Intercropping Halophytes to Mitigate Salinity Stress in Watermelon
Abstract
:1. Introduction
1.1. Salinization
1.2. Intercropping
1.3. Intercropping with Halophytes
2. Materials and Methods
2.1. Phase I: Greenhouse Evaluation
2.2. Phase II: Field Trial
2.3. Statistical Analysis
3. Results
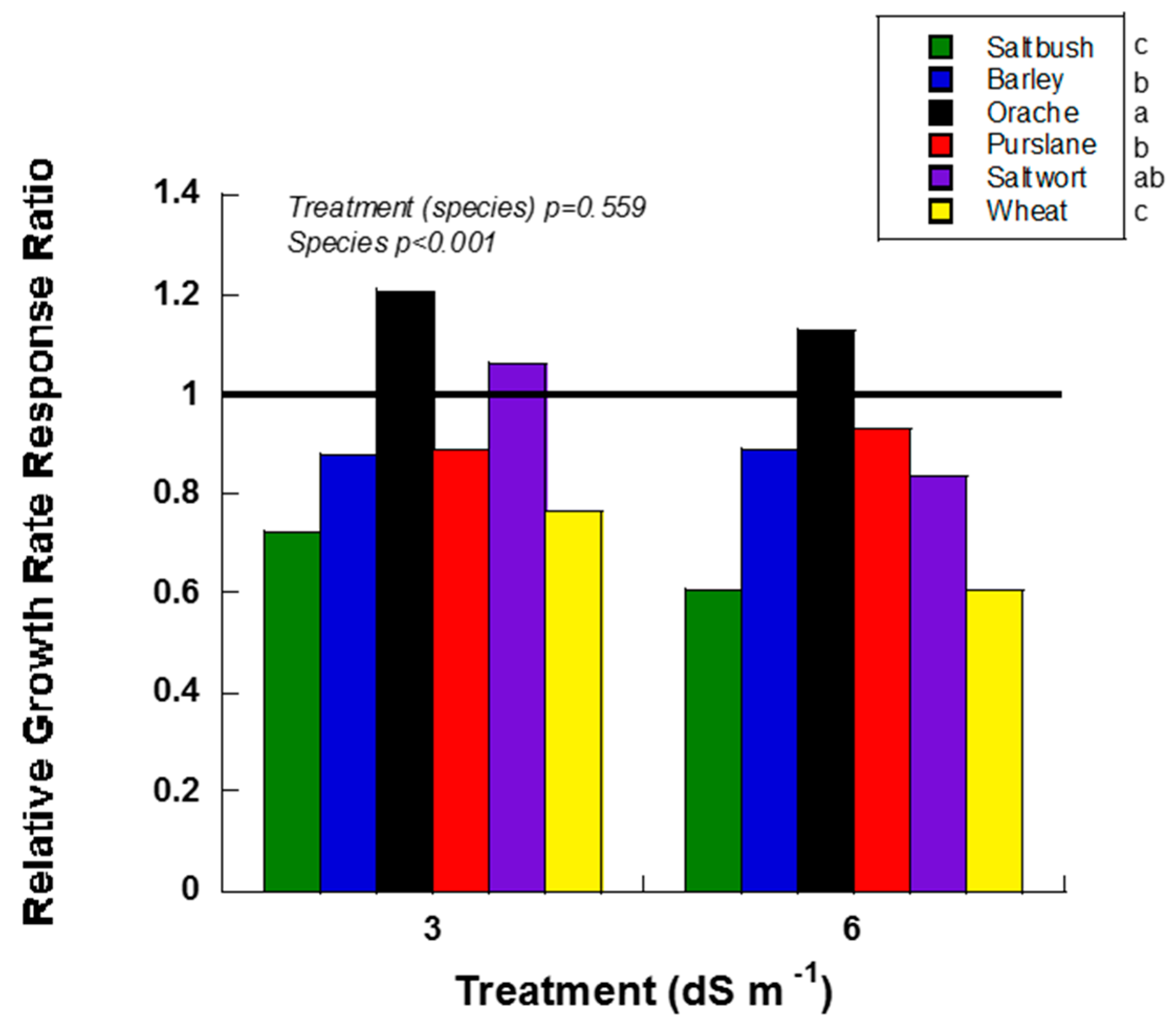
3.1. Phase I: Greenhouse Halophyte Evaluation
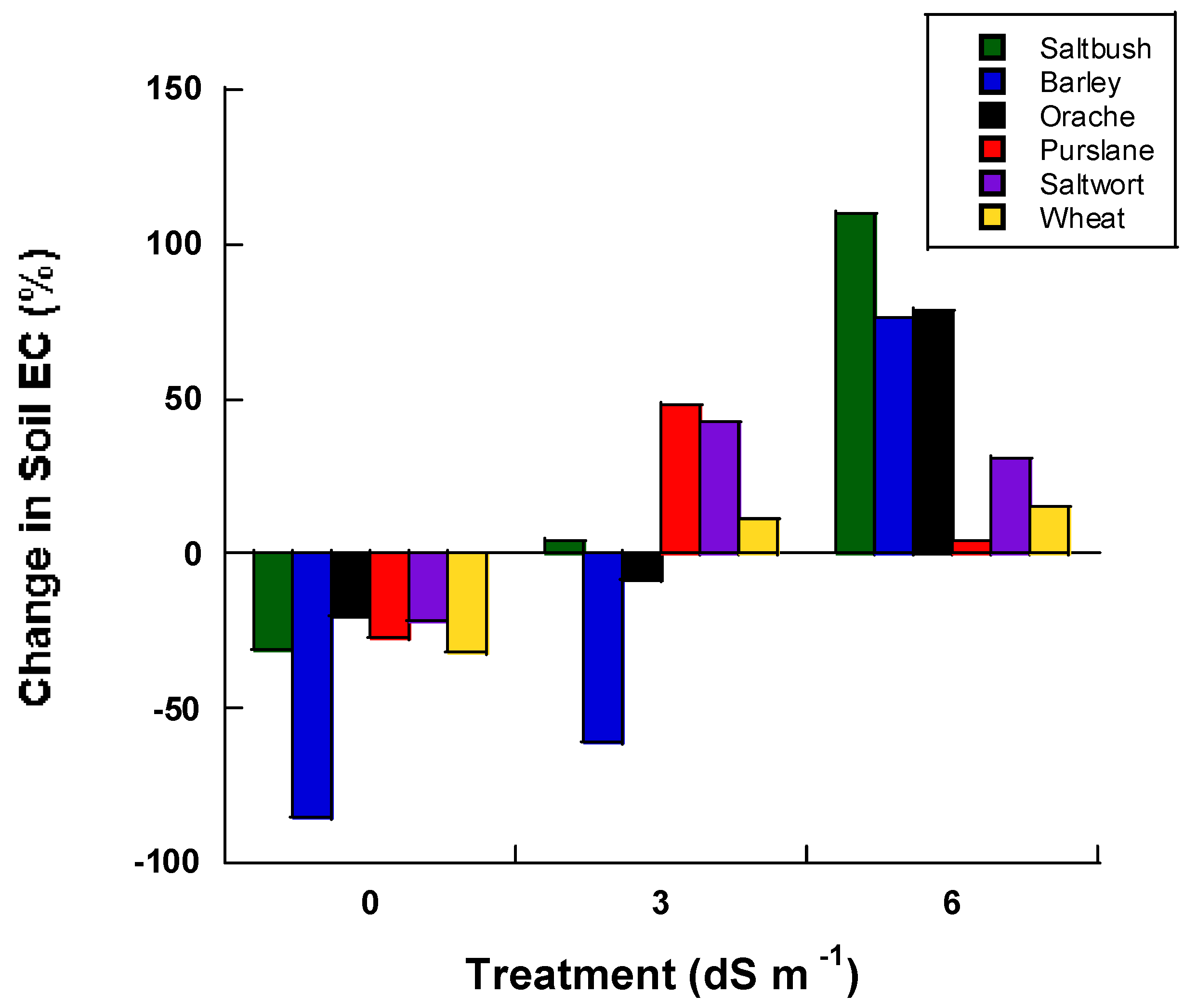
3.2. Phase II: Field Intercropping Trial
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Tissue * | Species | 0 dS m−1 | 3 dS m−1 | 6 dS m−1 |
|---|---|---|---|---|
| Leaves | Saltbush | 0.87 fgh | 1.87 defg | 1.43 efgh |
| Barley | 5.61 bc | 6.81 ab | 7.33 a | |
| Wheat | 5.24 c | 2.96 d | 5.67 bc | |
| Orache | 2.06 def | 2.40 de | 2.51 de | |
| Purslane | 2.06 def | 2.17 def | 1.63 defgh | |
| Saltwort * | 0.33 h | 0.60 gh | 0.32 h | |
| pSpecies×salinity = 0.014 | ||||
| Stems | Saltbush | 0.24 c | 0.67 c | 0.36 c |
| Orache | 1.84 b | 2.51 b | 2.42 b | |
| Purslane | 2.70 b | 4.48 a | 2.16 b | |
| pSpecies×salinity = 0.041 | ||||
| Roots | Saltbush | 0.22 | 0.39 | 0.37 |
| Barley | 3.75 | 3.09 | 3.35 | |
| Wheat | 3.44 | 4.26 | 3.82 | |
| Orache | 1.20 | 1.67 | 1.78 | |
| Purslane | 0.99 | 1.02 | 0.85 | |
| pSpecies×salinity = 0.865 | ||||
| Total plant | Saltbush | 1.32 | 2.94 | 2.16 |
| Barley | 9.36 | 9.90 | 10.68 | |
| Wheat | 8.68 | 7.22 | 9.49 | |
| Orache | 5.10 | 6.57 | 6.71 | |
| Purslane | 5.75 | 7.67 | 4.63 | |
| Saltwort * | 0.33 | 0.60 | 0.32 | |
| pSpecies×salinity = 0.353 | ||||
References
- Wedekind, L. Saline soils: Salt of the Earth. Available online: http://www.iaea.org/newscenter/features/salinesoil/saline_part05.shtml (accessed on 10 October 2014).
- United States Department of the Interior. Quality of Water Colorado River Basin; United States Department of the Interior: Washington, DC, USA, 2005. [Google Scholar]
- Scanlon, B.R.; Jolly, I.; Sophocleous, M.; Zhang, L. Global impacts of conversions from natural to agricultural ecosystems on water resources: Quantity versus quality. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef]
