Development and Evaluation of Topical Gabapentin Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Production of Topical Gabapentin Hydrogels
- For blank Carbopol® and 0% ethanol (EtOH) gels, sodium methyl and ethyl hydroxybenzoate were also dissolved in de-ionised water.
- For hydrogels containing a permeation enhancer, methyl and propyl hydroxybenzoate were dissolved in the required mass of permeation enhancer solvent. Permeation enhancers included EtOH, dimethyl sulfoxide (DMSO), dimethyl isosorbide (DMI), isopropyl myristate (IPM) or propylene glycol (PG). The permeation enhancer mixture was added to the aqueous mixture and pre-mixed for 5 min.
2.2.2. Production of Formulations Utilising Proprietary Bases
2.2.3. Viscosity Measurement
2.2.4. Formulation Release Studies
2.2.5. Preparation of Human Epidermal Membranes
2.2.6. Franz-Type Diffusion Cell Studies
2.2.7. High Performance Liquid Chromatography (HPLC) Analysis
2.2.8. Data Analysis
- Cr is the cumulative receptor chamber concentration (mcg/mL).
- Vr is the volume of the receptor chamber
- A is the diffusional surface area of the membrane.
3. Results
3.1. Production of Topical Gabapentin Formulations
3.2. Release of Gabapentin from Topical Formulations
3.3. Skin Permeation of Gabapentin from Saturated Hydroalcoholic Solutions
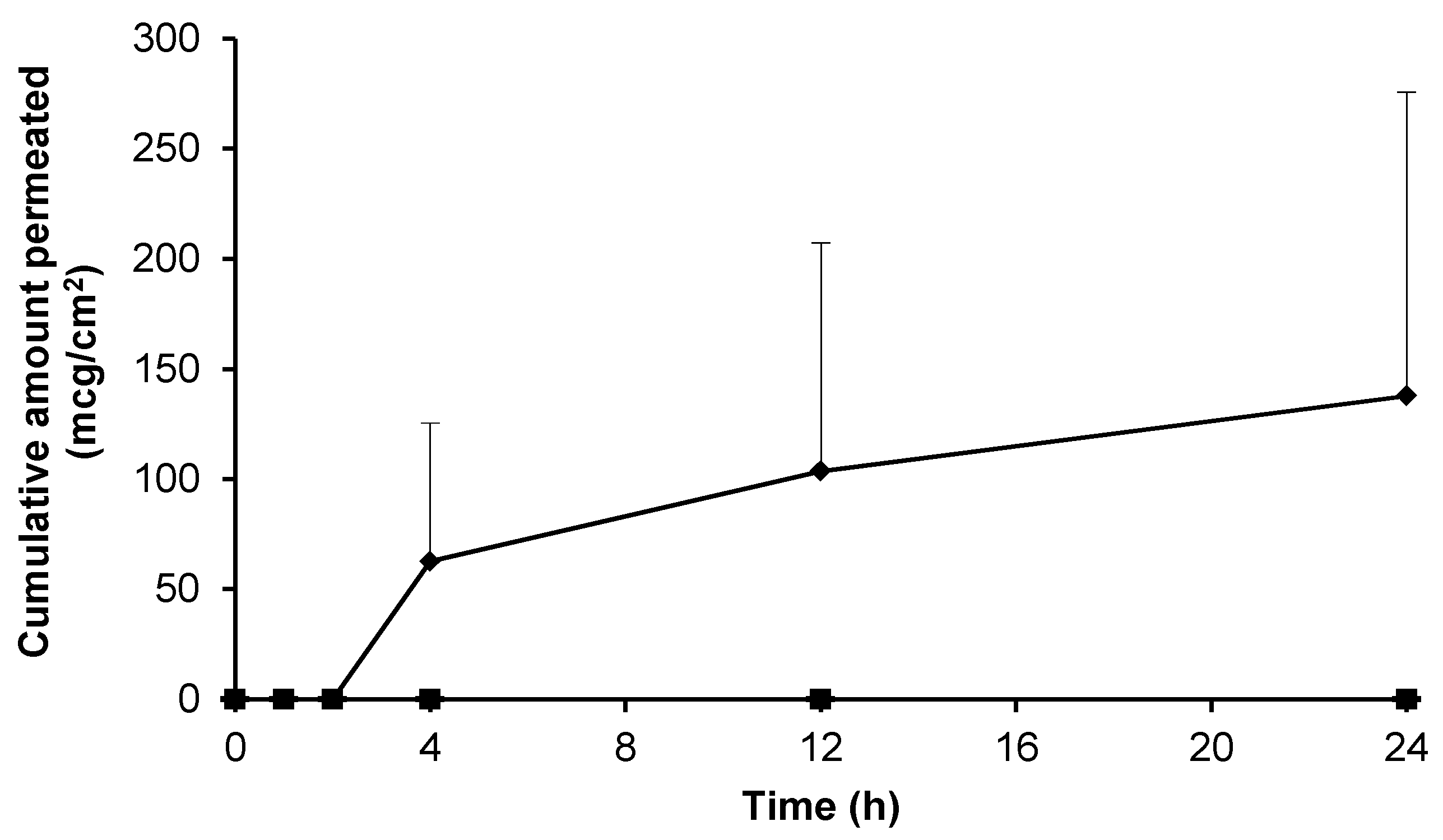
3.4. Skin Permeation of Gabapentin from Topical Formulations
3.5. Electrical Resistance Measurement of Epidermal Membranes
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rose, M.; Kam, P. Gabapentin: Pharmacology and its use in pain management. Anaesthesia 2002, 57, 451–462. [Google Scholar] [CrossRef] [PubMed]
- BMA; RPS. British National Formulary; BMJ Group: London, UK; Tavistock Square Pharmaceutical Press: London, UK, 2016; Available online: https://www-medicinescomplete-com.ezproxy1.bath.ac.uk/mc/bnf/current/PHP2930-gabapentin.htm (accessed on 25 August 2017).
