Investigation of Drug–Polymer Compatibility Using Chemometric-Assisted UV-Spectrophotometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
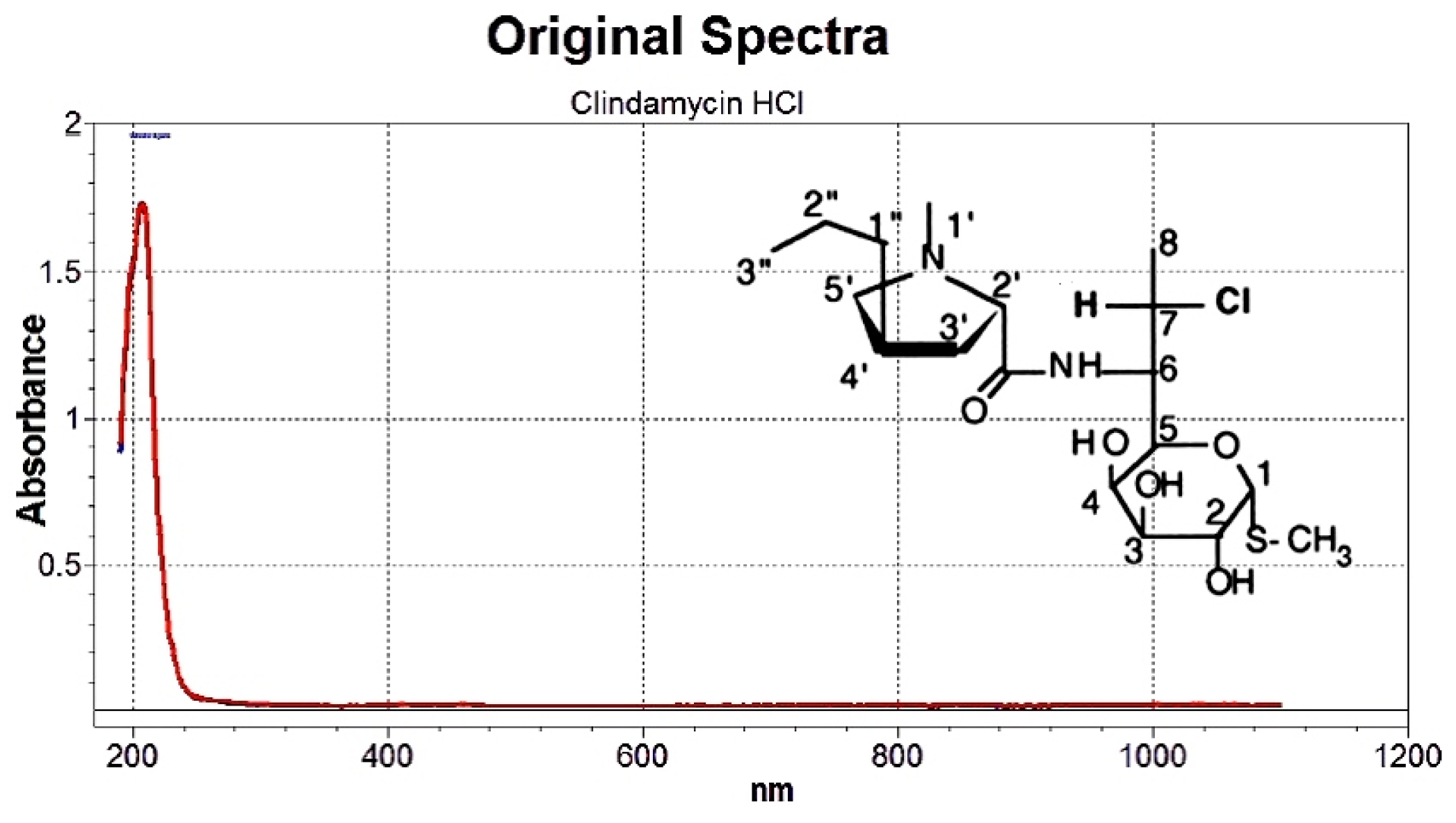
2.2.1. UV-Spectroscopic Studies
Preparation of Standard Stock Solutions
Selection of Appropriate Wavelength Range
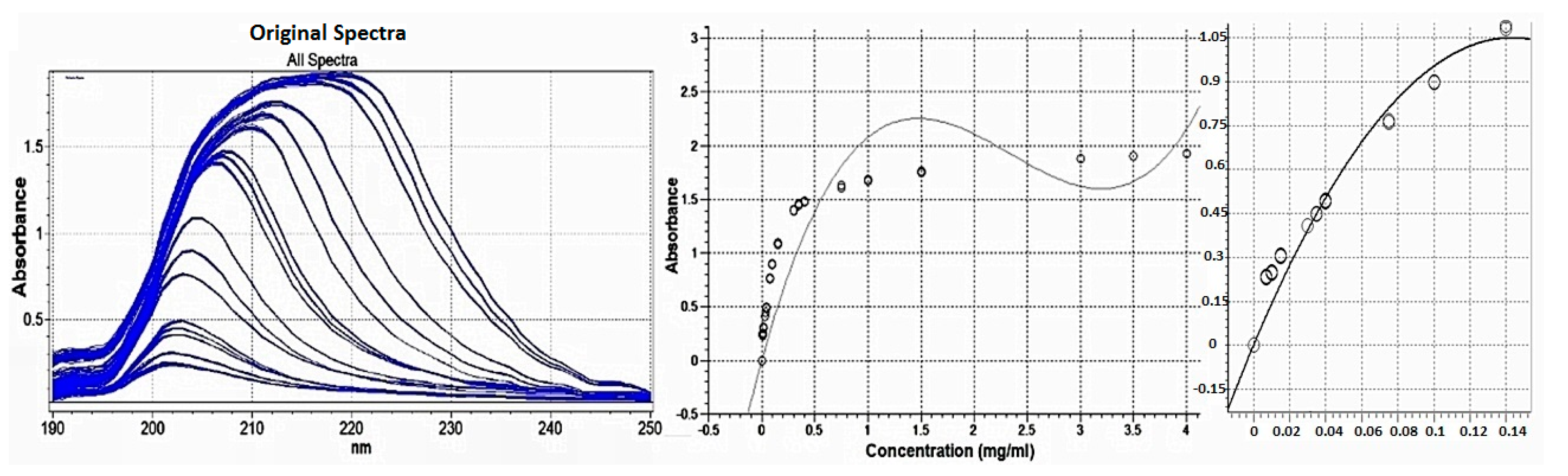
Selection of Linear Concentration Range
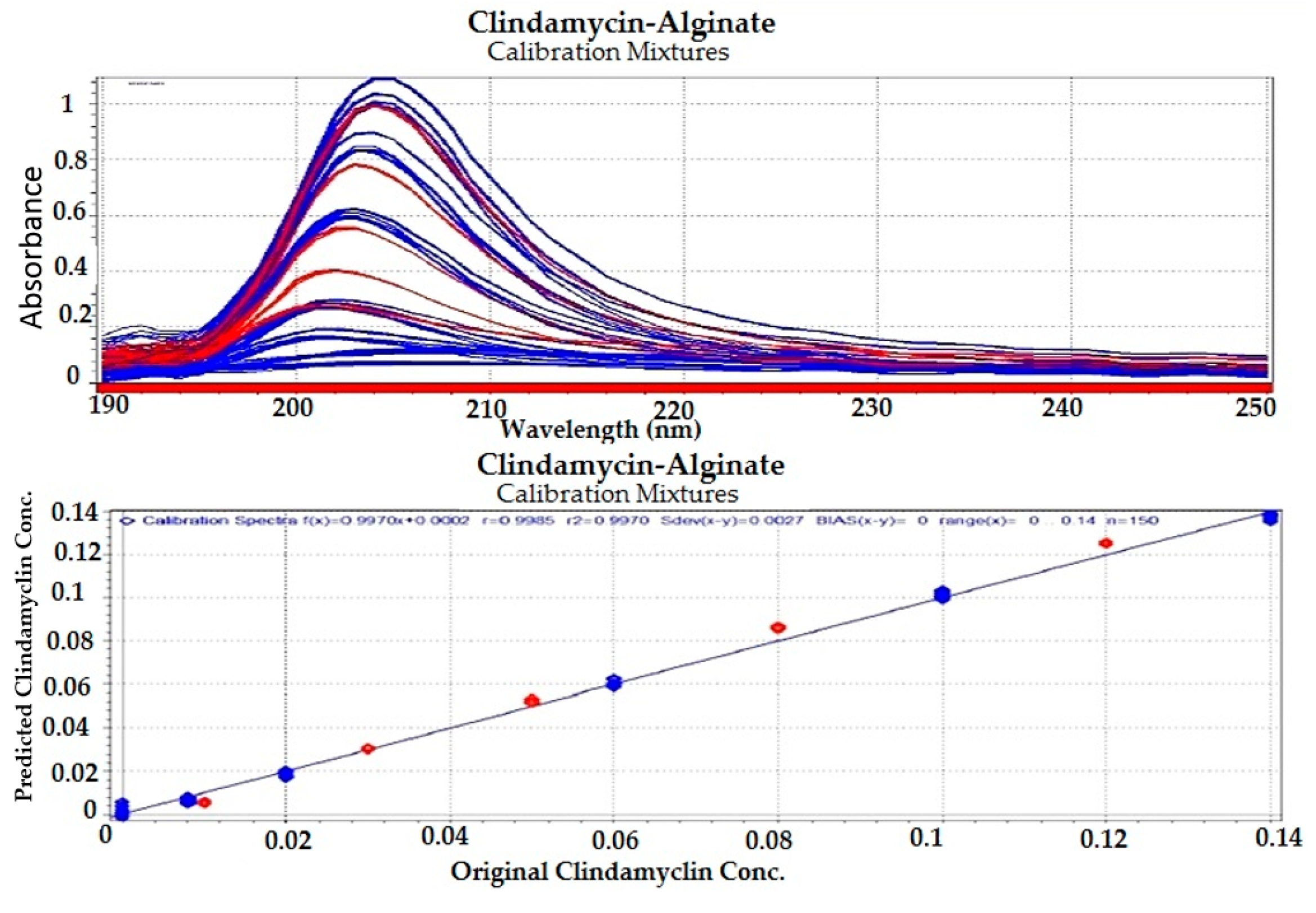
Preparation of Standard Calibration Drug–Polymer Mixtures
Preparation of Test Drug–Polymer Mixtures
Data Collection
Chemometric Analysis
Recovery Analysis
2.2.2. Physico-Chemical Analysis
Preparation of Drug–Polymer Physical Mixtures and Blend Films
Differential Scanning Calorimetry (DSC) Thermal Analysis
X-ray Diffraction Crystallography (XRD)
Fourier Transform Infrared (FTIR) Spectral Analysis
2.2.3. Effect of Polymers on Clindamycin Anti-Bacterial Activity against Staphylococcus aureus
Bacterial Isolates and Culture Conditions
Preparation of Sterile Stock Solutions (Drug or Polymer)
Preparation of Working Dilutions for Agar Susceptibility Tests
Bacterial Growth Inhibition Assay (Agar Dilution Susceptibility Method)
3. Results and Discussion
3.1. UV-Spectroscopic Studies
3.1.1. Selection of Wavelength Range