- NASS. Land in Farms, Harvested Cropland, and Irrigated Land, by Size of Farm: 2012 and 2007; NASS: Burr Ridge, IL, USA, 2012. [Google Scholar]
- Pitman, M.; Läuchli, A. Global impact of salinity and agricultural ecosystems. In Salinity: Environment–Plants–Molecules; Lauchli, A., Luttge, U., Eds.; Kluwer Academic Publisher: Norwell, MA, USA, 2002; pp. 1–51. [Google Scholar]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources : Human Causes, Extent, Management and Case Studies; Ghassemi, F., Jakeman, A.J., Nix, H.A., Eds.; University of New South Wales Press: Sydney, Australia, 1995; ISBN 0868401986. [Google Scholar]
- McCoy, T. Evaluation of Ground-Water Resources in the Lower Rio Grande Valley, Texas; Texas Water Development Board: Austin, TX, USA, 1990. [Google Scholar]
- Michelsen, A.; McGuckin, T.; Sheng, Z. Rio Grande Salinity Management Program: Preliminary Economic Impact Assessment; Texas A&M System: El Paso, TX, USA, 2009. [Google Scholar]
- Storey, R. Salt Tolerance, Ion Relations and the Effect of Root Medium on the Response of Citrus to Salinity. Aust. J. Plant Physiol. 1995, 22, 101–114. [Google Scholar] [CrossRef]
- Storey, R.; Walker, R. Citrus and salinity. Sci. Hortic. 1998, 78, 39–81. [Google Scholar] [CrossRef]
- Tozlu, I.; Guy, C.; Moore, G. Tolerance mechanisms to salinity stress in Citrus and Poncirus. In Proceedings IS on Salination for Horticultural Production; International Society for Horticultural Sciences: Leuven, Belgium, 2002; pp. 271–282. [Google Scholar]
- De La Fuente, E.B.; Suárez, S.A.; Lenardis, A.E.; Poggio, S.L. Intercropping sunflower and soybean in intensive farming systems: Evaluating yield advantage and effect on weed and insect assemblages. NJAS Wageningen J. Life Sci. 2014, 70, 47–52. [Google Scholar] [CrossRef]
- Bantie, Y.B.; Abera, F.A.; Woldegiorgis, T.D. Competition Indices of Intercropped Lupine (Local) and Small Cereals in Additive Series in West Gojam, North Western Ethiopia. AJPS 2014, 5, 1296–1305. [Google Scholar] [CrossRef]