- Hiom, S.; Patel, G.K.; Newcombe, R.G.; Khot, S.; Martin, C. Severe postherpetic neuralgia and other neuropathic pain syndromes alleviated by topical gabapentin. Br. J. Dermatol. 2015, 173, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Chen, L. Gabapentin in pain management. Anesth. Analg. 2000, 91, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Vadaurri, V. Topical treatment of neuropathic pain. Int. J. Pharm. Compd. 2008, 12, 183–190. [Google Scholar]
- Ali, G.; Subhan, F.; Abbas, M.; Zeb, J.; Shahid, M.; Sewell, R.D.E. A streptozotocin-induced diabetic neuropathic pain model for static or dynamic mechanical allodynia and vulvodynia: Validation using topical and systemic gabapentin. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Boardman, L.A.; Cooper, A.S.; Blais, L.R.; Raker, C.A. Topical gabapentin in the treatment of localized and generalized vulvodynia. Obstet. Gynecol. 2008, 112, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Datapharm. Electronic Medicines Compendium. 2016. Available online: http://www.medicines.org.uk/emc/ (accessed on 25 August 2017).
- Steluti, R.; De Rosa, F.; Collett, J.; Tedesco, A.; Bentley, M. Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. Eur. J. Pharm. Biopharm. 2005, 60, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.; Nnadi, C. Transdermal delivery of gabapentin: Effect of cosolvent and microemulsion on permeation through the rat skin. Pharmacol. Pharm. 2014, 5, 471–478. [Google Scholar] [CrossRef]
- Arellano, A.; Santoyo, S.; Martı́n, C.; Ygartua, P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur. J. Pharm. Sci. 1999, 7, 129–135. [Google Scholar] [CrossRef]
- Taş, C.; Ozkan, Y.; Savaşer, A.; Baykara, T. In vitro and ex vivo permeation studies of chlorpheniramine maleate gels prepared by carbomer derivatives. Drug Dev. Ind. Pharm. 2004, 30, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, G.E.; Williams, A.; Barry, B. Skin hydration and possible shunt route penetration in controlled estradiol delivery from ultradeformable and standard liposomes. J. Pharm. Pharmacol. 