3.1.2. Selection of Concentration Range
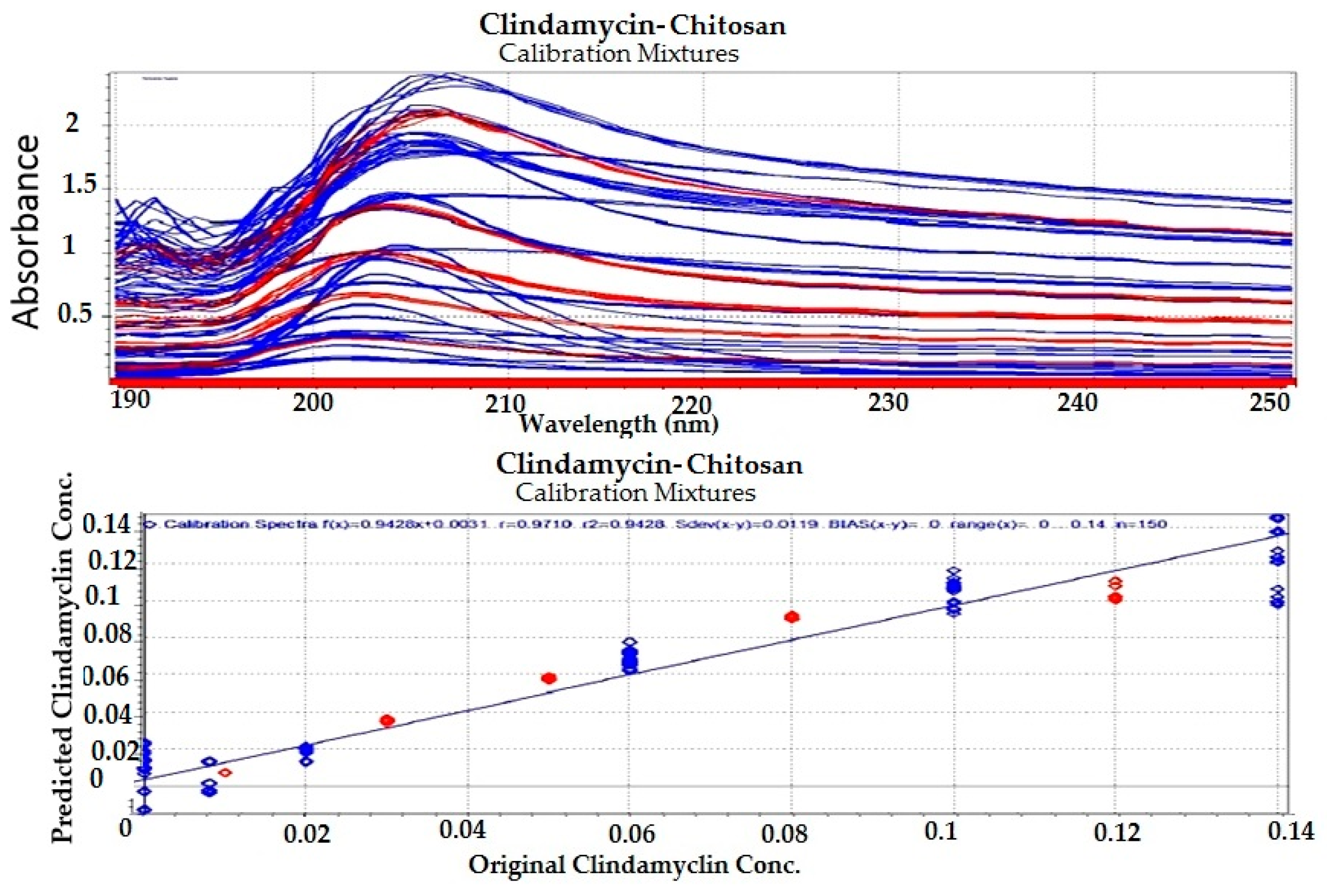
3.1.3. Preparation of Calibration and Test Drug–Polymer Mixtures
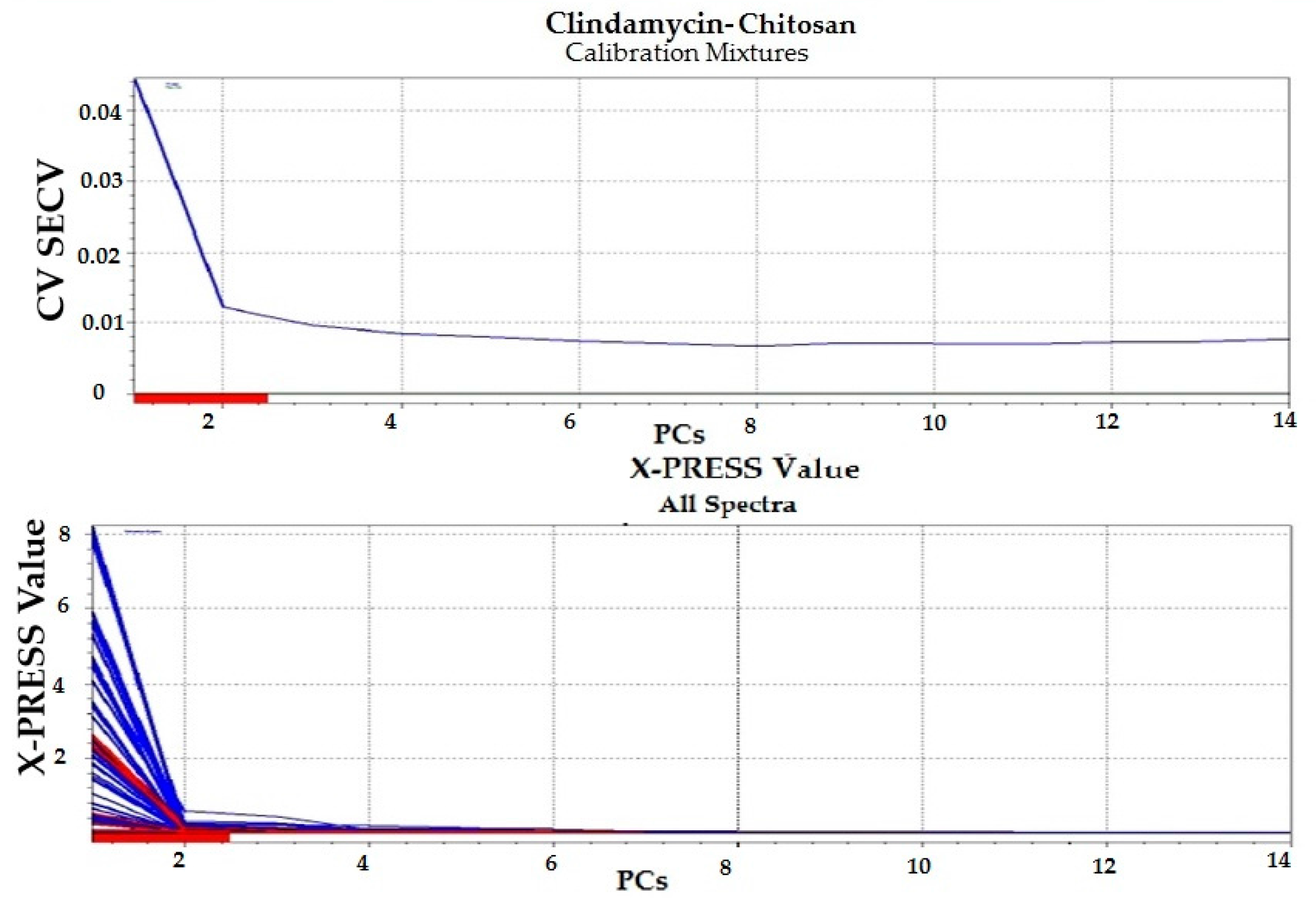
3.1.4. Chemometric Analysis
3.1.5. Recovery Analysis
3.2. Physico-Chemical Analysis
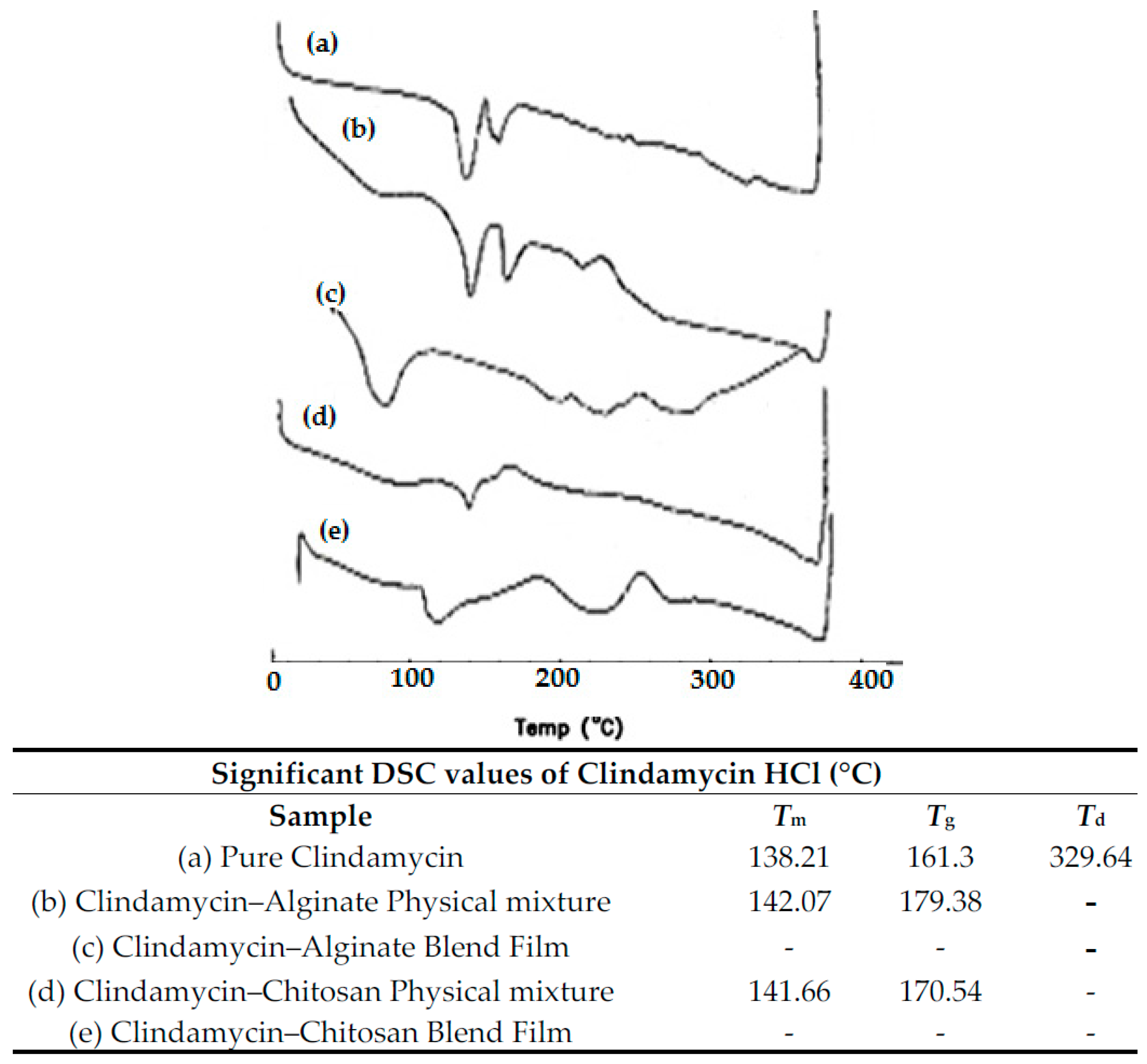
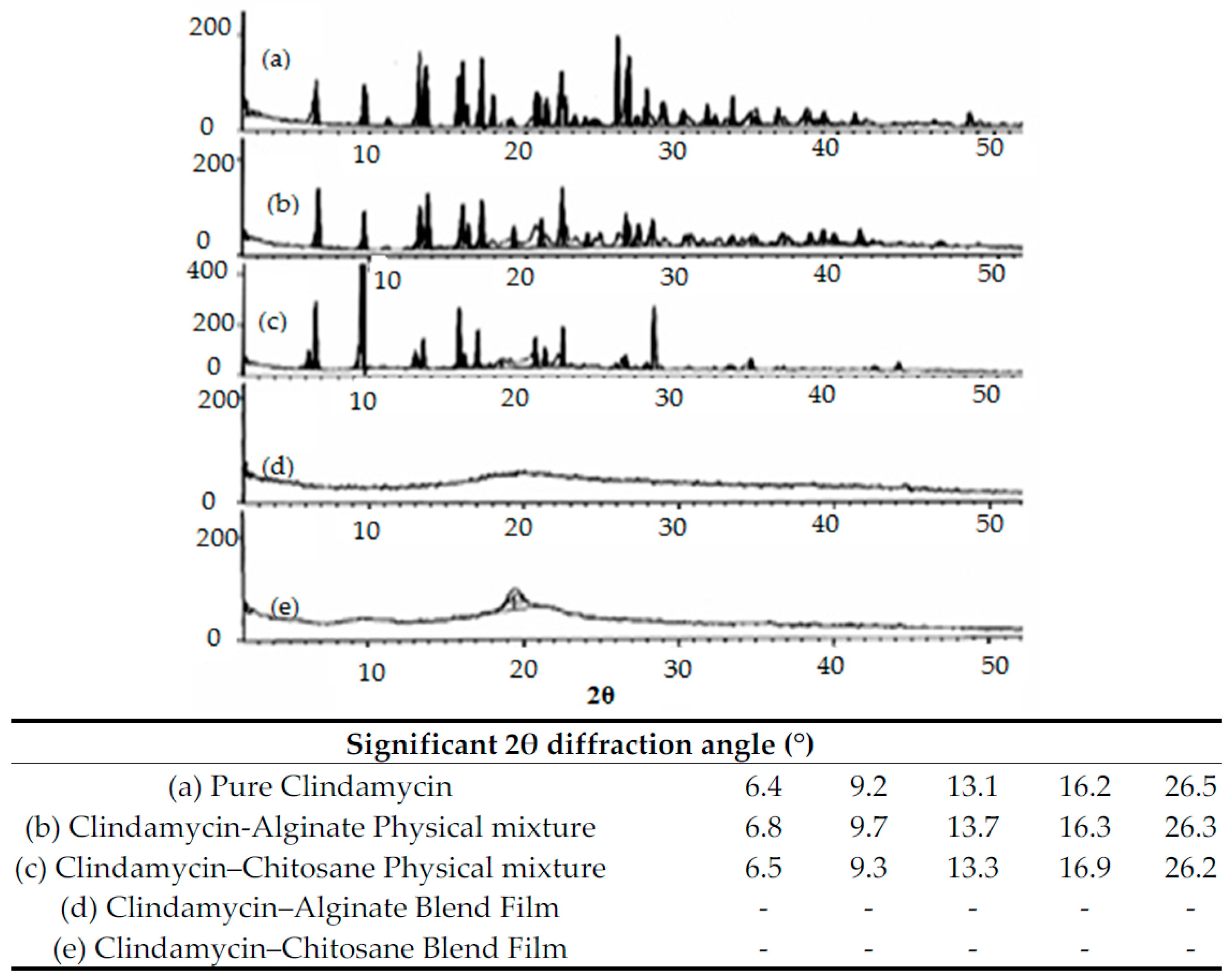
3.2.1. DSC Thermal Analysis and X-ray Diffraction
3.2.2. Fourier Transform Infrared (FTIR) Spectral Analysis
3.3. Effect of Polymers on Clindamycin Anti-Bacterial Activity against Staphylococcus aureus
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kumar, N.; Goindi, S.; Saini, B.; Bansal, G. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J. Therm. Anal. Calorim. 2014, 115, 2375–2383. [Google Scholar] [CrossRef]
- Dinte, E.; Bodoki, E.; Leucuta, S. Compatibility studies between drugs and excipients in the preformulation phase of buccal mucoadhesive systems. Farmacia 2013, 61, 703–712. [Google Scholar]
- Dinte, E.; Tomuţǎ, I.; Muţ, E.M.; Iovanov, R.I.; Leucuţa, S.E. Chemometric methods for the simultaneous assay of chloramphenicol, chlorhexidine and metronidazole during in vitro dissolution of drugs from mucoadhesive buccal gels. Farmacia 2010, 58, 572–582. [Google Scholar]