- Machado, S. Does intercropping have a role in modern agriculture? J. Soil Water Conserv. 2009, 64, 55A–57A. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Interspecific competition, N use and interference with weeds in pea-barley intercropping. Field Crop. Res. 2001, 70, 101–109. [Google Scholar] [CrossRef]
- Franco, J.G.; King, S.R.; Masabni, J.G.; Volder, A. Plant functional diversity improves short-term yields in a low-input intercropping system. Agric. Ecosyst. Environ. 2015, 203, 1–10. [Google Scholar] [CrossRef]
- Vandermeer, J.H. The Ecology of Intercropping. John Vandermeer; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1989; ISBN 0521345928. [Google Scholar]
- Fukai, S.; Trenbath, B.R. Processes determining intercrop productivity and yields of component crops. Field Crop. Res. 1993, 34, 247–271. [Google Scholar] [CrossRef]
- Banik, P.; Midya, A.; Sarkar, B.K.; Ghose, S.S. Wheat and chickpea intercropping systems in an additive series experiment: Advantages and weed smothering. Eur. J. Agron. 2006, 24, 325–332. [Google Scholar] [CrossRef]
- Sobkowicz, P. Competition between triticale (Triticosecale Witt.) and field beans (Vicia faba var minor L.) in additive intercrops. Plant Soil Environ. 2006, 52, 47–54. [Google Scholar] [CrossRef]
- Brennan, E.B. Agronomic aspects of strip intercropping lettuce with alyssum for biological control of aphids. Biol. Control 2013, 65, 302–311. [Google Scholar] [CrossRef]
- Baumann, D.T.; Bastiaans, L.; Kropff, M.J. Competition and crop performance in a leek-celery intercropping system. Crop Sci. 2001, 41, 764–774. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E. Strawberry Intercropping with Vegetables for Proper Utilization of Space and Resources. J. Sustain. Agric. 2009, 33, 107–116. [Google Scholar] [CrossRef]
- Albaho, M.S.; Green, J.L. Suaeda salsa, A Desalinating Companion Plant for Greenhouse Tomato. HortScience 2000, 35, 620–623. [Google Scholar]
- Zuccarini, P. Ion uptake by halophytic plants to mitigate saline stress in Solarium lycopersicon L., and different effect of soil and water salinity. Soil Water Res. 2008, 3, 62–73. [Google Scholar] [CrossRef]