2001, 53, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Achrai, B.; Libster, D.; Aserin, A.; Garti, N. Solubilization of Gabapentin into HII Mesophases. J. Phys. Chem. B 2011, 115, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Le, U.; Truong, L.; Nguyen, H.; Tran, M.; Vuong, H.-P.; Tran, H. Gabapentin in elastic liposomes: Preparation, drug release, and penetration through porcine skin. In Proceedings of the 2012 AAPS Annual Meeting and Exposition, San Diego, CA, USA, 21–23 May 2012. [Google Scholar]
- Wang, X.; Black, L. Ex vivo percutaneous absorption of ketamine, bupivacaine, diclofenac, gabapentin, orphenadrine and pentoxifylline: Comparison of versatile cream vs. reference cream. Int. J. Pharm. Compd. 2013, 17, 520–525. [Google Scholar] [PubMed]
- Pearton, M.; Allender, C.; Brain, K.; Anstey, A.; Gateley, C.; Wilke, N.; Morrissey, A.; Birchall, J. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharm. Res. 2008, 25, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol. In Vitro 2004, 18, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, A.; Gupta, A.; Sayeed, V.; Khan, M.; Faustino, P. Development and application of a validated HPLC method for the determination of gabapentin and its major degradation impurity in drug products. J. Pharm. Biomed. Anal. 2007, 43, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.R.; Boissy, Y.L.; Lilly, N.A.; Spears, M.J.; McKillop, K.; Marshall, J.L.; Stone, K.J. Water disrupts stratum corneum lipid lamellae: Damage is similar to surfactants. J. Investig. Dermatol. 1999, 113, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Professional Compounding Centers of America. Technical Report: PCCA Lipoderm® Delivers Four Drugs Simultaneously in Transdermal Study. 2011. Available online: http://www.pccarx.com/patients/item/93-pcca-lipoderm-and-four-drugs-transdermal-study (accessed on 25 August 2017).