- Abdel-Hamid, M.E. Applications of spectrometric full spectrum quantitation (FSQ) software for multicomponent analysis and stability studies of pharmaceuticals. Anal. Lett. 2000, 33, 2719–2735. [Google Scholar] [CrossRef]
- Mohamed, A.E.-M.I.; Mikre, W. Determination of lamivudine and stavudine in pharmaceutical preparations using chemometrics-assisted spectrophotometry. Saudi Pharm. J. 2009, 17, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sankar, A.; Vetrichelvan, T.; Venkappaya, D. Simultaneous estimation of ramipril, acetylsalicylic acid and atorvastatin calcium by chemometrics assisted UV-spectrophotometric method in capsules. Acta Pharm. 2011, 61, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Martelo-Vidal, M.; Vázquez, M. Classification of red wines from controlled designation of origin by ultraviolet-visible and near-infrared spectral analysis. Ciênc. Téc. Vitivinic. 2014, 29, 35–43. [Google Scholar] [CrossRef]
- Gomes, C.L.; Lima, A.C.A.; Cândido, M.C.L.; Silva, A.B.R.; Loiola, A.R.; Nascimento, R.F. Multivariate Analysis of Perfumes by Ultraviolet Spectrophotometry. J. Braz. Chem. Soc. 2015, 26, 1730–1736. [Google Scholar] [CrossRef]
