | |||||||||||
| H. brevisubulatum | 4x | P | 4 | 1 | 1 | 1 (1) | 4 | 1 (1) | 4 | - | |
| H. bulbosum | 4x | P | 12,22 | 2 | 1 | 2 (2) | 12,22 | 2 (2) | 12,22 | - | |
| H. pubiflorum | 2x | P | 54 | 1 | 1 | 0 (1) | 0 (1) | 0 (1) | |||
| H. vulgare | 2x | A | 69,70 | 2 | 2 | 0 (2) | 0 (2) | 0 (2) | |||
| Species | Presence of Leaves | Presence of Spikes | ||||
|---|---|---|---|---|---|---|
| May 2014 | August 2014 | July 2015 | August 2016 | August 2014 | July 2015 | |
| Two-year-old plants | ||||||
| H. bogdanii | 2.5 ± 0.5 | 3.0 ± 0.0 | 2.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | |
| H. brachyantherum | 1.6 ± 0.5 | 1.8 ± 0.5 | 0.5 ± 0.3 | 1.0 ± 0.0 | 0 | |
| H. brevisubulatum | 4.0 ± 0.0 | 3.5 ± 0.5 | 4.5 ± 0.5 | 2.5 ± 0.5 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| H. bulbosum | 2.9 ± 0.2 | 2.6 ± 0.1 | 1.9 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.0 | 0.7 ± 0.1 |
| H. capense | 0.8 ± 0.2 | 0.2 ± 0.2 | 0 | 0.5 ± 0.5 | 0 | |
| H. chilense | 0.8 ± 0.2 | 1.2 ± 0.6 | 0.5 ± 0.3 | 0.8 ± 0.2 | 0.2 ± 0.2 | |
| H. comosum | 1.0 ± 1.0 | 1.0 ± 1.0 | 1.0 ± 1.0 | 0.5 ± 0.5 | 0.5 ± 0.5 | |
| H. cordobense | 0.5 ± 0.5 | 0.5 ± 0.5 | 0 | 0.5 ± 0.5 | 0 | |
| H. erectifolium | 0.5 ± 0.5 | 0.5 ± 0.5 | 0 | 0.5 ± 0.5 | 0 | |
| H. fuegianum | 2.5 ± 0.5 | 2.5 ± 0.5 | 3.0 ± 0.0 | 1.0 ± 1.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| H. jubatum | 1.2 ± 0.2 | 1.8 ± 0.2 | 0.9 ± 0.1 | 0.5 ± 0.5 | 0.8 ± 0.2 | 0.5 ± 0.3 |
| H. lechleri | 2.5 ± 0.5 | 4.5 ± 0.5 | 3.0 ± 0.0 | 1.0 ± 1.0 | 0 | 1.0 ± 0.0 |
| H. muticum | 0 | 0 | 0 | 0 | 0 | |
| H. patagonicum | 2.5 ± 0.5 | 2.5 ± 0.5 | 0.2 ± 0.2 | 1.0 ± 0.0 | 0 | |
| H. pubiflorum | 2.5 ± 0.5 | 2.0 ± 1.0 | 2.5 ± 0.5 | 1.0 ± 0.0 | 1.0 ± 0.0 | |
| H. secalinum | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 1.5 ± 1.5 | 1.0 ± 0.0 | 0.5 ± 0.5 |
| H. stenostachys | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0 | |
| Young plants, vernalized | ||||||
| H. bulbosum (2x) | 1.5 ± 0.3 | 1.8 ± 0.5 | 0.3 ± 0.2 | 0 | 1.0 ± 0.0 | 0.0 ± 0.0 |
| H. bulbosum (4x) | 2.4 ± 0.1 | 2.7 ± 0.2 | 1.0 ± 0.3 | 0.5 ± 0.2 | 0.9 ± 0.1 | 0.4 ± 0.1 |
| H. chilense | 0.3 ± 0.2 | 0 | 0 | 0 | 0 | |
| H. stenostachys | 0.4 ± 0.2 | 0.6 ± 0.5 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.2 ± 0.2 | |
| Young plants, not vernalized | ||||||
| H. brevisubulatum | 2.5 ± 0.5 | 3.0 ± 1.0 | 3.0 ± 0.0 | 1.0 ± 1.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| H. muticum | 0.2 ± 0.2 | 0 | 0 | 0 | 0 | |
| H. pubiflorum | 0 | 0 | 0 | 0 | 0 | |
| Seeds | ||||||
| H. brevisubulatum | 1.0 ± 2.0 | 1.0 ± 2.0 | 1.5 ± 2.0 | 0 | 0 | |
| H. bulbosum | 1.3 ± 0.3 | 0.7 ± 0.3 | 2.0 ± 1.0 | 0.3 ± 0.3 | 0.3 ± 0.3 | |
| H. pubiflorum | 0 | 0 | 0 | 0 | 0 | |
| Plant Material/ Species | Accession ID a | No. of Accessions and ID with Spikes b (no. of Accessions with Regrowth) | |||
|---|---|---|---|---|---|
| Year | ID | Year | ID | ||
| 2014 | 2015 | ||||
| Two-year old plants | |||||