- Graifenberg, A.; Botrini, L.; Giustiniani, L.; Filippi, F.; Curadi, M. Tomato growing in saline conditions with biodesalinating plants: Salsola soda L., and Portulaca oleracea L. Acta Hortic. 2003, 609, 301–305. [Google Scholar] [CrossRef]
- Kurdali, F. Growth and N 2 fixation in mixed cropping of Medicago arborea and Atriplex halimus grown on a salt-affected soil using a 15 N tracer technique. J. Plant Interact. 2010, 5, 37–44. [Google Scholar] [CrossRef]
- Kurdali, F.; Janat, M.; Khalifa, K. Growth and nitrogen fixation and uptake in Dhaincha/Sorghum intercropping system under saline and non-saline conditions. Commun. Soil Sci. Plant Anal. 2003, 34, 17–18. [Google Scholar] [CrossRef]
- Rawson, H.M.; Richards, R.A.; Munns, R. An examination of selection criteria for salt-tolerance in wheat, barley, and triticale genotypes. Aust. J. Agric. Res. 1988, 759–772. [Google Scholar] [CrossRef]
- Suaire, R.; Durickovic, I.; Framont-Terrasse, L.; Leblain, J.Y.; De Rouck, A.C.; Simonnot, M.O. Phytoextraction of Na+ and Cl− by Atriplex halimus L. and Atriplex hortensis L.: A promising solution for remediation of road runoff contaminated with deicing salts. Ecol. Eng. 2016, 94, 182–189. [Google Scholar] [CrossRef]
- Uygur, V.; Yetisir, H. Effects of Rootstocks on Some Growth Parameters, Phosphorous and Nitrogen Uptake Watermelon under Salt Stress. J. Plant Nutr. 2009, 32, 629–643. [Google Scholar] [CrossRef]
- Colla, G.; Roupahel, Y. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. HortScience 2006, 41, 622–627. [Google Scholar]
- Kiliç, C.C.; Kukul, Y.S.; Anaç, D. Performance of purslane (Portulaca oleracea L.) as a salt-removing crop. Agric. Water Manag. 2008, 95, 854–858. [Google Scholar] [CrossRef]
- Rozema, E.R.; Gordon, R.J.; Zheng, Y. Plant Species for the Removal of Na+ and Cl− from Greenhouse Nutrient Solution. Hortscience 2014, 49, 1071–1075. [Google Scholar]
- Alharby, H.F.; Colmer, T.D.; Barrett-Lennard, E.G. Salt accumulation and depletion in the root-zone of the halophyte Atriplex nummularia Lindl.: Influence of salinity, leaf area and plant water use. Plant Soil 2014, 382, 31–41. [Google Scholar] [CrossRef]