- Shalaby, S.; Shukr, M. The influence of the type and concentration of alcohol on the rheological and mucoadhesive properties of carbopol 940 hydroalcoholic gels. Der Pharm. Sin. 2011, 2, 161–171. [Google Scholar]
- PubChem. Open Chemistry Database; Gabapentin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/gabapentin (accessed on 25 August 2017).
- Zour, E.; Lodhi, S.A.; Nesbitt, R.U.; Silbering, S.B.; Chaturvedi, P.R. Stability studies of gabapentin in aqueous solutions. Pharm. Res. 1992, 9, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Annex 2 Stability testing of active pharmaceutical ingredients and finished pharmaceutical products. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2009; Volume 953, pp. 87–130. [Google Scholar]
- Pokharkar, V.; Dhar, S.; Singh, N. Effect of Penetration Enhancers on Gel Formulation of Zidovudine: In Vivo and Ex Vivo Studies. PDA J. Pharm. Sci. Technol. 2010, 64, 337–347. [Google Scholar] [PubMed]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Heard, C.; Screen, C. Probing the permeation enhancement of mefenamic acid by ethanol across full-thickness skin, heat-separated epidermal membrane and heat-separated dermal membrane. Int. J. Pharm. 2008, 349, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Skelly, J.P.; Yacobi, A.; Shah, V.P.; Flynn, G.L.; Maibach, H.I.; Wester, R.C.; Guy, R.H. FDA and AAPS Report of the Workshop on Principles and Practices of In Vitro Percutaneous Penetration Studies: Relevance to Bioavailability and Bioequivalence. Pharm. Res. 1987, 4, 265–267. [Google Scholar] [CrossRef]
- Aggarwal, N.; Goindi, S. Development of hydroalcoholic gels of griseofulvin containing terpenes for the treatment of topical fungal infections. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1415–1423. [Google Scholar]
- Aupova, R.; Sakipova, Z.; Zemlicka, M. Study of rheological properties of carbomer gels. Life Sci. J. 2014, 11, 25–27. [Google Scholar]
- Goates, C.Y.; Knutson, K. Enhanced permeation of polar compounds through human epidermis. I. Permeability and membrane structural changes in the presence of short chain alcohols. Biochim. Biophys. Acta 1994, 1195, 169–179. [Google Scholar] [CrossRef]