| H. bogdanii | 1 | 1 (1) | 1 | 1 (1) | 1 |
| H. brachyantherum | 2,3 | 2 (2) | 2,3 | 0 (1) | |
| H. brevisubulatum | 4 | 1 (1) | 4 | 1 (1) | 4 |
| H. bulbosum | 5–27 | 23 (23) | 5–27 | 15 (20) | 5,7,9,10,12–19,22–24 |
| H. capense | 34 | 0 (1) | 0 (0) | ||
| H. chilense | 35,36 | 2 (2) | 35,36 | 0 (2) | |
| H. comosum | 38 | 1 (1) | 38 | 1 (1) | 38 |
| H. cordobense | 39 | 1 (1) | 39 | 0 (0) | |
| H. depressum | 40 | 0 (1) | 0 (0) | ||
| H. erectifolium | 41 | 0 (1) | 0 (0) | ||
| H. euclaston | 42 | 0 (1) | 0 (0) | ||
| H. fuegianum | 44 | 1 (1) | 44 | 1 (1) | 44 |
| H. intercedens | 45–47 | 0 (3) | 0 (0) | ||
| H. jubatum | 48,49 | 1 (2) | 49 | 1 (2) | 49 |
| H. lechleri | 50 | 0 (1) | 1 (1) | 50 | |
| H. muticum | 51 | 0 (0) | 0 (0) | ||
| H. patagonicum | 53 | 1 (1) | 53 | 0 (1) | |
| H. pubiflorum | 54 | 1 (1) | 54 | 1 (1) | 54 |
| H. secalinum | 58 | 1 (1) | 58 | 1 (1) | 58 |
| H. stenostachys | 59–67 | 6 (9) | 59,60,62,63,65,67 | 0 (3) | |
| Young plants, vernalized | |||||
| H. bulbosum (2x) | 29,30 | 2 (2) | 29,30 | 0 (1) | |
| H. bulbosum (4x) | 10,13,28,31–33 | 6 (6) | 10,13,28,31–33 | 3 (3) | 10,31,32 |
| H. chilense | 37 | 0 (1) | 0 (0) | ||
| H. euclaston | 42,43 | 0 (2) | 0 (0) | ||
| H. pusillum | 55–57 | 0 (3) | 0 (0) | ||
| H. stenostachys | 59–61 | 1 (3) | 59 | 1 (1) | 59 |
| H. vulgare | 68 | 0 (1) | 0 (0) | ||
| Young plants, not vernalized | |||||
| H. brevisubulatum | 4 | 1 (1) | 4 | 1 (1) | 4 |
| H. muticum | 52 | 0 (1) | 0 (0) | ||
| H. pubiflorum | 54 | 0 (1) | 0 (0) | ||
| Seed | |||||
| H. brevisubulatum | 4 | 0 (1) | 0 (1) | ||
| H. bulbosum | 12,22 | 1 (2) | 22 | 0 (2) | |
| H. pubiflorum | 54 | 0 (1) | 0 (0) | ||
| H. vulgare | 69,70 | 0 (2) | 0 (0) | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westerbergh, A.; Lerceteau-Köhler, E.; Sameri, M.; Bedada, G.; Lundquist, P.-O. Towards the Development of Perennial Barley for Cold Temperate Climates—Evaluation of Wild Barley Relatives as Genetic Resources. Sustainability 2018, 10, 1969. https://doi.org/10.3390/su10061969
Westerbergh A, Lerceteau-Köhler E, Sameri M, Bedada G, Lundquist P-O. Towards the Development of Perennial Barley for Cold Temperate Climates—Evaluation of Wild Barley Relatives as Genetic Resources. Sustainability. 2018; 10(6):1969. https://doi.org/10.3390/su10061969
Chicago/Turabian StyleWesterbergh, Anna, Estelle Lerceteau-Köhler, Mohammad Sameri, Girma Bedada, and Per-Olof Lundquist. 2018. "Towards the Development of Perennial Barley for Cold Temperate Climates—Evaluation of Wild Barley Relatives as Genetic Resources" Sustainability 10, no. 6: 1969. https://doi.org/10.3390/su10061969
APA StyleWesterbergh, A., Lerceteau-Köhler, E., Sameri, M., Bedada, G., & Lundquist, P.-O. (2018). Towards the Development of Perennial Barley for Cold Temperate Climates—Evaluation of Wild Barley Relatives as Genetic Resources. Sustainability, 10(6), 1969. https://doi.org/10.3390/su10061969





