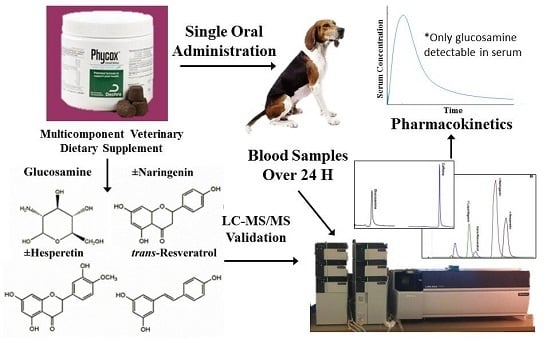
Pharmacokinetic Analysis of an Oral Multicomponent Joint Dietary Supplement (Phycox®) in Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Analytical System and Conditions
2.3. Glucosamine
2.4. Polyphenols
2.5. Stock and Working Standard Solutions
2.6. Sample Preparation for Standard Curves
2.6.1. Glucosamine
2.6.2. Polyphenols
2.7. Precision, Accuracy and Recovery
2.8. Content Analysis of Polyphenols in Phycox®
2.9. Animals and Compliance with Ethical Standards
2.10. Pharmacokinetic Studies
2.11. Treatment of Pharmacokinetic Samples
2.11.1. Glucosamine
2.11.2. Polyphenols
2.12. Pharmacokinetic Analysis
2.13. Data Analysis
3. Results
3.1. LC-MS/MS Analyses
3.1.1. Glucosamine
3.1.2. Polyphenols
3.2. Linearity and Limit of Quantification
3.2.1. Glucosamine
3.2.2. Polyphenols
3.3. Precision, Accuracy and Recovery
3.3.1. Glucosamine
3.3.2. Polyphenols
3.3.3. Content Analysis of Polyphenols in Phycox®
3.4. Glucosamine
3.5. Polyphenols
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adebowale, A.; Du, J.; Liang, Z.; Leslie, J.L.; Eddington, N.D. The bioavailability and pharmacokinetics of glucosamine hydrochloride and low molecular weight chondroitin sulfate after single and multiple doses to beagle dogs. Biopharm. Drug Dispos. 2002, 23, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Sanchez, C.; Balligand, M. Pharmaceutical and nutraceutical management of canine osteoarthritis: present and future perspectives. Vet. J. 2005, 170, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rychel, J.K. Diagnosis and treatment of osteoarthritis. Top. Companion Anim. Med. 2010, 25, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hulse, D. Treatment methods for pain in the osteoarthritic patient. Vet. Clin. North. Am. Small Anim. Pract. 1998, 28, 361–375. [Google Scholar] [CrossRef]
- Day, R.O.; Graham, G.G. Non-steroidal anti-inflammatory drugs (NSAIDs). Br. Med. J. 2013, 3195, 1–7. [Google Scholar]
- Dingle, J.T. The effect of nonsteroidal antiinflammatory drugs on human articular cartilage glycosaminoglycan synthesis. Osteoarthr. Cartil. 1999, 7, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.M.; Lichtenstein, D.R.; Singh, G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1999, 340, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Vandeweerd, J.M.; Coisnon, C.; Clegg, P.; Cambier, C.; Pierson, A.; Hontoir, F.; Saegerman, C.; Gustin, P.; Buczinski, S. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J. Vet. Intern. Med. 2012, 26, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Zie, G.; Jia, W. Towards polypharmacokinetics: pharmacokinetics of multicomponent drugs and herbal medicines using a metabolomics approach. Evid. Based Complement. Alternat. Med. 2013, 2013, 819147. [Google Scholar] [CrossRef] [PubMed]
- He, S.-M.; Chan, E.; Zhou, S.F. Properties of herbal medicines in humans: evidence, challenges andstrategies. Curr. Pharm. Des. 2011, 17, 357–407. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.E.; Chen, Y.; Ho, E.A.; Martinez, S.A.; Davies, N.M. Pharmacological effects of a C-phycocyanin-based multicomponent nutraceutical in an in vitro canine chondrocyte model of osteoarthritis. Can. J. Vet. Res. 2015, 79, 241–249. [Google Scholar] [PubMed]