- Finnin, B.C.; Morgan, T.M. Transdermal penetration enhancers: Applications, limitations, and potential. J. Pharm. Sci. 1999, 88, 955–958. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Kumar, S.; Malick, A.; Meltzer, N.; Mouskountakis, J.; Behl, C. Studies of in vitro skin permeation and retention of a leukotriene antagonist from topical vehicles with a hairless guinea pig model. J. Pharm. Sci. 1992, 81, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Sheskey, P.; Cook, W.; Cable, C. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2016; p. 239. ISBN 9780853697923. [Google Scholar]
- Bronaugh, R.; Stewart, R.; Congdon, E. Methods for in vitro percutaneous absorption studies II. Animal models for human skin. Toxicol. Appl. Pharmacol. 1982, 62, 481–488. [Google Scholar] [CrossRef]
- Harada, K.; Murakami, T.; Kawasaki, E.; Higashi, Y.; Yamamoto, S.; Yata, N. In-vitro permeability to salicylic acid of human, rodent, and shed snake skin. J. Pharm. Pharmacol. 1993, 45, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Schmook, F.; Meingassner, J.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar] [CrossRef]
- Barry, B.W. Mode of action of penetration enhancers in human skin. J. Control. Release 1987, 6, 85–97. [Google Scholar] [CrossRef]
- Heylings, J.R.; Diot, S.; Esdaile, D.J.; Fasano, W.J.; Manning, L.A.; Owen, H.M. A prevalidation study on the in vitro skin irritation function test (SIFT) for prediction of acute skin irritation in vivo: Results and evaluation of ECVAM Phase III. Toxicol. In Vitro 2003, 17, 123–138. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; McCarthy, H.O.; Moan, J. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: Potential for enhanced topical photodynamic therapy. J. Control. Release 2008, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Davis, S.P.; Holiday, N.R.; Wang, J.; Gill, H.S.; Prausnitz, M.R. Transdermal delivery of insulin using microneedles in vivo. Pharm. Res. 2004, 21, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Verbaan, F.J.; Bivas-Benita, M.; Bungener, L.; Huckriede, A.; van den Berg, D.J.; Kersten, G.; Bouwstra, J.A. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J. Control. Release 2009, 136, 71–78. [Google Scholar] [CrossRef] [PubMed]
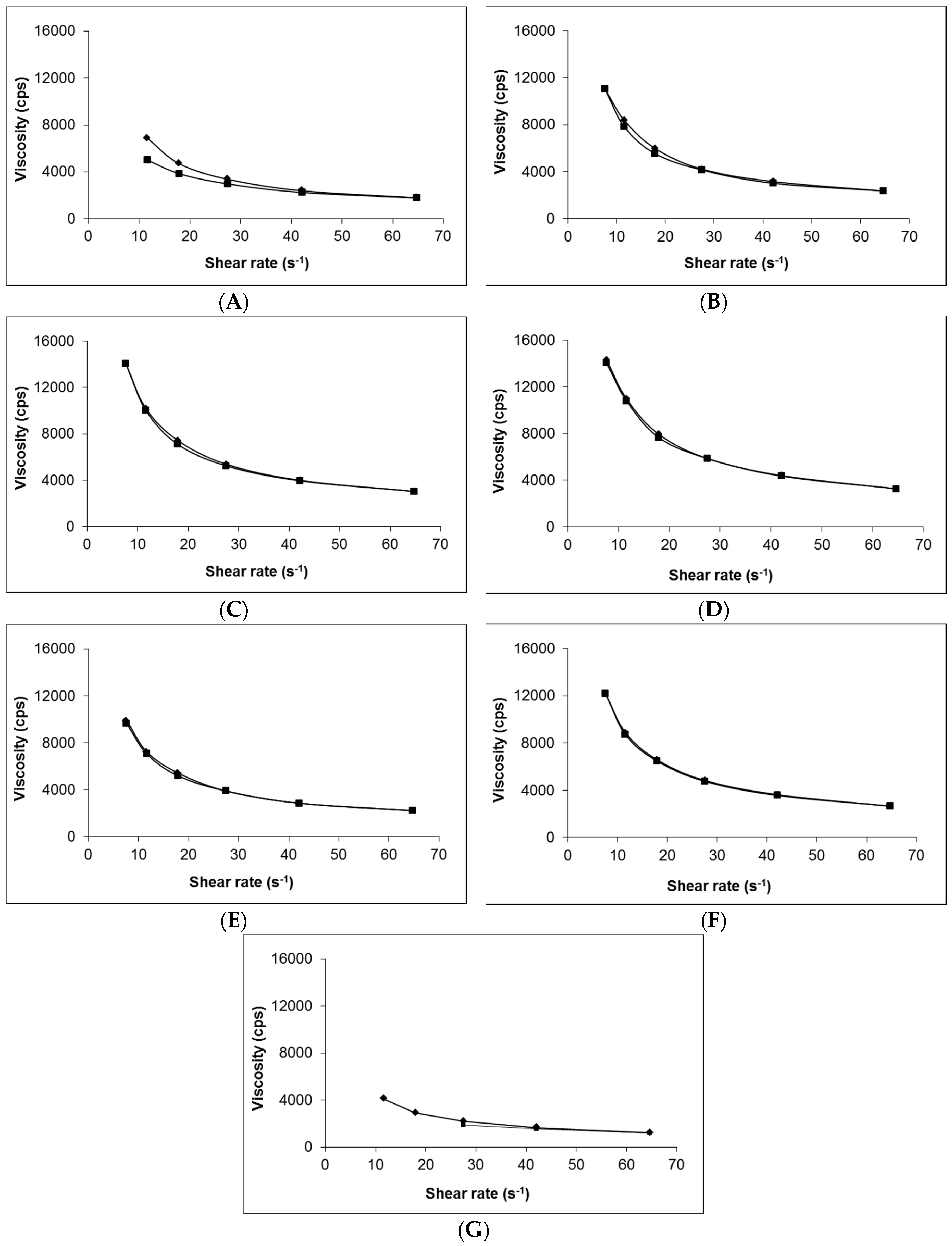
 ) and blank Carbopol® 1.5% (w/w) (). Data presented as mean ± standard error of mean (SEM) (n = 3).
) and blank Carbopol® 1.5% (w/w) (). Data presented as mean ± standard error of mean (SEM) (n = 3).
 ) and blank Carbopol® 1.5% (w/w) (). Data presented as mean ± standard error of mean (SEM) (n = 3).
) and blank Carbopol® 1.5% (w/w) (). Data presented as mean ± standard error of mean (SEM) (n = 3).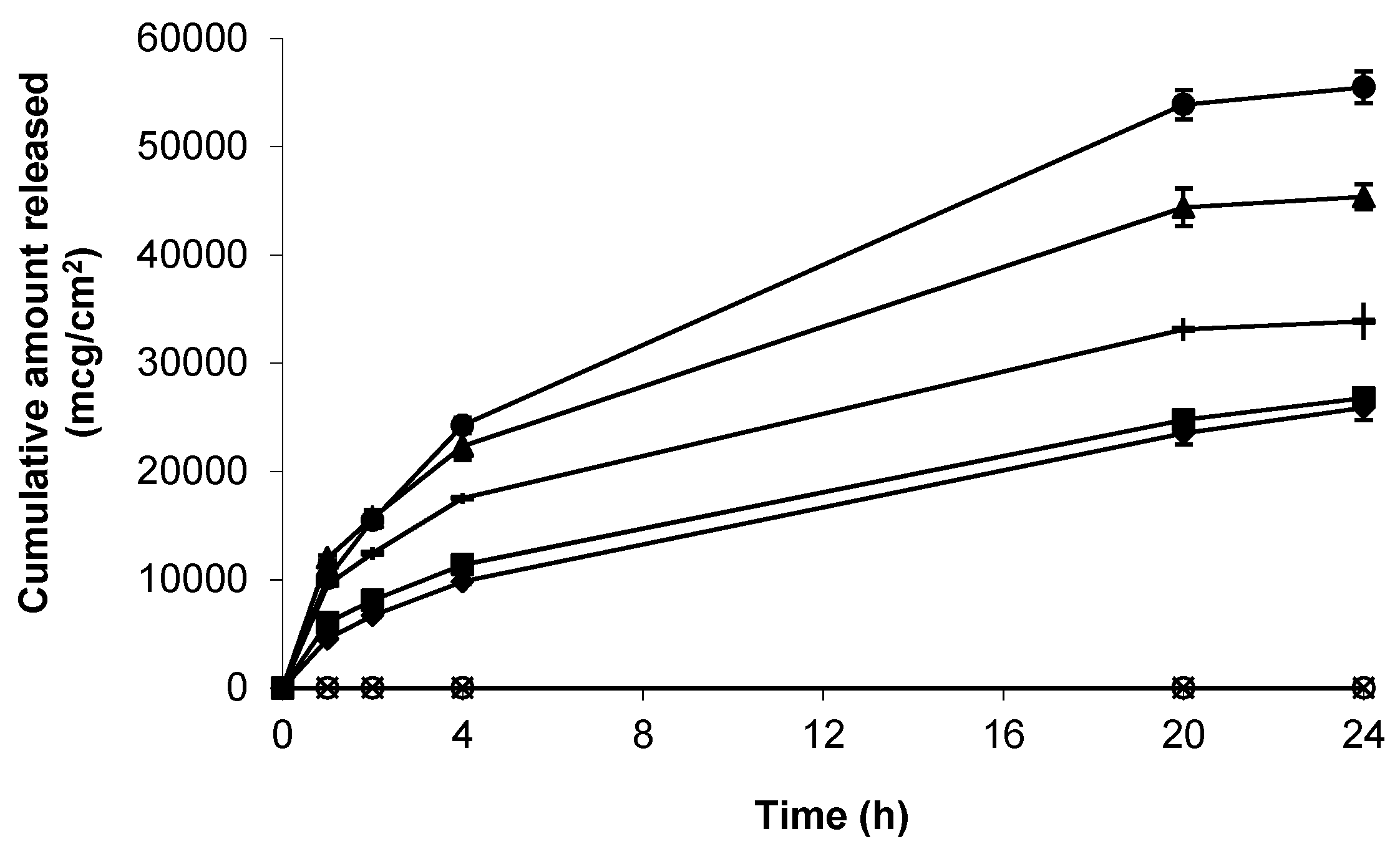
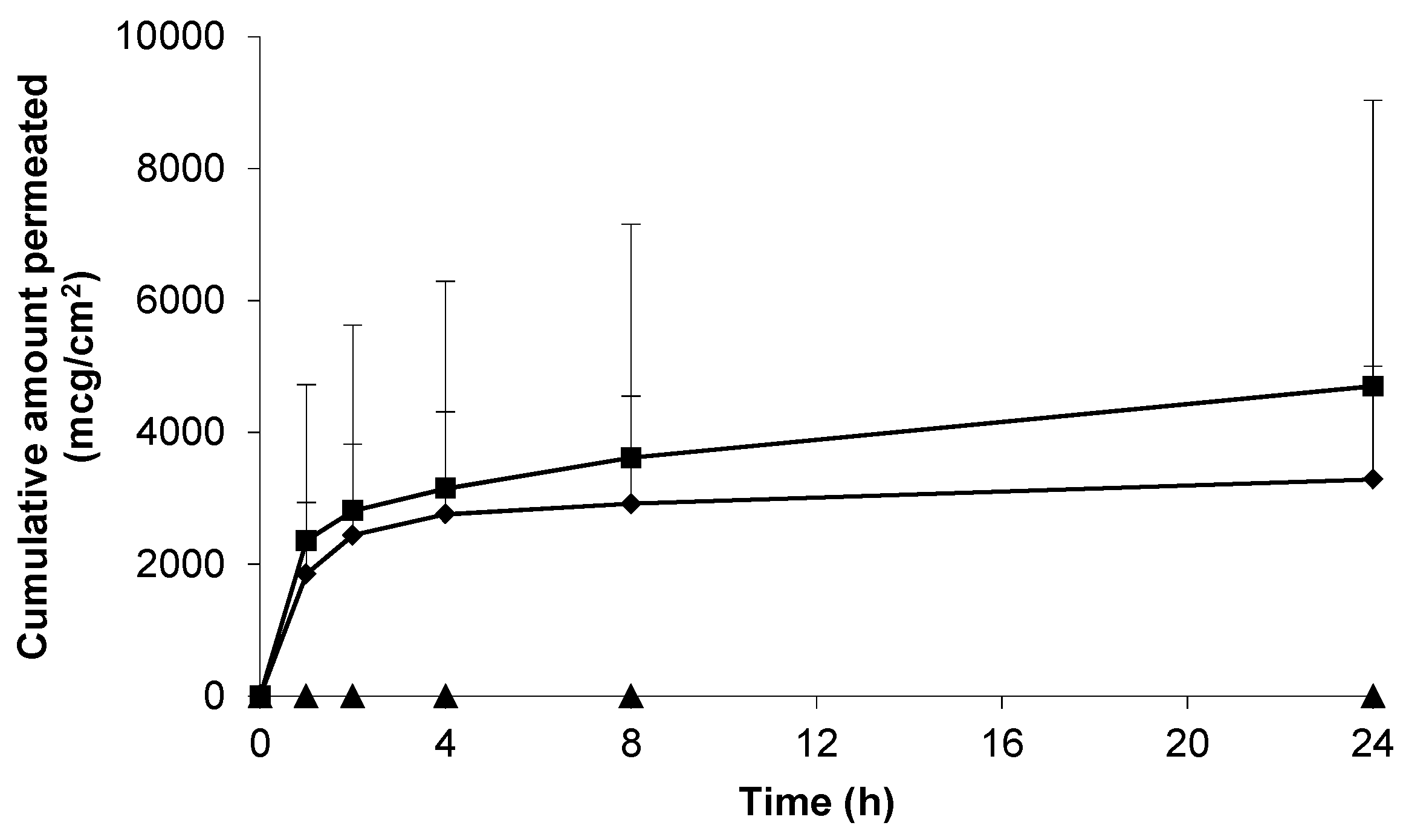

 , n = 3) and blank 0.75% (w/w) gel containing 5% (w/w) DMSO (-, n = 2) across non-hydrated human epidermal membrane. Data presented as mean ± SEM.
, n = 3) and blank 0.75% (w/w) gel containing 5% (w/w) DMSO (-, n = 2) across non-hydrated human epidermal membrane. Data presented as mean ± SEM.
 , n = 3) and blank 0.75% (w/w) gel containing 5% (w/w) DMSO (-, n = 2) across non-hydrated human epidermal membrane. Data presented as mean ± SEM.
, n = 3) and blank 0.75% (w/w) gel containing 5% (w/w) DMSO (-, n = 2) across non-hydrated human epidermal membrane. Data presented as mean ± SEM.

| Formulation | |||||
|---|---|---|---|---|---|
| Gabapentin (%(w/w)) | Base (%(w/w)) | Permeation Enhancer (%(w/w)) | Characteristics | Viscosity (cps × 103) | pH |
| 0 | Carbopol® (1.5) | - | Transparent, homogeneous | - | - |
| 0 | Lipoderm® | - | Off-white, homogeneous | 1.74 | - |
| 0 | Carbopol® (0.75) | - | Transparent, homogeneous | 2.37 | 6.19 |
| 10 | Carbopol® (1.5) | - | Transparent, homogeneous | - | - |
| 10 | Lipoderm® | - | Off-white, homogeneous | 1.20 | 6.00 |