- Mata-González, R.; Abdallah, M.A.B.; Trejo-Calzada, R.; Wan, C. Growth and leaf chemistry of Atriplex species from Northern Mexico as affected by salt stress. Arid Land Res. Manag. 2017, 31, 57–70. [Google Scholar] [CrossRef]
- Plaut, Z.; Edelstein, M.; Ben-Hur, M. Overcoming Salinity Barriers to Crop Production Using Traditional Methods. Crit. Rev. Plant Sci. 2013, 32, 250–291. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Fallovo, C.; Caradrelli, M.; Graifenberg, A. Use of Salsola soda as a companion plant to improve greenhouse pepper (Capsicum annuum) performance under saline conditions. N. Z. J. Crop Hortic. Sci. 2006, 34, 283–290. [Google Scholar] [CrossRef]
- Wang, C.M.; Xia, Z.R.; Wu, G.Q.; Yuan, H.J.; Wang, X.R.; Li, J.H.; Tian, F.P.; Zhang, Q.; Zhu, X.Q.; He, J.J.; et al. The coordinated regulation of Na+ and K+ in Hordeum brevisubulatum responding to time of salt stress. Plant Sci. 2016, 252, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Qasem, J.R. Nutrient accumulation by weeds and their associated vegetable crops. J. Hortic. Sci. 1992, 67, 189–195. [Google Scholar] [CrossRef]
- Franco, J.G.; King, S.R.; Volder, A. Component crop physiology and water use efficiency in response to intercropping. Eur. J. Agron. 2018, 93, 27–39. [Google Scholar] [CrossRef]

| Crop | Common Name | Growth form and Potential Added Value |
|---|---|---|
| Atriplex canescens | four-wing saltbush | Upright growth, windbreak, forage |
| Atriplex hortensis | garden orache | Low growing, human consumption, forage |
| Hordeum vulgare | barley | Upright growth, windbreak, human consumption, forage |
| Portulaca oleracea | purslane | Low prostrate, smother crop (weed suppression), forage |
| Salsola komarovii | saltwort | Low prostrate, smother crop (weed suppression), forage, biofuel |
| Triticum aestivum | wheat | Upright growth, windbreak, forage/feed crop |
| Test | Na | Cl |
|---|---|---|
| Prob > F | Prob > F | |
| Species | <0.001 | 0.071 |
| Salinity treatment | <0.001 | 0.016 |
| Species × salinity treatment | 0.014 | 0.903 |