- Comblain, F.; Serisier, S.; Barthelemy, N.; Balligand, M.; Henrotin, Y. Review of dietary supplements for the management of osteoarthritis in dogs in studies from 2004 to 2014. J. Vet. Pharmacol. Ther. 2016, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.K.; Regier, P.; Satyanarayana, A. Comparison of glucosamine absorption after administration of oral liquid, chewable, and tablet formulations to dogs. J. Am. Anim. Hosp. Assoc. 2016, 52, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Pieloch, M.J. Method of use and dosage composition of bluegreen algae extract for inflammation in animals. US Patent 7025965 B1, 11 April 2006. [Google Scholar]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Neil, K.M.; John, P.; Orth, M.W. The role of glucosamine and chondroitin sulfate in treatment for and prevention of osteoarthritis in animals. J. Am. Vet. Med. Assoc. 2005, 226, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Debbi, E.M.; Agar, G.; Fichman, G.; Ziv, Y.B.; Kardosh, R.; Halperin, N.; Elbaz, A.; Beer, Y.; Debi, R. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: a randomized controlled study. BMC Complement. Altern. Med. 2011, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; Cho, M.L.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Najafi, M.; Hamzeiy, H.; Maleki-Dizaji, N.; Pezeshkian, M.; Sadeghi-Bazargani, H.; Darabi, M.; Mostafalou, S.; Bohlooli, S.; Garjani, A. Protective effects of methylsulfonylmethane on hemodynamics and oxidative stress in monocrotaline-induced pulmonary hypertensive rats. Adv. Pharmacol. Sci. 2012, 507278. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Enazi, M.M.; Ahmed, M.M.; Al-Rejaie, S.S. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 2013, 19, 5633–5644. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.N.; Spelman, K. Clinical utility of curcumin extract. Altern. Ther. Health Med. 2013, 19, 20–22. [Google Scholar]
- Muzzio, M.; Huang, Z.; Hu, S.; Johnson, W.D.; McCormick, D.L.; Kapetanovic, I.M. Determination of resveratrol and its sulfate and glucuronide metbaolites in plasma by LC-MS/MS and their pharmaockinetics in dogs. J. Pharm. Biomed. Anal. 2012, 59, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mata-Bilbao; Mde, L.; Andres-Lacueva, C.; Roura, E.; Jáuregui, O.; Escribano, E.; Torre, C.; Lamuela-Raventós, R.M. Absorption and pharmacokinetics of grapefruit flavanones in beagles. Br. J. Nutr. 2007, 98, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Liu, M.H.; Zou, W.; Guan, X.L.; Lai, L.; Su, W.W. Toxicokinetics of naringin and its metabolite naringenin after 180-day repeated oral administration in beagle dogs assayed by rapid resolution liquid chromatogrpahy/tandem mass spectrometric method. J. Asian Nat. Prod. Res. 2012, 14, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Remsberg, C.M.; Good, R.L.; Davies, N.M. Ingredient consistency of commercially available polyphenol and tocopherol nutraceuticals. Pharmaceutics 2010, 2, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Roupe, K.A.; Yáñez, J.A.; Teng, X.W.; Davies, N.M. Pharmacokinetics of selected stilbenes: Rhapontigenin, piceatannol and pinosylvin in rats. J. Pharm. Pharmacol. 2006, 58, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tsai, S.; Chao, P.L.; Yen, H.; Chien, T.; Hsiu, S. Determination of hesperetin and its conjugate metabolites in serum and urine. J. Food Drug Anal. 2002, 10, 143–148. [Google Scholar] [CrossRef]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.; Davies, N.M. Stereospecific high-performance liquid chromatographic analysis of naringenin in urine. J. Pharm. Biomed. Anal. 2005, 39, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.; Teng, X.W.; Roupe, K.; Davies, N.M. Stereospecific high-performance liquid chromatographic analysis of hesperetin in biological matrices. J. Pharm. Biomed. Anal. 2005, 37, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Sabatini, L.; Barbieri, A.; Guardigli, M.; Locatelli, M.; Violante, F.S.; Rovati, L.C.; Persiani, S. Development and validation of a sensitive HPLC-ESI-MS/MS method for the direct determination of glucosamine in human plasma. J. Chromatogr. B 2006, 844, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Pastorini, E.; Rotini, R.; Guardigli, M.; Vecchiotti, S.; Persiani, S.; Trisolino, G.; Antonioli, D.; Rovati, L.C.; Roda, A. Development