| 10 | Versatile™ | - | Off-white, homogeneous | - | - |
| 10 | Doublebase™ | - | White, homogeneous | - | - |
| 6 | Carbopol® (0.75) | - | Transparent, homogeneous | 2.98 | 6.34 |
| 6 | Carbopol® (0.75) | Ethanol (30.0) | Transparent, homogeneous, characteristic EtOH smell | 3.28 | 6.65 |
| 6 | Carbopol® (0.75) | Ethanol (70.0) | Translucent, homogeneous, characteristic EtOH smell | 2.24 | 7.15 |
| 6 | Carbopol® (0.75) | DMSO (5.0) | Transparent, homogeneous, characteristic DMSO smell | 2.68 | 7.00 |
| 6 | Carbopol® (0.75) | IPM (5.0) | Transparent, biphasic | - | - |
| 6 | Carbopol® (0.75) | DMI (10.0) | Transparent, homogeneous | - | - |
| 6 | Carbopol® (0.75) | PG (5.0) | Transparent, homogeneous | - | - |
| Vehicle | Skin MODEL | Approximate Gabapentin Dose Applied (mg) | Mean Jss(4–24 h) ± SEM (mcg/cm2/h) | Reference |
|---|---|---|---|---|
| 5% (50 mg/mL) gabapentin in EtOH:H20 (70:30) co-solvent solution | Human pre-hydrated breast skin epidermis | 25 | 26.30 ± 11.00 | This paper |
| 5% (50 mg/mL) gabapentin in EtOH:H20 (70:30) co-solvent solution | Human non-hydrated breast skin epidermis | 25 | 77.56 ± 59.80 | This paper |
| 0.5% (5 mg/mL) gabapentin in EtOH:H20 (70:30) co-solvent solution | Rat skin epidermis | 5 | 63.29 ± 1.62 | [11] |
| Gabapentin aqueous solution | Porcine skin | Unknown | Insignificant | [16] |
| Gabapentin water solution (100 mg/mL) | Dermatomed porcine skin | 10 | 262.50 | [15] |
| Compounded 10% (w/w) gabapentin in Lipoderm® base | Human non-hydrated breast skin epidermis | 100 | 23.82 ± 3.51 | This paper |
| Combination 10% gabapentin in Lipoderm® base | Dermatomed human trunk skin | 0.5 | 0.11 a | [22] |
| 10% (w/w) gabapentin 1.5% (w/w) Carbopol® gel | Human non-hydrated breast skin epidermis | 100 | Insignificant | This paper |
| 6% (w/w) gabapentin 70% (w/w) EtOH 0.75%(w/w) Carbopol® gel | Human pre-hydrated breast skin epidermis | 60 | 3.75 ± 3.75 | This paper |
| 6% (w/w) gabapentin 5% (w/w) DMSO 0.75% (w/w) Carbopol® gel | Human non-hydrated breast skin epidermis | 60 | 7.56 ± 5.50 | This paper |