- Terrile, A.E.; Marcheafave, G.; Oliveira, G.; Rakocevic, M.; Brunsd, R.; Scarminio, I. Chemometric analysis of UV characteristic profile and infrared fingerprint variations of Coffea arabica green beans under different space management treatments. J. Braz. Chem. Soc. 2016, 27, 1–10. [Google Scholar]
- Sheraz, M.; Kazi, S.H.; Ahmed, S.; Qadeer, K.; Khan, M.F.; Ahmad, I. Multicomponent spectrometric analysis of riboflavin and photoproducts and their kinetic applications. Cent. Eur. J. Chem. 2014, 12, 635–642. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.A.; Trissel, L.A.; Williams, K.Y. Compatibility and stability of linezolid injection admixed with three quinolone antibiotics. Ann. Pharmacother. 2000, 34, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Bosso, J.A.; Townsend, R.J. Stability of clindamycin phosphate and ceftizoxime sodium, cefoxitin sodium, cefamandole nafate, or cefazolin sodium in two intravenous solutions. Am. J. Hosp. Pharm. 1985, 42, 2211–2214. [Google Scholar] [PubMed]
- Kamble, M.S.; Mane, O.R.; Borwandkar, V.G.; Mane, S.S.; Chaudhari, P.D.; College, P.E.S.M. Formulation and evaluation of clindamycin HCL—Chitosan microspheres for dry powder inhaler formulation. Drug Invent. Today 2012, 4, 527–530. [Google Scholar]