| Tissue | 0.009 | <0.001 |
| Species × tissue | 0.001 | <0.001 |
| Salinity treatment × tissue | 0.297 | 0.014 |
| Species × salinity treatment × tissue | <0.001 | <0.001 |
| Tissue * | Species | Na (mg g−1) | Cl (mg g−1) | Total Na Uptake (mg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 dS m−1 | 3 dS m−1 | 6 dS m−1 | 0 dS m−1 | 3 dS m−1 | 6 dS m−1 | 0 dS m−1 | 3 dS m−1 | 6 dS m−1 | ||
| Leaves | Saltbush | 0.90 j | 4.79 hi | 7.17 h | 0.0010 g | 0.0008 g | 0.0011 fg | 0.86 e | 10.19 e | 10.82 e |
| Barley | 4.43 hi | 10.54 g | 10.56 g | 0.0022 ef | 0.0029 de | 0.0033 cd | 25.26 cde | 76.93 b | 78.71 b | |
| Wheat | 0.44 j | 2.22 ij | 3.04 ij | 0.0013 fg | 0.0040 bc | 0.0052 a | 2.31 e | 6.32 e | 16.98 de | |
| Orache | 26.71 d | 61.40 b | 64.88 a | 0.0018 fg | 0.0036 bcd | 0.0039 bcd | 53.75 bc | 146.76 a | 163.69 a | |
| Purslane | 3.63 ij | 21.38 e | 34.69 c | 0.0045 ab | 0.0045 ab | 0.0056 a | 7.39 e | 46.40 bcd | 56.07 bc | |
| Saltwort | 3.49 ij | 15.30 f | 17.74 f | 0.0005 fg | 0.0008 fg | - | 1.17 e | 9.70 e | 5.53 e | |
| pspecies×salinity < 0.001 | pspecies×salinity < 0.001 | pspecies×salinity < 0.003 | ||||||||
| Stems | Saltbush | 0.34 f | 0.81 f | 0.43 f | 0.0006 ab | 0.0004 b | - | 0.07 c | 0.69 c | 0.15 c |
| Orache | 11.85 d | 24.03 c | 30.63 ab | 0.0002 c | 0.0004 b | 0.0004 b | 21.18 c | 60.99 b | 70.52 b | |
| Purslane | 5.83 e | 27.94 bc | 34.09 a | 0.0008 a | 0.0006 a | 0.0007 a | 16.19 c | 126.90 a | 73.17 b | |
| pspecies×salinity < 0.001 | pspecies×salinity < 0.001 | pspecies×salinity < 0.001 | ||||||||
| Roots | Saltbush | 4.18 c | 9.17 b | 13.72 ^ | - | 0.0003 | - | 0.80 | 3.06 | 5.02 |
| Barley | 6.31 bc | 13.27 a | 15.76 a | 0.0002 | 0.0002 | 0.0003 | 23.69 | 46.41 | 53.00 | |
| Wheat | 5.93 bc | 5.95 bc | 8.55 b | 0.0002 | 0.0003 | 0.0004 | 21.31 | 27.30 | 33.13 | |
| Orache | 7.79 b | 8.17 b | 8.57 b | 0.0004 | 0.0006 | 0.0008 | 10.04 | 13.45 | 14.42 | |
| Purslane | 5.86 bc | 14.68 a | 15.61 a | 0.0003 | 0.0005 | 0.0008 | 5.71 | 15.23 | 13.41 | |
| pspecies×salinity < 0.001 | pspecies×salinity = 0.054 | pspecies×salinity < 0.24 | ||||||||