| 6% (w/w) gabapentin 10% (w/w) DMI 0.75% (w/w) Carbopol® gel | Human pre-hydrated breast skin epidermis | 60 | Insignificant | This paper |
| 6% gabapentin 5% (w/w) PG 0.75% (w/w) Carbopol® gel | Human non-hydrated breast skin epidermis | 60 | Insignificant | This paper |
| 0.5% (5 mg/mL) gabapentin w/o microemulsion | Rat skin epidermis | 5 | 128.22 ± 1.84 | [11] |
| 0.7% (6.9 mg/mL) gabapentin liposomes | Porcine skin | Unknown | 219.90 ± 48.20 | [16] |
| Gabapentin pluronic lecithin organogel | Porcine skin | Unknown | 19.00 ± 10.60 | [16] |
| Versatile™ cream (10% gabapentin) | Dermatomed human trunk skin | 3 | 0.10 ± 0.10 a | [17] |
| Inverted hexagonal liquid crystals(2% gabapentin) | Dermatomed porcine skin | 2 | 56.25 a | [15] |
| Lamellar liquid crystals (6% gabapentin) | Dermatomed porcine skin | 6 | 92.31 a | [15] |
| 10% (w/w) gabapentin in Versatile™ cream | Human non-hydrated breast skin epidermis | 100 | Insignificant | This paper |
| 10% (w/w) gabapentin Doublebase™ cream | Human non-hydrated breast skin epidermis | 100 | Insignificant | This paper |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, C.J.; Alcock, N.; Hiom, S.; Birchall, J.C. Development and Evaluation of Topical Gabapentin Formulations. Pharmaceutics 2017, 9, 31. https://doi.org/10.3390/pharmaceutics9030031
Martin CJ, Alcock N, Hiom S, Birchall JC. Development and Evaluation of Topical Gabapentin Formulations. Pharmaceutics. 2017; 9(3):31. https://doi.org/10.3390/pharmaceutics9030031
Chicago/Turabian StyleMartin, Christopher J., Natalie Alcock, Sarah Hiom, and James C. Birchall. 2017. "Development and Evaluation of Topical Gabapentin Formulations" Pharmaceutics 9, no. 3: 31. https://doi.org/10.3390/pharmaceutics9030031
APA StyleMartin, C. J., Alcock, N., Hiom, S., & Birchall, J. C. (2017). Development and Evaluation of Topical Gabapentin Formulations. Pharmaceutics, 9(3), 31. https://doi.org/10.3390/pharmaceutics9030031