- Mohamed, A.I.; Ahmed, O.A.A.; Amin, S.; Elkadi, O.A.; Kassem, M.A. In vivo evaluation of clindamycin release from glyceryl monooleate-alginate microspheres by NIR spectroscopy. Int. J. Pharm. 2015, 494, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Nyamweya, N.; Gönen, S.; Martı́nez-Miranda, L.J.; Hoag, S.W. Influence of various drugs on the glass transition temperature of poly(vinylpyrrolidone): A thermodynamic and spectroscopic investigation. Int. J. Pharm. 2001, 225, 83–96. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Ioele, G.; Spatari, C.; Ragno, G. Optimization of wavelength range and data interval in chemometric analysis of complex pharmaceutical mixtures. J. Pharm. Anal. 2016, 6, 64–69. [Google Scholar] [CrossRef]
- Chen, C.S.; Brown, C.W. A drug dissolution monitor employing multiple fiber optic probes and a UV/visible diode array spectrophotometer. Pharm. Res. 1994, 11, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Uhrich, K.E. Concurrent release of admixed antimicrobials and salicylic acid from salicylate-based poly(anhydride-esters). J. Biomed. Mater. Res. A 2009, 91, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Daihom, B.; Alayoubi, A.; Ma, D.; Wang, L.; Mishra, S.; Helms, R.; Almoazen, H. Development