| May | June | July | August | September | Total/Mean |
|---|---|---|---|---|---|
| Precipitation (mm) | |||||
| 100 | 29 | 21 | 15 | 148 | 313 |
| Mean air temperature (°C) | |||||
| 23 | 29 | 29 | 30 | 28 | 28 |
| Month | Intercrop | Pre-Dawn Stem Ψ (Mpa) | Mid-Day Stem Ψ (Mpa) | ||||
|---|---|---|---|---|---|---|---|
| 0 dS m−1 | 3 dS m−1 | 6 dS m−1 | 0 dS m−1 | 3 dS m−1 | 6 dS m−1 | ||
| June | W | −0.79 | −0.75 | −1.13 | −1.00 | −1.42 | −1.25 |
| W/O | −0.71 | −1.30 | −0.88 | −1.33 | −1.04 | −1.17 | |
| W/P | −0.92 | −1.04 | −0.92 | −1.21 | −1.25 | −1.04 | |
| W/O/P | −0.67 | −1.04 | −0.96 | −1.00 | −1.04 | −1.08 | |
| psalinity(treatment) = 0.174 | psalinity(treatment) = 0.966 | ||||||
| July * | W | −1.96 | −1.46 | −1.58 | |||
| W/O | −1.38 | −1.54 | −2.29 | ||||
| W/P | −1.29 | −1.13 | −1.79 | ||||
| W/O/P | −1.42 | −1.75 | −2.17 | ||||
| psalinity(treatment) = 0.966 | |||||||
| August | W | −1.75 | −1.79 | −2.30 | −1.79 | −2.71 | −2.50 |
| W/O | −3.17 | −1.79 | −2.17 | −3.17 | −2.75 | −2.33 | |
| W/P | −2.33 | −2.42 | −1.83 | −2.42 | −2.92 | −2.38 | |
| W/O/P | −2.83 | −1.46 | −1.21 | −2.83 | −3.08 | −1.67 | |
| psalinity(treatment) = 0.499 | psalinity(treatment) = 0.101 | ||||||
| Month | Pre-Dawn Stem Ψ (Mpa) | Mid-Day Stem Ψ (Mpa) |
|---|---|---|
| June | −0.92 b | −1.15 c |
| July * | −1.62 b | |
| August | −2.17 a | −2.54 a |
| pmonth < 0.001 | pmonth < 0.001 |
| Measurement | Salinity | W | W/O | W/P | W/O/P |
|---|---|---|---|---|---|
| Fruit mass (kg) | 0 dS m−1 | 8.68 | 9.27 | 8.42 | 8.79 |
| 3 dS m−1 | 8.09 | 9.71 | 9.26 | 8.65 | |
| 6 dS m−1 | 9.19 | 8.46 | 9.41 | 10.52 | |
| psalinity(treatment) = 0.512 | |||||
| Brix (°) | 0 dS m−1 | 10.31 | 10.63 | 10.98 | 10.28 |
| 3 dS m−1 | 11.06 | 11.00 | 10.81 | 10.80 | |
| 6 dS m−1 | 10.64 | 10.75 | 11.02 | 11.10 | |
| psalinity(treatment) = 0.424 | |||||
| Firmness (N) | 0 dS m−1 | 1.89 | 1.90 | 0.99 | 1.21 |
| 3 dS m−1 | 1.28 | 1.28 | 0.98 | 1.28 | |
| 6 dS m−1 | 1.43 | 0.99 | 1.13 | 1.11 | |
| psalinity(treatment) = 0.355 | |||||
| Rind thickness (mm) | 0 dS m−1 | 13.37 | 14.25 | 13.09 | 11.97 |