and validation of a HPLC-ES-MS/MS method for the determination of glucosamine in human synovial fluid. J. Pharm. Biomed. Anal. 2009, 50, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhong, D.; Chen, X. Improved and simplified liquid chromatography/electrospray ionization mass spectrometry method for the analysis of underivatized glucosamine in human plasma. J. Chromatogr. B 2007, 854, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Gilzad-kohan, M.H.; Aghazadeh-Habashi, A.; Jamali, F. Absorption and Bioavailability of Glucosamine in the Rat. J. Pharm. Sci. 2012, 101, 2574–2583. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zhang, Q.; Wang, Y.; Lee, B.; Betageri, G.V.; Chow, M.S.; Huang, M.; Zuo, Z. Bioavailability enhancement of glucosamine hydrochloride by chitosan. Int. J. Pharm. 2013, 455, 365–373. [Google Scholar] [CrossRef] [PubMed]




| Active Ingredients Per Soft Chew | |
|---|---|
| Glucosamine hydrochloride | 450 mg |
| Methylsulfonylmethane | 400 mg |
| Creatine monohydrate | 250 mg |
| Alpha-linolenic acid | 200 mg |
| Proprietary blend of citrus bioflavonoids, calcium phosphate, manganese sulfate, ascorbic acid (vitamin C), zinc sulfate, alpha lipoic acid, and grape seed extract | 132 mg |
| Turmeric | 50 mg |
| Phycox active | 30 mg |
| Eicosapentaenoic acid (EPA) | 9 mg |
| Docosahexaenoic acid (DHA) | 6 mg |
| Boron | 100 μg |
| Selenium | 10 μg |
| Alpha tocopheryl acetate (vitamin E) | 25 IU |
| Inactive ingredients | |
| Flaxseed oil, hydrolyzed vegetable protein, magnesium stearate, marine lipid concentrates, natural liver flavor, and sucrose | |
| Compound | Parent Ion m/z | Daughter Ion m/z | Collision Energy (eV) |
|---|---|---|---|
| Glucosamine | 180.20 | 162.10 | 10 |
| Caffeine (internal standard) | 194.90 | 138.00 42.00 110.15 | 19 36 24 |
| ±Hesperetin | 302.80 | 153.10 | 24.0 |
| ±Naringenin | 273.90 | 153.10 | 24.0 |
| trans-Resveratrol | 228.80 | 135.10 107.20 | 13.0 23.0 |
| ±Liquiritigenin (internal standard) | 257.90 | 137.0 | −22.0 |
| Analyte | Nominal Value (μg/mL) | Measured Value (μg/mL) (Mean ± SEM) | CV (%) | Bias (%) |
|---|---|---|---|---|
| Glucosamine | ||||
| Low | 0.075 | 0.063 ± 0.000 | 2.93 | −16.4 |
| Medium | 1.5 | 1.46 ± 0.102 | 12.2 | −2.91 |
| High | 15 | 14.6 ± 0.989 | 11.7 | −2.45 |
| ±Hesperetin | ||||
| Low | 0.075 | 0.087 ± 0.000 | 0.662 | 14.2 |
| Medium | 1.5 | 1.44 ± 0.062 | 7.40 | −3.64 |
| High | 15 | 13.7 ± 0.155 | 1.97 | −9.87 |
| trans-Resveratrol | ||||
| Low Medium High ±Naringenin Low Medium High | 0.075 1.5 15 0.075 1.5 15 | 0.088 ± 0.000 1.47 ± 0.063 14.6 ± 0.219 0.083 ± 0.004 1.55 ± 0.102 14.3 ± 0.219 | 0.267 7.49 2.57 7.98 11.4 2.78 | 17.7 −2.87 −1.50 10.1 3.30 −2.28 |
| Pharmacokinetic Parameter | Mean ± SEM |
|---|---|
| AUC0–∞ (µg·h/mL) | 20.4 ± 2.34 |
| Vd/F (L/kg) | 2.10 ± 0.254 |
| CL/F (L/h/kg) | 2.56 ± 0.446 |
| t1/2 (h) | 0.584 ± 0.034 |
| Tmax (h) | 2.00 ± 0.000 |
| Cmax (μg/mL) | 9.69 ± 1.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, S.E.; Lillico, R.; Lakowski, T.M.; Martinez, S.A.; Davies, N.M. Pharmacokinetic Analysis of an Oral Multicomponent Joint Dietary Supplement (Phycox®) in Dogs. Pharmaceutics 2017, 9, 30. https://doi.org/10.3390/pharmaceutics9030030
Martinez SE, Lillico R, Lakowski TM, Martinez SA, Davies NM. Pharmacokinetic Analysis of an Oral Multicomponent Joint Dietary Supplement (Phycox®) in Dogs. Pharmaceutics. 2017; 9(3):30. https://doi.org/10.3390/pharmaceutics9030030
Chicago/Turabian StyleMartinez, Stephanie E., Ryan Lillico, Ted M. Lakowski, Steven A. Martinez, and Neal M. Davies. 2017. "Pharmacokinetic Analysis of an Oral Multicomponent Joint Dietary Supplement (Phycox®) in Dogs" Pharmaceutics 9, no. 3: 30. https://doi.org/10.3390/pharmaceutics9030030