and physicochemical characterization of clindamycin resinate for taste masking in pediatrics. Drug Dev. Ind. Pharm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.; Karkhane, R.; Riazi-Esfahani, M.; Dorkoosh, F.; Rafiee-Tehrani, M.; Tamaddon, L. Thermoanalytical characterization of clindamycin-loaded intravitreal implants prepared by hot melt extrusion. Adv. Biomed. Res. 2015, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Chen, D.; Ma, X.; Liu, Y. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int. J. Pharm. 2005, 294, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.W.; Beyer, W.F. Clindamycin Hydrochloride. Anal. Profiles Drug Subst. 1981, 10, 75–91. [Google Scholar]
- Kacuráková, M. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Bergo, V.; Mamaev, S.; Olejnik, J.; Rothschild, K.J. Methionine changes in bacteriorhodopsin detected by FTIR and cell-free selenomethionine substitution. Biophys. J. 2003, 84, 960–966. [Google Scholar] [CrossRef]
- Bharathi, C.; Jayaram, P.; Raj, J.S.; Kumar, M.S.; Bhargavi, V.; Handa, V.K.; Dandalaa, R.; Naidub, A. Identification, isolation and characterization of impurities of clindamycin palmitate hydrochloride. J. Pharm. Biomed. Anal. 2008, 48, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