| 3 dS m−1 | 12.41 | 14.31 | 15.46 | 13.25 | |
| 6 dS m−1 | 12.15 | 15.28 | 10.92 | 13.29 | |
| psalinity(treatment) = 0.667 | |||||
| Intercrop | Salinity | Pre-Plant | Post-Harvest | % Change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conductivity | Na | Cl | Conductivity | Na | Cl | Conductivity | Na | Cl | ||
| (dS m−1) | (mg g−1) | (mg g−1) | (dS m−1) | (mg g−1) | (mg g−1) | (dS m−1) | (mg g−1) | (mg g−1) | ||
| W | 0 dS m−1 | 0.074 | 0.016 | 0.031 | 0.115 | 0.246 | 0.092 | 35.8 | 93.6 | 66.8 |
| 3 dS m−1 | 0.175 | 0.014 | 0.048 | 0.149 | 0.276 | 0.168 | 50.1 | 95.1 | 71.3 | |
| 6 dS m−1 | 0.079 | 0.009 | 0.047 | 0.160 | 0.270 | 0.081 | 50.5 | 96.6 | 41.8 | |
| W/O | 0 dS m−1 | 0.054 | 0.012 | 0.032 | 0.111 | 0.240 | 0.078 | 51.4 | 95.1 | 59.4 |
| 3 dS m−1 | 0.090 | 0.011 | 0.048 | 0.133 | 0.256 | 0.115 | 32.5 | 95.6 | 58.5 | |
| 6 dS m−1 | 0.084 | 0.009 | 0.062 | 0.162 | 0.283 | 0.074 | 48.3 | 96.8 | 15.5 | |
| W/P | 0 dS m−1 | 0.053 | 0.013 | 0.057 | 0.095 | 0.227 | 0.069 | 44.1 | 94.3 | 17.7 |
| 3 dS m−1 | 0.077 | 0.015 | 0.062 | 0.163 | 0.282 | 0.126 | 53.0 | 94.6 | 51.3 | |
| 6 dS m−1 | 0.087 | 0.011 | 0.045 | 0.153 | 0.277 | 0.074 | 43.5 | 96.2 | 39.6 | |
| W/O/P | 0 dS m−1 | 0.064 | 0.011 | 0.035 | 0.095 | 0.216 | 0.083 | 32.7 | 94.9 | 57.4 |
| 3 dS m−1 | 0.065 | 0.011 | 0.075 | 0.161 | 0.276 | 0.097 | 59.8 | 96.1 | 23.4 | |
| 6 dS m−1 | 0.081 | 0.011 | 0.053 | 0.157 | 0.272 | 0.082 | 48.4 | 96.1 | 35.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simpson, C.R.; Franco, J.G.; King, S.R.; Volder, A. Intercropping Halophytes to Mitigate Salinity Stress in Watermelon. Sustainability 2018, 10, 681. https://doi.org/10.3390/su10030681
Simpson CR, Franco JG, King SR, Volder A. Intercropping Halophytes to Mitigate Salinity Stress in Watermelon. Sustainability. 2018; 10(3):681. https://doi.org/10.3390/su10030681
Chicago/Turabian StyleSimpson, Catherine R., Jose G. Franco, Stephen R. King, and Astrid Volder. 2018. "Intercropping Halophytes to Mitigate Salinity Stress in Watermelon" Sustainability 10, no. 3: 681. https://doi.org/10.3390/su10030681