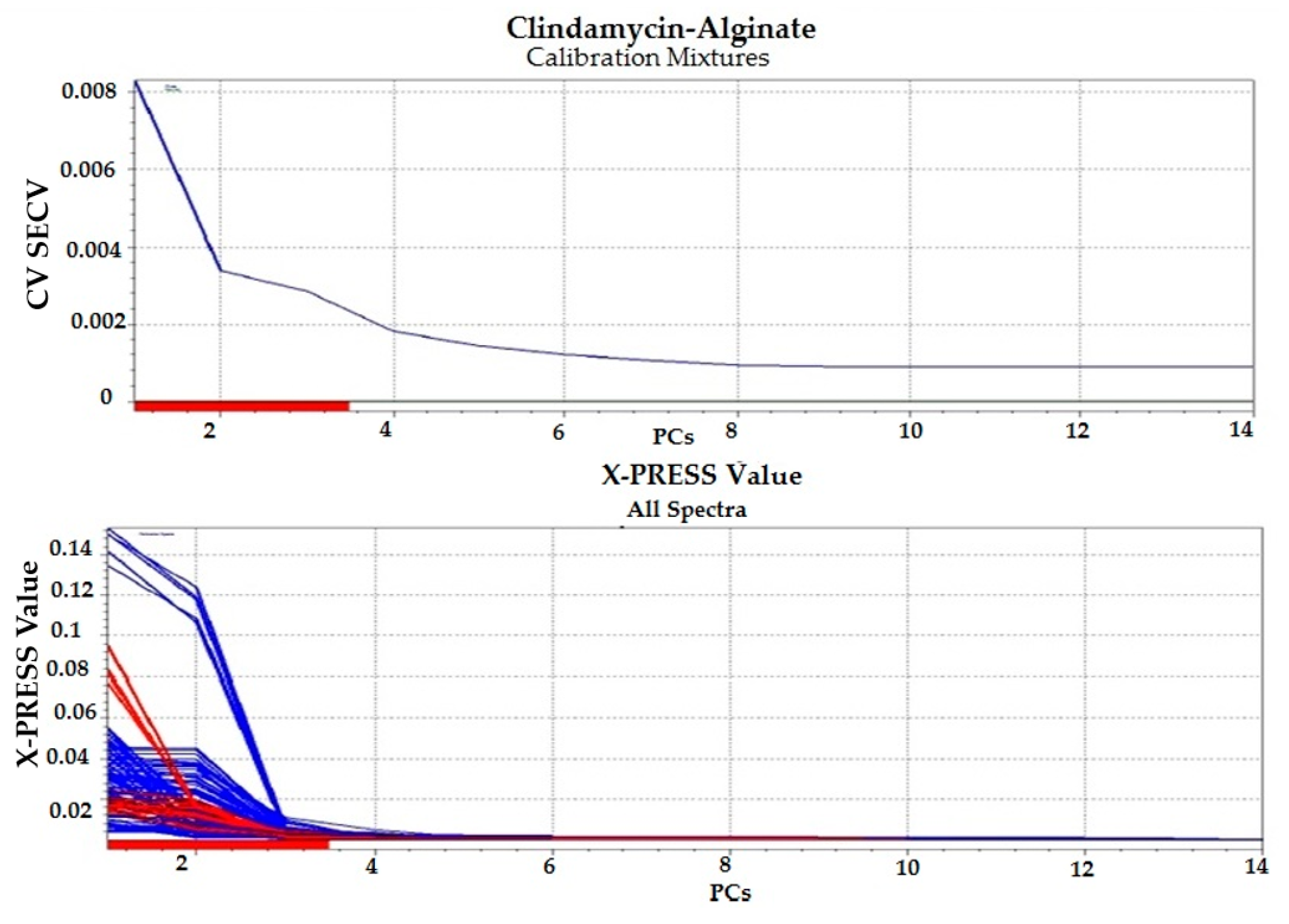
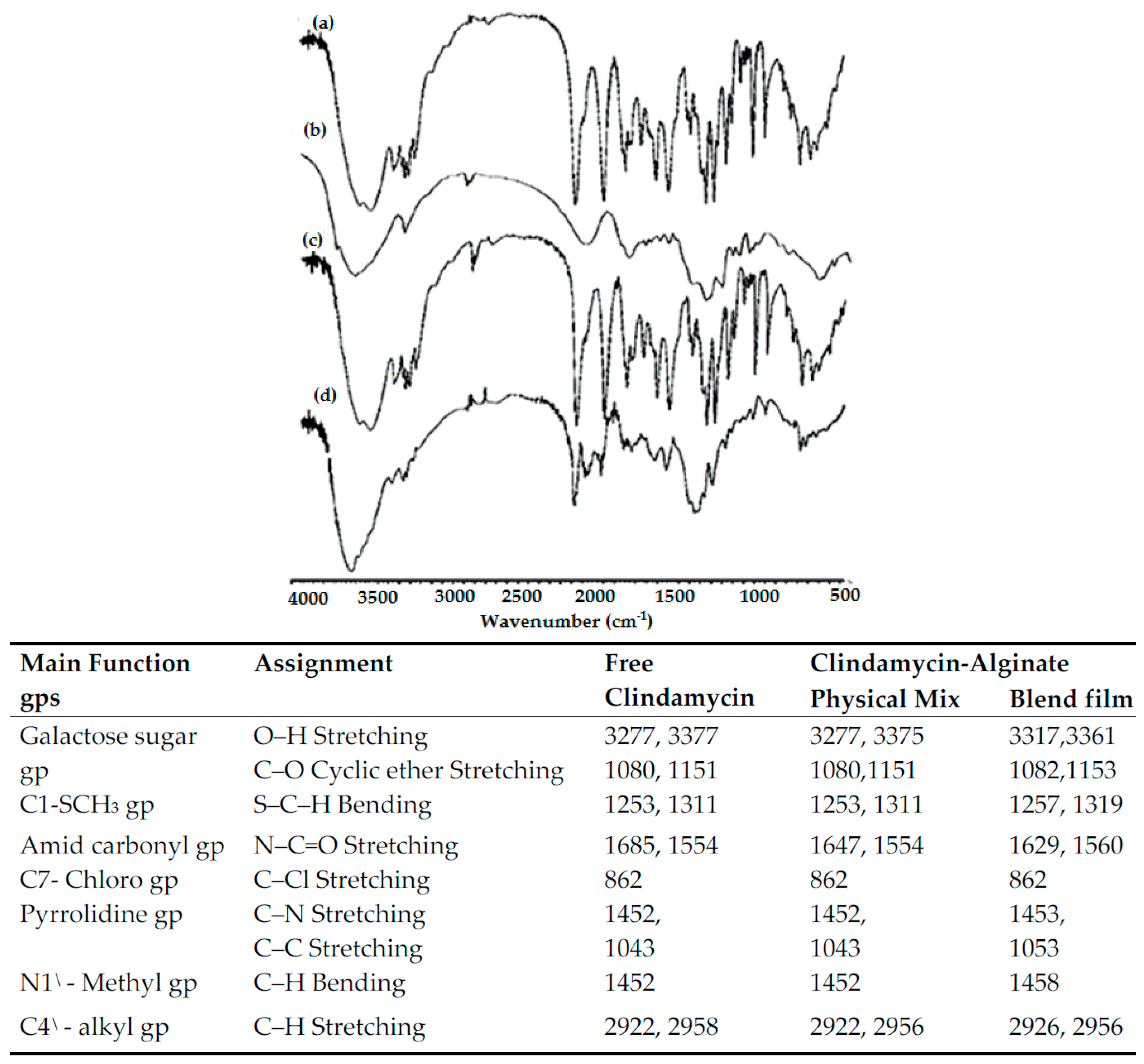
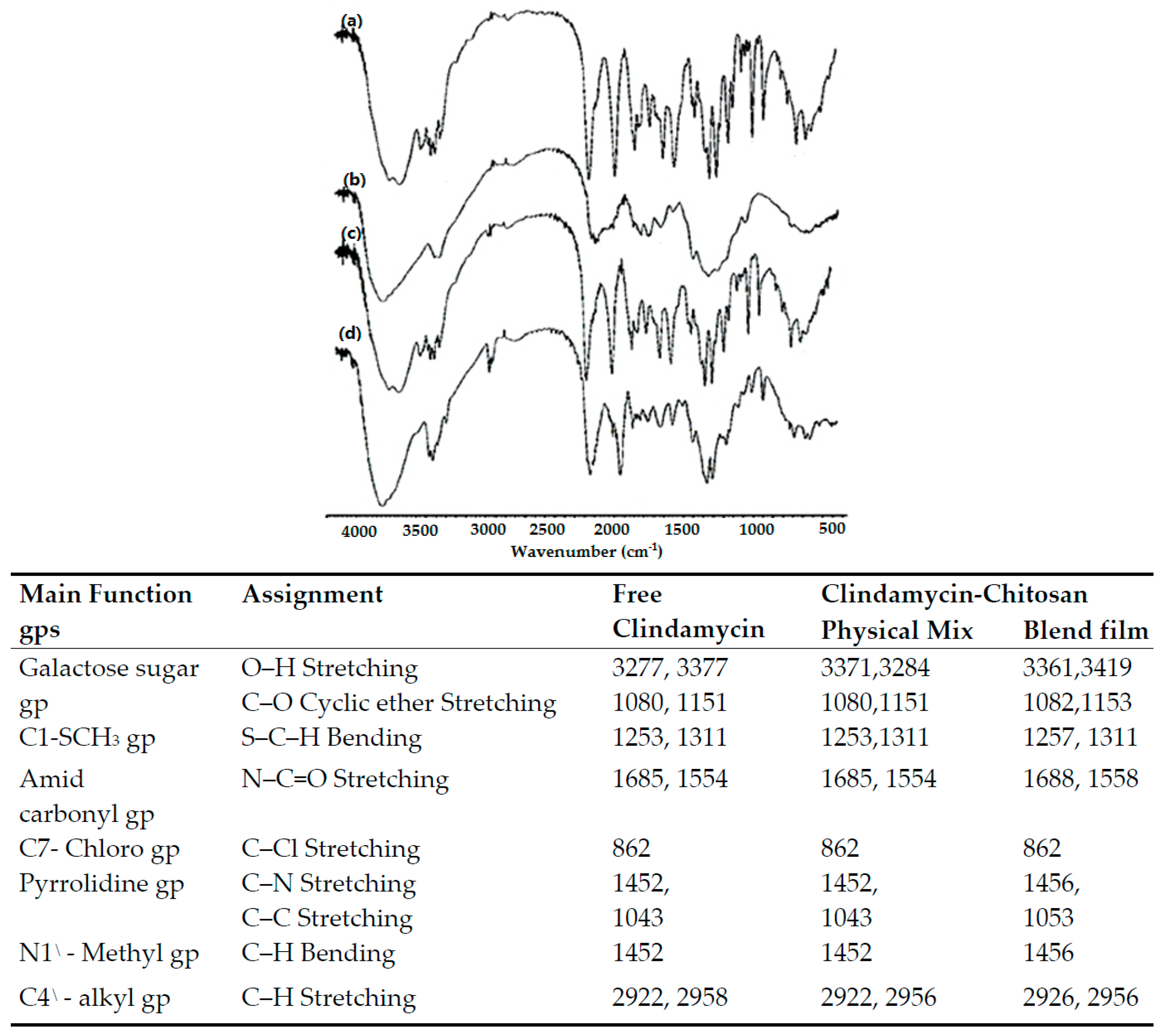
| Model | PCs | RMSECV | R2 | Offset | Bias |
|---|---|---|---|---|---|
| Clindamycin-Alginate Calibration Mixtures | 3 | 0.00284 | 0.996 | −0.0000774 | −0.0000338 |
| Clindamycin-Chitosan Calibration Mixtures | 2 | 0.01228 | 0.942 | −0.0052874 | −0.0013723 |
| Model | Clindamycin Concentration Average Recovery (%) * ± (SD) ** | ||
|---|---|---|---|
| Sample | After 3 Days | After 7 Days | |
| Clindamycin-Alginate Calibration Mixtures | Pure Clindamycin | 99.1 ± 1.4 | 97.1 ± 0.6 |
| Clindamycin-Alginate Test Mixtures | 98.1 ± 2.9 | 95.4 ± 4.0 | |
| Clindamycin-Chitosan Calibration Mixtures | Pure Clindamycin | 101.8 ± 1.5 | 100.6 ± 2.5 |
| Clindamycin–Chitosan Test Mixtures | 90.4 ± 3.0 | 81.3 ± 4.2 | |
| Clindamycin–Chitosan Test Mixtures after filtration | 97.2 ± 2.1 | 91.3 ± 3.8 | |
| Sample No. | Composition | MIC (μg/mL) | Remarks |
|---|---|---|---|
| 1- Control | Distilled water | - | Effect of solvent |
| 2- Solvent control | 0.5% acetic acid | - | Effect of solvent |
| 2- Sodium alginate/water | Sodium alginate/water | - | Effect of polymer |
| 3- Chitosan/water | Chitosan/water | - | Effect of polymer |
| 4- Free Clindamycin | Clindamycin/water | 4 | Drug activity |
| 5- Clindamycin + Alginate | Clindamycin/Alginate | 4 | Drug–polymer blending effect |
| 6- Clindamycin + Chitosan | Clindamycin/Chitosan | 4 | Drug–polymer blending effect |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.I.; Abd-Motagaly, A.M.E.; Ahmed, O.A.A.; Amin, S.; Mohamed Ali, A.I. Investigation of Drug–Polymer Compatibility Using Chemometric-Assisted UV-Spectrophotometry. Pharmaceutics 2017, 9, 7. https://doi.org/10.3390/pharmaceutics9010007
Mohamed AI, Abd-Motagaly AME, Ahmed OAA, Amin S, Mohamed Ali AI. Investigation of Drug–Polymer Compatibility Using Chemometric-Assisted UV-Spectrophotometry. Pharmaceutics. 2017; 9(1):7. https://doi.org/10.3390/pharmaceutics9010007
Chicago/Turabian StyleMohamed, Amir Ibrahim, Amr Mohamed Elsayed Abd-Motagaly, Osama A. A. Ahmed, Suzan Amin, and Alaa Ibrahim Mohamed Ali. 2017. "Investigation of Drug–Polymer Compatibility Using Chemometric-Assisted UV-Spectrophotometry" Pharmaceutics 9, no. 1: 7. https://doi.org/10.3390/pharmaceutics9010007





